Review Article - (2021) Volume 12, Issue 11
A Brief Review on Mucormycosis (Black Fungus Infection)
Vibhavari M Chatur*, Sanjay G Walode, Mithun Rudrapal, Sanjog Gandhi, Srushti Doshi and Shrawani GandhiAbstract
Mucormycosis is a life-threatening fungal (Black Fungus) and mould infection of the order Mucorales (Rhizopus, Mucor, Rhizomucer, Absidia, Saksenaea) especially affecting mune compromised or diabetic patients, patients with neutropenia or with malignancy and recently patients suffered with COVID-19 infection or In severe cases it may reach the brain and prove to be fatal in such cases. Mucormycosis presents with rhino-orbito-cerebral, pulmonary, disseminated, cutaneous or gastrointestinal involvement. Mortality rates can approach 100% depending on the patient's underlying diseases and form of mucormycosis. Early diagnosis along with treatment of the underlying medical condition, surgery and an Amphotericin B (anti-fungal) product are needed for the treatment. This review paper provides an update of therapy management and medication and treatment of mucormycosis. New approaches assessing relationships between host, fungi and antifungal drugs, and new routes of administration such as aerosoles could improve mucormycosis treatment.
Keywords
Mucormycosis, Black fungus, Amphotericin-B, Posacanazole, Antifungal drugs, Polyenes
Introduction
Mucormycosis is a life threatening Invasive Fungal Disease (IFD) due to fungi belonging to Mucorales order from class zygomycetes which is post COVID-19 effect (Brunet K and Rammaert B, 2020). Mucormycosis leads to many clinical manifestations, ranging from localized to disseminated infection. In general, members of the order Mucorales cause acute, angioinvasive infections in immunocompromised patients with mortality rates exceeding 60%. After aspergillosis and candidiasis, mucormycosis is the third most common invasive fungal infection. It produces 8.3%-13% of all fungal infections encountered at autopsy in hematology patients (Prabhu RM and Patel R, 2004). The most commonly recovered general includes Mucor, Rhizopus, Rhizomucor, Absidia, Apophysomyces, cunninghamella and saksenaea (Eucker J, et al., 2001). Different types of mucormycosis are Rhino, Orbito, Cerebral, Pulmonary, Disseminated, Cutaneous, and Gastrointestinal.
Spores enter the human host through inhalation, percutaneous inoculation or administration (Prabhu RM and Patel R, 2004; Ribes JA, etal., 2000). Mucormycosis predominantly affects patients with at least one of the immune compromising states hematologic malignancy, neutropenia, receipt of high dose corticosteroids, diabetes mellitus, diabetic ketoacidosis, organ transplantation, deferoxamine therapy, trauma and burns (Parfrey NA, 1986; Lee FY, etal., 1999). Immunocomponent patients rarely produce mucormycosis (Prabhu RM and Patel R, 2004).
Recommendations for the treatment of mucormycosis were rated according to the standard scoring system of the Infectious Diseases Society of America (IDSA) for eating recommendations in clinical guidelines (Chakrabarti A, etal., 2001; Skiada A, et al., 2013). Treatment is an emergency and combined surgery, which is frequently required owing to the angioinvasive and necrotic character of infection and antifungal treatment. Firstly in vitro resistance to several antifungal drugs limits therapeutic potions (Pilmis B, etal., 2018). However recent data gives the antifungal armamentarium with the U.S. Food and Drug Administration and European Medicines Agency approval of the new triazoles isavuconazole. However comparative clinical data are lacking and the respective places of polyenes and different azoles needs to be discussed (Pilmis B, etal., 2018).
Pathogenesis
When the spore enters the body from the environment it results in phagocytosis of mucorals with the help of polymorph nuclear phagocytes. In body the fungi shows its growth by killing the immune cells, the diseased conditions like acidosis and Hypergkycemia enhance the phagocytic activity. The enzyme (i.e. ketone reductase) required for the growth of fungi in acidic environment are secreted. The fungi then moves in blood vessels by up taking all the iron present in serum and causes the tissue damage and blood clots which results in major angioinvasion. Now the organisms enter the endothelial cell and extracellular matrix which is the most crucial step in pathogenesis. Epithelial Interaction-whenever mucorals enter the body epithelial cells are the one who acts against them the epithelial cells likes alveoli and skin epithelia but mucorals are found to germinate by adhering onto the basement membrane protein the Glucose Regulated Protein (GRP78) portray as recptor to enter in the cell and damage them. Since the infection can happen due to a various fungal species, the proper pathogenesis shall not be the same for all species; rather various forms can lead to different forms to mucormycosis (Hassan MI and Voigt K, 2019). The pathogenesis of mucormycosis is represented in Figure 1.
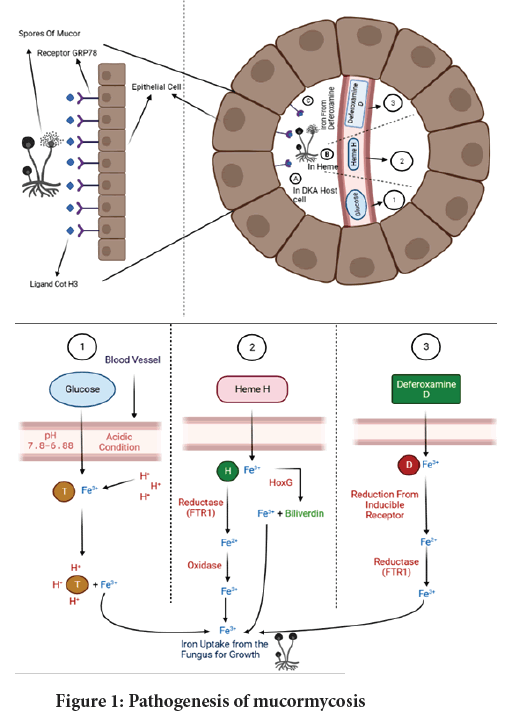
Figure 1: Pathogenesis of mucormycosis
Prevention
Patients should monitor their health for at least two weeks after recovering from coronavirus. To avoid contracting the Black Fungus, make sure to follow these precautions. When visiting dusty areas, use a mask and make sure you're well covered when handling soil, moss, or manure. Hyperglycemia must be managed. After being discharged from COVID-19, keep an eye on your blood glucose levels. To exercise caution when it comes to the timing and dosage of steroid use. While receiving oxygen therapy, keep humidifiers filled with clean water. Antibiotics and antifungals should be used with caution (Cornely OA, et al., 2019).
In the COVID-19 era, preventing mucormycosis requires the prudent use of steroids (both dose and duration), the management of co-morbidities (particularly diabetes), and the upkeep of sanitation and cleanliness. The use of prophylactic medications like posaconazole is currently not suggested in Indian COVID-19 recommendations. Posaconazole prophylaxis is only recommended for patients with neutropenia and graft vs. host disease, according to international guidelines. That too at a moderate level of strength (Chamilos G, et al., 2008).
Diagnosis
Mucormycosis is difficult to diagnose, and therapy should be started immediately so as to reduce mortality (Lass‐Flörl C, 2009). Although no adequately powered trials testing 1,3 beta-D-glucan in different types of mucormycosis have been performed, it is generally observed that 1,3 beta-Dglucan detection test is negative in Mucoralesinfections. No circulating antigen detection test (similar to galactomannan detection for invasive aspergillosis) is available for the diagnosis of mucor mycosis. These two tests, on the other hand, can help rule out invasive aspergillosis, which is the most common differential diagnosis, as well as mixed Aspergillus and Mucorales infections. There is currently no standardized blood Polymerase Chain Reaction (PCR) test. As a result, biological materials from clinically affected locations must be analyzed in order to diagnose the condition. Tissue biopsies for histopathology and culture should be obtained whenever possible. Unfortunately, due of severe thrombocytopenia, this is typically problematic in individuals with hematologic cancers. If a biopsy is not possible, all available specimens, such as sputum, should be examined directly and cultured. Sinus biopsies are required in cases of sinusitis. Endoscopy of the Ear, Nose, and Throat (ENT) should always be performed and repeated to assess the response to treatment. If sputum smear analysis is negative in the case of pulmonary involvement, endoscopic, Computed Tomography (CT)-guided, or surgical broncho-alveolar lavage or pulmonary biopsies (endoscopic, CT-guided, or surgical) should be performed based on the radiological results obtained by CT scans (Lass‐Flörl C, et al., 2007). Lass-Florl C, et al. found that CT guided percutaneous lung biopsy was highly effective in distinguishing aspergillosis from mucormycosis in hematologic patients (Jensen HE, et al., 1997). It should be noted, however, that no patients with platelet counts below 50 × 109/L were included in this study. A sinus and chest CT should be conducted in addition to brain imaging, regardless of the original clinical site involved, especially if there are suggestive signs and symptoms. This is significant since the therapeutic method for brain lesions differs. Because zygomycetes are fragile, the material obtained from biopsies should be handled with care so that it does not become crushed, resulting in a negative culture. Rapid growth happens after a 24-hour incubation period at 25°C-37°C.
Mucormycosis infection is confirmed by culture of a sterile location, which allows for exact genus and species identification. Blood cultures are almost invariably negative, and if they are positive, contamination should be suspected. Similarly, mucormycosis agents are rarely found in the cerebrospinal fluid, even when the central nervous system is infected. Direct microscopy is useful for detecting hyphae in clinical samples since it is quick and strongly indicative of illness. After treatment with potassium hydroxide, staining with an optical brightener (calcofluor white), or with Gomorimethaminesilver, specimens can be viewed (Jensen HE, et al., 1997). Hyphae are hyaline, non-or pau-ci-septate, ribbonlike, and have a great diameter (5-25 mm). The width is uneven, with 90-degree branching angles. Direct examination can be difficult when hyphae are fragmented, making a conclusive diagnosis of mucormycosis difficult, and culture is required to confirm the diagnosis (Jensen HE, et al., 1997). Gomorimethaminesilver or Periodic-acid Schiff can be used to stain tissue. Hyphae can be seen in necrotic tissue with symptoms of angioinvasion and infarction; neutrophilic infiltrates or granuloma formation can be seen in people who aren't granulocytopenic or who have a more persistent infection.
Immunohistochemistry using commercially available antizygomycete antibodies may occasionally aid in the diagnosis (Dannaoui E, et al., 2010).
When cultures are negative, tissue samples can be molecularly identified to validate the histopathological diagnosis. However, there is no standardized approach accessible at this time. Fresh or frozen samples are recommended; however, formalin-fixed paraffin-embedded tissues may also be employed, based on current inter-laboratory experimental and clinical findings (Rickerts V, et al., 2007; Dannaoui E, 2009). The fungus can be identified to the genus and species level using molecular identification of mucormycosis agents. DNA probes targeting the 18S subunit, ITS1 sequencing following Polymerase Chain Reaction (PCR) with pan-fungal primers, semi-nested PCR targeting the 18S subunit, and real-time PCR targeting the cytochrome b gene have all been reported (Torres-Narbona M, et al., 2007).
Signs and Symptoms
The sinuses (39 percent), lungs (24 percent), and skin are the most commonly reported locations of invasive mucormycosis (19 percent) Invasive mucormycosis is most commonly (Zaoutis TE, et al., 2007). Dissemination developed in 23 percent of these cases. Diabetics have a 44 percent mortality rate, patients without underlying diseases have a 35 percent mortality rate, and patients with cancer have a 66 percent mortality rate. The death rate differed depending on the infection site and the type of illness. 96 percent of patients had disseminated infections, and 85 percent had gastrointestinal illnesses, according to the researchers and 76 percent of those who had pulmonary infections died. In one study, mucormycosis presented itself in children as cutaneous, gastrointestinal, rhino cerebral, and pulmonary infections in 27%, 21%, 18%, and 16% of cases, respectively (Paes de Oliveira-Neto M, et al., 2006). The skin and gut are affected more frequently in children than in adults.
Pulmonary mucormycosis
Pulmonary mucormycosis has nonspecific clinical characteristics that are difficult to identify from pulmonary aspergillosis. Patients frequently present with a persistent high-grade fever (>38°C) that is resistant to medications. Hemoptysis, pleuritic chest pain, and dyspnea are the most prevalent symptoms, while haemoptysis, pleuritic chest pain, and dyspnea are less common.
Rhino cerebral mucormycosis
Rhino cerebral mucormycosis begins with symptoms similar to sinusitis and periorbital inflammation cellulitis symptoms include eye and/or facial pain, numbness, and Blurry vision (Chakrabarti A, et al., 2001).
Cutaneous mucormycosis
Superficial lesions with just slightly elevated circinate and squamous margins resembling tinea corporis are less common presentations of cutaneous mucormycosis (Rubin AI and Grossman ME, 2004), targeted plaques having erythematous rims on the outside and ecchymotic or necrotic interiors (Chawla R, et al., 2007), and in patients with open wounds, lesions with a cotton like appearance resembling that of bread mould (Kordy FN, et al., 2007; Michalak DM, et al., 1980).
Gastrointestinal mucormycosis
Anappendiceal, cecal, or ileac mass, as well as a stomach perforation, are common symptoms of the illness, which are often accompanied by significant upper gastrointestinal tract haemorrhage (Oliver MR, et al., 1996; Cherney CL, et al., 1999). GI mucormycosis manifests as necrotizing enterocolitis in premature neonates, whereas it manifests as a mass like appendiceal or ileal lesion in neutropenic patients (Chakrabarti A, et al., 2001; Petrikkos G, et al., 2012).
Disseminated mucormycosis
The host, as well as the location and degree of vascular invasion and tissue infarction in the affected organs, influence the symptoms and progression of disseminated mucormycosis (Gamaletsou MN, et al., 2012).
Conclusion
The above review article highlights on the very current, area of concerned disease, its treatment, causes etc. From above information we can hope that even though this mucormycosis is fatal to human life, with due precautions, care and treatment, it is curable too. In coming future we can overcome with this disease as well.
Treatment
Antifungal agents for mucormycosis
Polyenes
The therapeutic approach to mucormycosis includes Antifungal agents, the lipid formulation of Amphotericin B, the new triazoles posaconazole, and the echinocandins in combination with Amphotericin B (AMB). The only antifungal agent approved for the treatment of mucormycosis is Amphotericin B.
First-line antifungal options for mucormycosis
Table 1depicts first-line antifungal agents used to treat mucormycosis.
| Drug | Recommended dosage | Advantage and supporting studies | Disadvantage |
|---|---|---|---|
| AMB | 1.0-1.5 mg/kg/day | >5 decades clinical experience, only licensed agent for treatment of mucormycosis. | Highly toxic, poor CNS penetration |
| LAMB | 5-10 mg/kg/day | Improved CNS penetration compared to AMB | Expensive |
| ABLC | 5-7.5 mg/kg/day | Less nephrotoxic than AMB; murine and retrospective clinical data suggest benefit of combination therapy with echinocandins | More nephrotoxic than LAMB |
Table 1: First-line anti fungal agents for mucormycosis
Lipid formulations of Amphotericin B
For the primary therapy of mucormycosis, AMB is the medicine of choice (Dannaoui E, et al., 2003). Despite the lack of interpretative breakpoints for AMB, significant in vitro MICs for AMB have been recorded in clinical isolates of Cunninghamella species (Lamoth F, et al., 2016). A MIC of 0.5 g/mL for Amphotericin B was substantially related with better 6-week outcomes in a limited investigation of non-Aspergillus invasive mould infections (Tissot F, et al., 2017). The appropriate dosage for AMB and its formulations against mucormycosis is yet unknown, as it is for many antifungal drugs and mycoses According to current standards, the usual daily dose of LAMB (Lipid formulations of Amphotericin B) and ABLC (Amphotericin B Lipid Complex)is 5 mg/ kg/day (Cornely O, et al., 2013; Marty FM, et al., 2016).
New triazoles
Triazoles work by removing ergosterol from the cell membrane of fungi. Fluconazole, itraconazole, and voriconazole are triazole antifungals that have minimal or no effect against Mucorales. Posaconazole and isavuconazole, two newer triazoles, show better in vitro efficacy against Mucorales, as well as clinical data to support their usage in mucormycosis (Nagappan V and Deresinski S, 2007; Caramalho R, et al., 2015).
Posaconazole
Posaconazole's in vitro action against Mucorales varies according on the species (Lewis RE, et al., 2014). Posaconazole median MICs for several Mucorales species ranged from 1.0 g/mL to 8.0 g/mL, according to a study of 131 clinical isolates (Krishna G, et al., 2012). Mucor spp. infections were the most responding to posaconazole in laboratory animal investigations, but Rhizopus spp. infections were frequently non-responsive (Jung DS, et al., 2014). There are few clinical research on the efficacy of posaconazole for mucormycosis. As a result, posaconazole oral suspension absorption was frequently inadequate, resulting in treatment failures. A gastro-resistant tablet and an Intravenous (IV) solution have been developed to circumvent the oral solution's pharmacokinetic restrictions (Trang TP, et al., 2017). The tablet formulation has several advantages over the suspension formulation, including better bioavailability, which allows for a once-daily dose, no food requirements, and absorption that is unaffected by changes in gastric pH or motility; less interpatient variability, and more predictable plasma concentrations (Chitasombat MN and Kontoyiannis DP, 2015). Posaconazole (oral suspension 400 mg2/day with meals, or 200 mg4/ day if not taken with meals) is currently being explored as a salvage treatment for mucormycosis.
Isavuconazole
Isavuconazole is the biologically active agent of the prodrug is a vuconazonium sulphate and is a novel broad-spectrum triazole. In the United States, it is approved for the treatment of mucormycosis. While in Europe, it is approved for the treatment of mucormycosis when Amphotericin B is not an option. It comes in both intravenous and oral forms, with a loading dose of 200 mg three times a day for two days and then 200 mg daily after that. When compared to other azoles, isavuconazole has a number of pharmacokinetic and safety advantages, including linear pharmacokinetics; fewer drug-drug interactions; less toxicity, skin and ocular side effects, or QT prolongation; no nephrotoxic cyclodextrin in the IV formulation; no need for dose adjustments in kidney, liver, or obesity; and excellent oral bioavailability with no food requirements (Arendrup MC, et al., 2015). Isavuconazole, like posaconazole, has inconsistent in vitro efficacy against Mucorales that varies by species. It should be noted that the Mucorales MIC values of Isavuconazole are 2-to 4-fold higher than those of posaconazole, and this should be considered in clinical practice (Peixoto D, et al., 2014; Graves B, et al., 2016). Isavuconazole has been successfully utilized as a salvage therapy for mucormycosis in immunocompromised patients, including cases of posaconazole failure, according to several case reports (Spellberg B, et al., 2012; Ibrahim AS, et al., 2005).
Combinational therapy
Despite the lack of reliable clinical evidence, treating mucormycosis in immunocompromised individuals with a combination of antifungals is becoming more prevalent. Synergistic impact and larger coverage are the benefits of such a therapeutic approach, whereas antagonism, drug interactions, toxicity, and cost are the drawbacks (Gebremariam T, et al., 2016).
Synergy between polyenes and echinocandins has been demonstrated in vitro and in vivo animal model studies. Although fundamentally inactive against Mucorales, in vitro echinocandins are thought to have some invivoeffect, possibly through the breakdown of a small quantity of glucan on the fungus's cell wall, immune epitope unmasking, and phagocytosis facilitation (Reed C, et al., 2008; Kyvernitakis A, et al., 2016). The combination of AMB+echinocandin was successful in 6 of 7 diabetic patients with rhino-orbital or rhino-cerebral mucormycosis, compared to just 7 of 22 patients treated with ABLC alone (p=0.02 in one retrospective research) (Ballester F, et al., 2008). The evidence regarding the effectiveness of the AMB+triazole combination in the treatment of mucormycosis is mixed. The combination of a polyene with posaconazole has shown synergy in vitro, but in vivo investigations in mouse models of mucormycosis showed no benefit when the drugs were taken simultaneously.
References
- Brunet K, Rammaert B. Mucormycosis treatment: Recommendations, latest advances, and perspectives. J Mycol Med. 2020; 30(3): 101007.
- Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: A review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004; 10: 31-47.
- Eucker J, Sezer O, Graf B, Possinger K. Mucormycoses. Mycoses. 2001; 44(7‐8): 253-260.
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000; 13(2): 236-301.
- Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine. 1986; 65(2): 113-123.
- Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: The last 30 years. Arch Intern Med. 1999; 159(12): 1301-1309.
- Chakrabarti A, Das A, Sharma A, Panda N, Das S, Gupta KL, et al. Ten years' experience in zygomycosis at a tertiary care centre in India. J Infect. 2001; 42(4): 261-266.
- Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013; 98(4): 492.
- Pilmis B, Alanio A, Lortholary O, Lanternier F. Recent advances in the understanding and management of mucormycosis. F1000research. 2018; 7.
- Hassan MI, Voigt K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med Mycol. 2019; 57(2): 245-256.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019; 19(12): 405-421.
- Chamilos G, Lewis RE, Kontoyiannis DP. Delaying Amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008; 47(4): 503-509.
- Lass‐Flörl C. Zygomycosis: Conventional laboratory diagnosis. Clin Microbiol Infect. 2009; 15: 60-65.
- Lass-Flörl C, Resch G, Nachbaur D, Mayr A, Gastl G, Auberger J, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis. 2007; 45(7): 101-104.
- Jensen HE, Salonen J, Ekfors TO. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J Pathol. 1997; 181(1): 100-105.
- Dannaoui E, Schwarz P, Slany M, Loeffler J, Jorde AT, Cuenca-Estrella M, et al. Molecular detection and identification of zygomycetes species from paraffin-embedded tissues in a murine model of disseminated zygomycosis: A collaborative European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) evaluation. J Clin Microbiol. 2010; 48(6): 2043-2046.
- Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, Presterl E, et al. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis. 2007; 44(8): 1078-1083.
- Dannaoui E. Molecular tools for identification of Zygomycetes and the diagnosis of zygomycosis. Clin Microb Infect. 2009; 15: 66-70.
- Torres-Narbona M, Guinea J, Martínez-Alarcón J, Muñoz P, Gadea I, Bouza E as a representative of the MYCOMED Zygomycosis Study Group. Impact of zygomycosis on microbiology workload: A survey study in Spain. J Clin Microbiol. 2007; 45(6): 2051-2053.
- Zaoutis TE, Roilides E, Chiou CC, Buchanan WL, Knudsen TA, Sarkisova TA, et al. Zygomycosis in children: A systematic review and analysis of reported cases. Pediatr Infect Dis J. 2007; 26(8): 723-727.
- Paes de Oliveira-Neto M, da Silva M, Cezar Fialho Monteiro P, Lazera M, de Almeida Paes R, Beatriz Novellino A, et al. Cutaneous mucormycosis in a young, immunocompetent girl. Med Mycol. 2006; 44(6): 567-570.
- Rubin AI, Grossman ME. Bull's-eye cutaneous infarct of zygomycosis: A bedside diagnosis confirmed by touch preparation. J Am Acad Dermatol. 2004; 51(6): 996-1001.
- Chawla R, Sehgal S, Kumar SR, Mishra B. A rare case of mucormycosis of median sternotomy wound caused by Rhizopus arrhizus. Indian J Med Mycol. 2007; 25(4): 419-421.
- Kordy FN, Al-Mohsen IZ, Hashem F, Almodovar E, Al Hajjar S, Walsh TJ. Successful treatment of a child with posttraumatic necrotizing fasciitis caused by Apophysomyces elegans: Case report and review of literature. Pediatr Infect Dis J. 2004; 23(9): 877-879.
- Michalak DM, Cooney DR, Rhodes KH, Telander RL, Kleinberg F. Gastrointestinal mucormycoses in infants and children: A cause of gangrenous intestinal cellulitis and perforation. J Pediatr Surg. 1980; 15(3): 320-324.
- Oliver MR, van Voorhis WC, Boeckh M, Mattson D, Bowden RA. Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clin Infect Dis. 1996; 22(3): 521-524.
- Cherney CL, Chutuape A, Fikrig MK. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: Case report and literature review. Am J Gastroenterol. 1999; 94(1): 252-256.
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012; 54(1): 23-34.
- Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ. Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep. 2012; 14(4): 423-434.
- Dannaoui E, Meletiadis J, Mouton JW, Meis JF, Verweij PE. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J Antimicrob Chemother. 2003; 51(1): 45-52.
- Lamoth F, Damonti L, Alexander BD. Role of antifungal susceptibility testing in non-Aspergillus invasive mold infections. J Clin Microbiol. 2016; 54(6): 1638-1640.
- Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017; 102(3): 433.
- Cornely O, Arikan‐Akdagli SE, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014; 20: 5-26.
- Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson III GR, et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016; 16(7): 828-837.
- Nagappan V, Deresinski S. Posaconazole: A broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007; 45(12): 1610-1617.
- Caramalho R, Maurer E, Binder U, Araújo R, Dolatabadi S, Lass-Flörl C, Lackner M. Etest cannot be recommended for in vitro susceptibility testing of Mucorales. Antimicrob Agents Chemother. 2015; 59(6): 3663-3665.
- Lewis RE, Albert ND, Kontoyiannis DP. Comparative pharmacodynamics of posaconazole in neutropenic murine models of invasive pulmonary aspergillosis and mucormycosis. Antimicrob Agents Chemother. 2014; 58(11): 6767-6772.
- Krishna G, Ma L, Martinho M, Preston RA, O'mara E. A new solid oral tablet formulation of posaconazole: A randomized clinical trial to investigate rising single-and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012; 67(11): 2725-2730.
- Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014; 58(11): 6993-6995.
- Trang TP, Hanretty AM, Langelier C, Yang K. Use of isavuconazole in a patient with voriconazole‐induced QT c prolongation. Transpl Infect Dis. 2017; 19(4): 12712.
- Chitasombat MN, Kontoyiannis DP. The ‘cephalosporin era’of triazole therapy: Isavuconazole, a welcomed newcomer for the treatment of invasive fungal infections. Expert Opin Pharmacother. 2015; 16(10): 1543-1558.
- Arendrup MC, Jensen RH, Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales order. Antimicrob Agents Chemother. 2015; 59(12): 7735-7742.
- Peixoto D, Gagne LS, Hammond SP, Gilmore ET, Joyce AC, Soiffer RJ, et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol. 2014; 52(3): 1016-1019.
- Graves B, Morrissey CO, Wei A, Coutsouvelis J, Ellis S, Pham A, et al. Isavuconazole as salvage therapy for mucormycosis. Med Mycol Case Rep. 2016; 11: 36-39.
- Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, et al. Combination therapy for mucormycosis: Why, what, and how?. Clin Infect Dis. 2012; 54(1): 73-78.
- Ibrahim AS, Bowman JC, Avanessian V, Brown K, Spellberg B, Edwards Jr JE, et al. Caspofungin inhibits Rhizopus oryzae 1, 3- β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob Agents Chemother. 2005; 49(2): 721-727.
- Gebremariam T, Wiederhold NP, Alqarihi A, Uppuluri P, Azie N, Edwards Jr JE, et al. Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis. J Antimicrob Chemother. 2016; 433.
- Reed C, Bryant R, Ibrahim AS, Edwards Jr J, Filler SG, Goldberg R, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008; 47(3): 364-371.
- Kyvernitakis A, Torres HA, Jiang Y, Chamilos G, Lewis RE, Kontoyiannis DP. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: A propensity score analysis. Clin Microbiol Infect. 2016; 22(9): 811-818.
- Ballester F, Pastor FJ, Guarro J. In vitro activities of combinations of Amphotericin B, posaconazole and four other agents against Rhizopus. J Antimicrob Chemother. 2008; 61(3): 755-757.
Author Info
Vibhavari M Chatur*, Sanjay G Walode, Mithun Rudrapal, Sanjog Gandhi, Srushti Doshi and Shrawani GandhiReceived: 28-Jul-2021 Accepted: 11-Aug-2021 Published: 18-Aug-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






