Review Article - (2021) Volume 12, Issue 11
A Brief Review on CAR-T Cell Therapy
Bodi Gayathri*, Sariki Ashok Krishnan and Korimelli SrideviAbstract
Cancer is one of the significant causes of death around the world. Chimeric Antigen Receptor-T cell therapy is the immunotherapeutic treatment for cancer involving collection of T cells from patient’s blood and is genetically modified in-vitro to express Chimeric Antigen Receptor. These modified T cells are reinfused into patient where they can recognize the tumor specific antigen resulting anti-tumor activity. This cytotoxic effect can also result in toxic effects like Cytokine Release Syndrome, neurotoxicity and off-tumor toxicity which can be fatal. Further research and development are required to improve the anti-cancer efficacy. This review describes about the structure of Chimeric Antigen Receptor, production of Chimeric Antigen Receptor-T cells, major adverse effects and their management.
Keywords
Cancer, Chimeric Antigen Receptor-T cell therapy, Genetic engineering, Cytokine Release Syndrome, Neurotoxicity, Off-tumor toxicity
Abbreviations
ALL: Acute Lymphoblastic Leukemia; BBB: Blood Brain Barrier; CAR-T therapy: Chimeric Antigen Receptor-T Cell Therapy; CD3: Cluster Differentiation 3; CRES: CAR-T Cell Related Encephalopathy Syndrome; CRP: C-Reactive Protein; CRS: Cytokine Release Syndrome; CSF: Cerebrospinal Fluid; CT: Computed Tomography; CTLA-4: Cytotoxic-T Lymphocyte Associated Antigen 4; EEG: Electroencephalogram; HLH: Hemophagocytic Lymphohistiocytosis; IL-6: Interleukin-6; ICAR: Inhibitory Chimeric Antigen Receptor; MRI: Magnetic Resonance Imaging; NHL: Non-Hodgkin Lymphoma; PD-1: Programmed Cell Death Protein-1; WHO: World Health Organization
Introduction
Cancer is the malignant growth resulting from uncontrollable cell division and potentially invades to other parts of body. According to World Health Organization (WHO) 18 million new cases of cancer are diagnosed every year and is second world wide cause of death about 8.8 million deaths in a year (Mattiuzzi C and Lippi G, 2019). Treatment for cancer includes chemotherapy, radiotherapy, immunotherapy and surgical treatment. Chimeric Antigen Receptor T cell (CAR-T) therapy is a type of Immunotherapy which utilizes the body’s own immune system to fight against cancer and improve body’s ability to detect and kill cancer cells.
T cells are the vital part of immune system that recognizes abnormal cells in the body. Cancer has developed mechanisms to evade killing by T cells. CAR-T therapy involves T cells that have been genetically engineered to enable the T cells to recognize cancerous cells. The receptors are chimeric because they combine both antigen binding and T cell activating functions into a single receptor.
Literature Review
Structure of Chimeric Antigen Receptor (CAR)
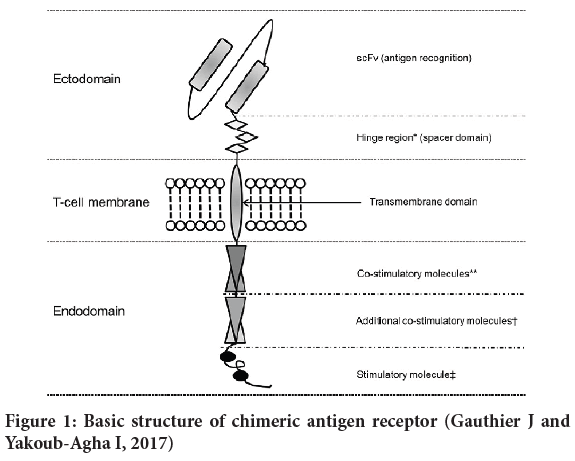
The structure of CAR consists of an antigen domain, hinge, one or more costimulatory domains and main signaling domain. The first generation CARs has main signaling domain whereas second and third generation CARs include one or two co-stimulatory domains along with main signaling domain, respectively. Second and third generation CAR-T cells are more potent (Gauthier J and Yakoub-Agha I, 2017) (Figure 1).
Figure 1: Basic structure of chimeric antigen receptor (Gauthier J and Yakoub-Agha I, 2017)
Production of CAR-T cells
The first step in the production of CAR-T cells involves isolation of T cells through a process called Leukapheresis, where white blood cells are isolated from either patient’s blood (autologous) or from a healthy donor (allogenic) (Martínez Bedoya D, et al., 2021). These T cells are taken to lab and are activated using anti-CD3 (Cluster Differentiation 3) or anti-CD3/anti-CD28 monoclonal antibody coated beads and are later genetically modified by transduction with viral vector to express CAR (Hay KA and Turtle CJ, 2017; Fesnak A and O’Doherty U, 2017). The virus used is inactive and are not able to replicate or cause any disease and the viral vector can be lentiviral which delivers gene construct to non-dividing cells or retroviral that delivers gene construct to dividing cells (Gauthier J and Yakoub-Agha I, 2017).
These modified T cells are then grown and expanded and are reinfused into patient. Prior to infusion, patient is treated with chemotherapy for lymphodepletion i.e., to prepare for the reception of CAR T cells, as the CAR T cells may not persist for longer time and may have decreased efficacy if not treated with chemotherapy (Hay KA and Turtle CJ, 2017).
Persistence can be vital in case of Acute Lymphoblastic Leukemia (ALL) where as it may not be necessarily important in Non-Hodgkin’s lymphoma (NHL) (Hay KA and Turtle CJ, 2017; Maude SL, et al., 2014). There are three factors that have impact on CAR T cell proliferation and persistence, they are lymphodepletion regimen, quantity of CAR T cells and cell product composition. Homogenization of cell product composition can be done by 1:1 ratio CD4+/CD8+ (Turtle CJ, et al., 2016).
Toxicities of CAR T cell therapy and their management
Though CAR T cell therapy has promising antitumor effects it has many adverse effects.
Cytokine Release Syndrome (CRS): It is the common side effect that occurred due to over activation of immune system and release of cytokines (Abken H, 2017; Brudno JN, Kochenderfer JN, 2016). These cytokines activate other cells of immune system especially leading to elevation of Interleukin-6 (IL-6) (Maude SL, et al., 2014). First stage of CRS includes signs of flu, muscle aches and patient can develop tachycardia, respiratory distress and arrhythmias, severe Hemophagocytic Lymphohistiocytosis (HLH). Severity of CRS depends on number of cells infused, degree of in-vitro CAR T cell proliferation and patients with comorbidities, those who develop early onset of CRS within 3 days of CAR-T cells infusion (Graham C, et al., 2018; Neelapu SS, et al., 2018). CRS after CAR-T cell therapy can occur in 54%-91% of patients and severe CRS in 8%-43% (Miliotou AN and Papadopoulou LC, 2018; Hay KA, et al., 2017).
Only the patients with limited comorbidities and who are able to tolerate severe CRS should be infused with CAR-T cell therapy (Miliotou AN and Papadopoulou LC, 2018).when an infection is suspected, patient should be assessed using blood, urine cultures and other tests like CT scan of chest, respiratory viral screening should be done. The infusion of CAR-T cells is carried out only when infection has been controlled or ruled out (Neelapu SS, et al., 2018).
After infusion of CAR-T cells patients should be monitored continuously and vital signs are checked for every 4 hours. If patient experiencing persistent heart rate of 115 beats per minute, vital signs are checked for every 2 hours (Brudno JN, Kochenderfer JN, 2016). Daily review of metabolic profiles, complete blood count, serum C-Reactive Protein (CRP) and ferritin levels should be done (Neelapu SS, et al., 2018). Management of acute symptoms of CRS can be done through grading system to assess the severity and provide treatment accordingly (Lee DW, et al., 2014). Fever can be managed by acetaminophen and hypotension by vasopressors like norepinephrine (Brudno JN, Kochenderfer JN, 2016).
Tocilizumab and siltuximab are IL-6 antagonists used as off-label drugs for reversal of CRS symptoms (Neelapu SS, et al., 2018; Maude SL, et al., 2014). Tocilizumab is the most commonly used and is effective for treating severe to life threatening CRS gly (Lee DW, et al., 2014). When the condition of patient is not stabilized after use of tocilizumab then immunosuppressive agents like corticosteroids are considered. Corticosteroids can have adverse effect on antitumor activity of adaptively transferred T cells gly (Lee DW, et al., 2014; Ledford H, 2014).
Neurotoxicity: It involves CAR-T Cell Related Encephalopathy Syndrome (CRES) with symptoms of cognitive disorders, confusion, restlessness, anxiety, aphasia, seizures, delirium, and can develop to fatal cerebral edema. Incidence of neurotoxicity in CAR-T cell treated patients can be 40% (12). This toxicity is due to systemic cytokines that cross Blood Brain Barrier (BBB). CAR-T cells are found in Cerebrospinal Fluid (CSF) of patients and increase in CAR-T cell infiltration in CSF is seen due to release of IL-6 during CRS and hyperthermia (Prudent V and Breitbart WS, 2017).
Continuous monitoring of patient given with CAR-T cell therapy through neuroimaging involving MRI, CT, EEG, to determine the severity and funduscopic examination to assess for papilledema. CRES with concurrent CRS can be treated with IL-6 antagonists whereas corticosteroids are preferred for severe CRES (Neelapu SS, et al., 2018; Lee DW, et al., 2014). Convulsive and non-convulsive status epilepticus is treated with benzodiazepines and other antiepileptics especially levetiracetam (Neelapu SS, et al., 2018).
On target on-tumor toxicity is due to rapid destruction of a large tumor mass and massive release of tumor cell components into circulation causing electrolyte and metabolic disturbances. This type of toxicity is seen in leukemia treatment and the risk is less in treatment to solid tumors (Abken H, 2017).
On target off-tumor toxicity, most of targets of CAR-T cells are also expressed in normal tissues, resulting in engagement of target antigen on non-pathogenic tissue leading to on target off-tumor toxicity (Bonifant CL, et al., 2016; Zhang Q, et al., 2020).This type of toxicity is majorly seen in solid tumors compared to leukemia and lymphoma (Miliotou AN and Papadopoulou LC, 2018). A fatal example of On target off-tumour toxicity where a patient treated with CAR-T cells for cancer associated with antigen HER-2/neu has developed respiratory failure and multi-organ dysfunctions leading to death due to reactivity against HER-2/neu expressed on pulmonary tissue (Bonifant CL, et al., 2016; Morgan RA, et al., 2010).
Off target off-tumor toxicity is the inflammatory reaction beyond the targeted tumor tissue. It is mediated through the CAR-T cell independently of target engagement (Miliotou AN and Papadopoulou LC, 2018).
Some advancements in CAR-T cell therapy for limiting toxicity
Transduction of CAR-T cells with inducible caspase-9 suicide gene that will switch off CAR-T cell proliferation in severe toxicity through gene expression and triggering apoptosis (Gauthier J and Yakoub-Agha I, 2017; Minagawa K, et al., 2016).
Bispecific CAR is a bispecific receptor containing two distinct intracellular signaling domains that are expressed as two different CARs on single cell surface (Mohanty R, et al., 2019).
Tandem CARs are Chimeric Antigen Receptors where two particular antigen recognition sites joined by linker and placed in tandem on a single intracellular domain and expressed as single CAR on a cell surface (Mohanty R, et al., 2019). Bispecific targeting can reduce antigen escape mechanism (Hegde M, et al., 2016).
Inhibitory CARs (ICARs) are antigen specific Inhibitory Chimeric Antigen Receptor intended to control T cell responses (Fedorov VD, et al., 2013). Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)-and programmed cell death protein 1 (PD-1)-based iCARs can limit cytotoxicity and cytokine secretion induced by chimeric receptor activation (Fedorov VD, et al., 2013).
Conclusion
AR-T cell therapy has revolutionized immunotherapeutic treatment of cancer and has a promising future. However, it can cause many toxic effects which require continuous monitoring and supplement treatments. Advancements in CAR designs and further developments in managing toxicities can enhance anti-tumor efficacy of the therapy.
Declarations
Availability of data and material
The datasets used during the current study are available from the corresponding author on request.
Author’s contribution
BG is the main contributor in writing the article. SA and KS helped in reviewing the article. All authors have read and approved the article.
References
- Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019; 9(4): 217.
- Gauthier J, Yakoub-Agha I. Chimeric Antigen-Receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr Res Transl Med. 2017; 65(3): 93-102.
- Martínez Bedoya D, Dutoit V, Migliorini D. Allogeneic CAR T cells: An alternative to overcome challenges of CAR T cell therapy in glioblastoma. Front Immunol. 2021; 12: 506.
- Hay KA, Turtle CJ. Chimeric Antigen Receptor (CAR) T cells: Lessons learned from targeting of CD19 in B-cell malignancies. Drugs. 2017; 77(3): 237-245.
- Fesnak A, O’Doherty U. Clinical development and manufacture of Chimeric Antigen Receptor t cells and the role of leukapheresis. Eur Oncol Haematol. 2017; 13(1): 28-34.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014; 371(16): 1507-1517.
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016; 126(6): 2123-2138.
- Abken H. Driving CARs on the highway to solid cancer: Some considerations on the adoptive therapy with CAR T cells. Hum Gene Ther. 2017; 28(11): 1047-1060.
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood. 2016; 127(26): 3321-3330.
- Graham C, Hewitson R, Pagliuca A, Benjamin R. Cancer immunotherapy with CAR-T cells-behold the future. Clin Med J R Coll Physicians London. 2018; 18(4): 324.
- Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol. 2018; 15(1): 47-62.
- Miliotou AN, Papadopoulou LC. CAR T-cell therapy: A new era in cancer immunotherapy. Curr Pharm Biotechnol. 2018; 19(1): 5-18.
- Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017; 130(21): 2295-2306.
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124(2): 188-195.
- Ledford H. Cancer treatment: The killer within. Nature. 2014; 508(7494): 24.
- Prudent V, Breitbart WS. Chimeric antigen receptor T-cell neuropsychiatric toxicity in acute lymphoblastic leukemia. Palliat Support Care. 2017; 15(4): 499-503.
- Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther-Oncolytics. 2016; 3: 16011.
- Zhang Q, Ping J, Huang Z, Zhang X, Zhou J, Wang G, et al. CAR-T cell therapy in cancer: tribulations and road ahead. J Immunol Res. 2020; 2020.
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010; 18(4): 843-851.
- Minagawa K, Jamil MO, Al-Obaidi M, Pereboeva L, Salzman D, Erba HP, et al. In vitro pre-clinical validation of suicide gene modified anti-CD33 redirected chimeric antigen receptor T-cells for acute myeloid leukemia. PLoS One. 2016; 11(12): e0166891.
- Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: A new era for cancer treatment. Oncol Rep. 2019; 42(6): 2183-2195.
- Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13R α 2 mitigate tumor antigen escape. J Clin Invest. 2016; 126(8): 3036-3052.
- Fedorov VD, Themeli M, Sadelain M. PD-1-and CTLA-4-based Inhibitory Chimeric Antigen Receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013; 5(215): 172.
Author Info
Bodi Gayathri*, Sariki Ashok Krishnan and Korimelli SrideviReceived: 21-Jun-2021 Accepted: 05-Jul-2021 Published: 12-Jul-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3