Case Report - (2023) Volume 14, Issue 8
Abstract
Ketamine has been shown to be a novel and exciting antidepressant medication in patients with treatment-resistant depression. A complication commonly seen in frequent and heavy recreational use of ketamine is ulcerative cystitis, which presents with Lower Urinary Tract Symptoms (LUTS) and upper renal tract damage, seen in over 25% of regular users.
Although Ketamine-Induced Cystitis (KIC) is a recognized complication in recreational use of ketamine, its occurrence in therapeutic use for depression has so far not been reported. The exact pathogenesis of ketamine induced cystitis is currently unknown, making treatment and prevention much more difficult. Early diagnosis of ketamine induced cystitis and immediate cessation of ketamine use has been shown to improve adverse urinary tract symptoms and prevent further damage. We present a case of a 28-year-old female who was started on ketamine treatment for depression and developed symptoms of cystitis, which was confirmed by urine microscopy, culture and analysis. To our knowledge, this is the first reported case of ketamine-induced cystitis in a patient receiving treatment-dose ketamine for antidepressant therapy.
Keywords
Ketamine, Treatment-resistant depression, Cystitis
Introduction
Ketamine has been shown to be a very effective anti-depressant in patients undergoing Treatment-Resistant Depression (TRD) with up to 71% positive response rate in these cohorts (Zarate CA, et al., 2006). It acts as an N-Methyl-D-Aspartate (NMDA) receptor antagonist with glutamate, blocking capacity and onset of action much faster than conventional antidepressant medications (Zarate CA, et al., 2006; Bahji A, et al., 2021). Depending on the mode of delivery, the anti-depressant effects from ketamine administration can be seen within minutes to hours and the benefits can last for days to weeks (Zarate CA, et al., 2006; Bahji A, et al., 2021).
Common symptoms of KIC include urinary urgency, polyuria, dysuria, incontinence and haematuria, progressing to incontinence, haematuria, ulcerative cystitis, hydronephrosis, bladder wall fibrosis and chronic kidney failure (Huang YC, et al., 2008; Yek J, et al., 2015; Anderson DJ, et al., 2022).
Regular ketamine use is associated with increase in LUTS by up to 3-4 times compared to healthy individuals. Urinary symptoms can occur in over 25% of those who use ketamine recreationally and this is directly correlated with dose and frequency of ketamine use (Winstock AR, et al., 2012). If ketamine cessation occurs early, the urinary symptoms can improve and early damage can reverse (Anderson DJ, et al., 2022).
We present a case of a 28-year-old female who was started on ketamine treatment for depression, who developed symptoms of cystitis, which was confirmed by urine microscopy, culture and analysis. To our knowledge, this is the first reported case of ketamine-induced cystitis in a patient receiving treatment-dose ketamine for antidepressant therapy.
Case Presentation
A 28-year-old female presented to Maudsley Hospital with a relapse of her treatment-resistant depression. She had a past psychiatric history of severe unipolar treatment-resistant depression, which had previously been successfully treated with Electroconvulsive Therapy (ECT) and medications. Her past medical history also included epilepsy, for which she was taking sodium valproate 600 mg with good effect.
She was already on a combination of antidepressant medications, mood stabilisers and an antipsychotic. Her medication regime included-Vortioxetine 20 mg, Levothyroxine 150 mcg, Lithium (Priadel) 800 mg, Valproate 600 mg and Quetiapine 500 mg. Up until this relapse, her mood had been stable and she had been taking her medications as prescribed.
On assessment, she was well-dressed and well-groomed. She spoke slowly and monotonously with one syllable words and appeared dysthymic in nature. On discussion, it was evident that she had negativistic cognitive distortions and ideas of worthlessness. She was at times not fully able to engage in the assessment but was generally coherent. There were no signs of hallucinations or abnormal sensory experiences, but she did appear more depressed than previously and more easily distracted.
Due to this relapse, ketamine augmentation therapy was started on 25/10/2021 in the form of sublingual lozenges 160 mg twice weekly. This was increased to three times a day on 10/01/2022 with good effect. However, she complained of nausea and vomiting from the sublingual preparation.
In September 2022, her depression began to decline again and on 27/09/2022 the treatment was switched to oral capsules 240 mg and escalated to four times a week. She reported that the nausea and vomiting improved significantly.
The ketamine showed excellent anti-depressant response (Figure 1). However, she began to complain of symptoms of dysuria which initially began insidiously. She described this as “stinging during and after peeing”. This discomfort could occasionally last for a few hours after voiding. These usually happened 12-24 hours after taking the ketamine dose. She was managing the pain with over-the-counter paracetamol and phenazopyridine hydrochloride, which she found very helpful. She was advised to stay well hydrated whenever taking the ketamine and to continue monitoring her symptoms closely. She reported taking the ketamine exactly as prescribed.
Figure 1: Structure of ketamine.
Unfortunately, the frequency and severity of the dysuria progressively worsened. A urine microscopy, culture and stain demonstrated sterile pyuria, with positive inflammatory cells, but no growths, nitrites or blood. No intimate examinations were performed. There were no indications of a urinary tract infection or a sexually transmitted infection.
Blood tests were unremarkable and renal function was normal. This included: Na 141 mmol/L, K 4.7 mmol/L, urea 3.2 mmol/L, creatinine 80 mmol/L, GFR 89, WCC 5.2 × 109/L.
Due to the concerns about irreversible bladder and renal tract injury, the decision was made to withdraw the ketamine. The patient reported that within 3 weeks, the symptoms of dysuria had completely resolved, but her mood had worsened. A repeat urine test carried out yielded normal results. A decision was made to begin ECT and to rationalise her medications, which included discontinuing the ketamine.
Patient’s perspective
“Ketamine was really helpful for my mood-it worked quickly and my family, friends and I all saw the difference straight away. My mood was better, I had more energy and motivation to do things. My thought processes took more positive swing and I was actually genuinely happy to be alive again. Sadly, the urinary symptoms were horrible-they felt like a really bad urinary tract infection and the pain lingered longer each time. In the end, the urinary symptoms made me start to dread taking each dose of the ketamine, as I knew that the pain would be there for most of the day/night afterwards. As a result, making the decision to stop the ketamine was a difficult decision, but so was the idea of continuing it.”
Results and Discussion
Over 350 million people in the world suffer from depression and about one third of these patients are believed to have TRD (Charlson F, et al., 2019). TRD is often defined as failure to two antidepressant medications (Popova V, et al., 2019).
Ketamine has been shown to be a novel and exciting anti-depressant in patients with TRD, with up to 71% positive response rate (Zarate CA, et al., 2006). It acts as an NMDA receptor antagonist with glutamate-blocking capacity and onset of action much faster than conventional anti-depressant medications (Zarate CA, et al., 2006; Bahji A, et al., 2021). Depending on the mode of delivery, the anti-depressant effects can be seen within minutes to hours and the benefits can last for days to weeks (Zarate CA, et al., 2006; Bahji A, et al., 2021). KIC typically starts with urinary symptoms, including dysuria, urgency, polyuria, nocturia and progressing to incontinence, haematuria, bladder wall fibrosis and ulcerative cystitis. Continuation of the drug can lead to involvement of the upper renal tract, including hydronephrosis and chronic kidney failure (Huang YC, et al., 2008; Yek J, et al., 2015; Anderson DJ, et al., 2022). Physical examination and investigations may show suprapubic pain, sterile pyuria and increased eosinophils within the bladder wall (Anderson DJ, et al., 2022).
Imaging of the bladder in severe cases may show a grossly constricted with thickened walls (Anderson DJ, et al., 2022). Cystoscopy often demonstrates a friable bladder mucosa that is prone to bleeding (Anderson DJ, et al., 2022). Microscopically, the urothelium may appear denuded, ulcerated and infiltrated by inflammatory cells, such as mast cells and eosinophils (Figure 2). Other findings include, submucosal fibrosis, muscle hypertrophy and collagen deposition (Yee CH, et al., 2017).
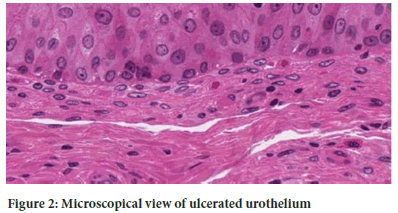
Figure 2: Microscopical view of ulcerated urothelium.
Although the exact pathogenesis of ketamine induced cystitis is not yet fully understood, various mechanisms have been postulated and it is likely that several pathways are involved concurrently. (Zhou J, et al., 2023).
One theory is that the ketamine and its metabolites, which are largely excreted by the urinary tract, can direct cause toxicity to the bladder. This disrupts the urothelial integrity of the bladder epithelium and initiate interstitial fibrosis and it has been demonstrated in animal models and the level of damage directly correlates with the dose of ketamine used (Lee WC, et al., 2018).
Another theory is an Immunoglobin E (IgE) mediated response. Bladder samples in ketamine users frequently show raised inflammatory cells and messengers, including mast cells, eosinophils, Cyclo-Oxygynase-2 (COX-2), Nitric Oxide Synthase (NOS) and IgE. These levels fall once the patient is in remission from ketamine use and rise again once ketamine use restarts. This suggests an inflammatory response or a hypersensitivity reaction leading to bladder damage (Anderson DJ, et al., 2022; Lee WC, et al., 2018).
Ketamine can also directly stimulate various chemicals, including adenosine triphosphate, antiproliferative factor, and oxidative stress which lead to changes in the bladder wall (Anderson DJ, et al., 2022). It has been reported that the N-Methyl-D-Aspartate Receptor (NMDAR) and angiogenic factors can also cause microvascular injury in the bladder (Anderson DJ, et al., 2022).
Other proposed theories include aberrant neurotrophic factors, protein kinase B, mTOR pathways and metadherin and MAPK pathways leading to downstream fibrosis of the bladder (Anderson DJ, et al., 2022). Early diagnosis of ketamine induced cystitis and immediate cessation of ketamine usage has been shown to improve symptoms, reverse early disease and prevent further damage (Anderson DJ, et al., 2022).
Conclusion
To our knowledge, this is the first reported case of ketamine-induced cystitis in a patient receiving treatment-dose ketamine for antidepressant therapy. Our case highlights the need to be aware and to monitor for symptoms of LUTS and KIC in all patients taking ketamine for depression, especially now that this therapy is becoming more and more widely used.
Further research is required to determine the safe frequency, dose, route and duration of antidepressant ketamine therapy to avoid ketamine induced cystitis. We also advise further research to identify individual susceptibility and risk factors that may play a significant role in determining the susceptibility to developing KIC. Furthermore, we recommend regular screening for such symptoms in all patients receiving ketamine treatment.
Authors’ Contributions
All data, information and interviews were conducted and collated by the lead author, Minna Chang.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report.
Availability of Data and Materials
If necessary, the data set used in the study will be accessible.
References
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006; 63(8): 856-864.
[Crossref] [Google Scholar] [PubMed]
- Bahji A, Vazquez GH, Zarate Jr CA. Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J Affect Disord. 2021; 278: 542-555.
[Crossref] [Google Scholar] [PubMed]
- Huang YC, Jeng CM, Cheng TC. Ketamine-associated ulcerative cystitis. Tzu Chi Med J. 2008; 20(2): 144-146.
- Yek J, Sundaram P, Aydin H, Kuo T, Ng LG. The clinical presentation and diagnosis of ketamine-associated urinary tract dysfunction in Singapore. Singapore Med J. 2015; 56(12): 660.
[Crossref] [Google Scholar] [PubMed]
- Anderson DJ, Zhou J, Cao D, McDonald M, Guenther M, Hasoon J, et al. Ketamine-induced cystitis: A comprehensive review of the urologic effects of this psychoactive drug. Health Psychol Res. 2022; 10(3).
[Crossref] [Google Scholar] [PubMed]
- Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012; 110(11): 1762-1766.
[Crossref] [Google Scholar] [PubMed]
- Charlson F, van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet. 2019; 394(10194): 240-248.
[Crossref] [Google Scholar] [PubMed]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A randomized double-blind active-controlled study. Am J Psychiatry. 2019; 176(6): 428-438.
[Crossref] [Google Scholar] [PubMed]
- Yee CH, Teoh JY, Lai PT, Leung VY, Chu WC, Lee WM, et al. The risk of upper urinary tract involvement in patients with ketamine-associated uropathy. Int Neurourol J. 2017; 21(2): 128.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Scott C, Miab ZR, Lehmann C. Current approaches for the treatment of ketamine‐induced cystitis. Neurourol Urodyn. 2023; 42(3): 680-689.
[Crossref] [Google Scholar] [PubMed]
- Lee WC, Su CH, Tain YL, Tsai CN, Yu CC, Chuang YC. Potential orphan drug therapy of intravesical liposomal onabotulinumtoxin-A for ketamine-induced cystitis by mucosal protection and anti-inflammation in a rat model. Sci Rep. 2018; 8(1): 5795.
[Crossref] [Google Scholar] [PubMed]
Author Info
Minna Chang*Received: 19-Jul-2023 Accepted: 02-Aug-2023 Published: 09-Aug-2023, DOI: 10.31858/0975-8453.14.8.511-513
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






