Review Article - (2021) Volume 12, Issue 12
Abstract
Various bioflavonoids present in plants are recognized as nutritional supplements such as myricetin, quercetin, Kaempferol, and Luteolin. Myricetin, a flavonoid has multiple physiological activities, which include antioxidant, Pro-oxidant, antimicrobial, anti-cancer, anti-platelet, antidiabetic, and anti-inflammatory properties. Myricetin exerts favorable effects on neuroprotection by decreasing aggregation of misfolded luciferase proteins, which has preclinical evidence. Recent studies reported the Protective role of myricetin against the African swine fever virus. Myricetin shows a protective effect against neurodegenerative diseases like Alzheimer's and Parkinson's. In this review, recent findings of in-vitro and in-vivo studies on myricetin molecules were included. Along with recent findings, this article includes the natural source, synthesis, pharmacological actions, and toxicity studies of myricetin. Thus, this review furnish perception of myricetin preclinical activities and part in clinical trials.
Keywords
Myricetin, Pro-oxidant, Neuroprotection, African swine fever virus, Perception
Abbreviations
IUPAC: International Union of Pure and Applied Chemistry; DMS: Dimethyl Sulfoxide; DMF: Dimethylformamide; THF: Tetrahydrofuran; OECD: Organization for Economic Co-operative and Development; ABTS+: 2,2’-azino-bis(3-ethyl- benzothiazoline-6-sulfonic acid); DPPH: 2,2-diphenyl-1-picryl- hydrazyl; I.P: Intraperitoneal; ROA: Route of Administration; CAT: Catalase; DNA: Deoxyribonucleic acid; PGE2: Prostaglan- din E2; MDA: Malondialdehyde; COX: Cyclooxygenase
Introduction
In the olden days, plant extracts have been used to treat definite communicable or contagious diseases. But in recent days few of those are used as standard treatments for various diseases. Holistic medicine attracts people due to its safety, potency, excitability, affordable or cost-effectiveness. In this review, we discuss different biological and pharmacological activities of the myricetin compounds.
Myricetin is a natural flavonoid IUPAC name of myricetin is 3,5,7-Trihydroxy-2-(3, 4, 5-trihydroxy phenyl)-4-chromenone. Myricetin was first isolated from Myricanagi, Family Myricaceae as yellow-colored crystals. Myricetin is solid in nature insoluble in water but soluble in organic solvents such as ethanol, DMS (Dimethyl Sulfoxide) and DMF (Dimethyl Formamide), THF (Tetrahydrofuran). Sparingly soluble in boiling water. Solubility of myricetin in different solvents including methanol, ethanol, acetone, ethyl acetate, chloroform, petroleum ether, toulene, hexene was measured using a water bath oscillator. Myricetin also known as cannabiscetin or myricetol, belongs to the class of organic compounds known as flavonols (Yao Y, et al., 2014). The molecular weight of Myricetin is 318.23 gm/mol. The statistical analysis states that natural fruits and vegetables reduce the incidence of degenerative diseases (Temple NJ and Gladwin KK, 2003). Chemically flavonoids are subdivided into 6 major subclasses-Flavones, Flavanols, Flavanones, Isoflavones, Flavanols, and anthocyanidins (Rive-Evans CA and Miller NJ, 1998) (Figures 1-7). Flavonoids obtain substantial attention due to their importance in oxidation antimicrobial and anticancer properties (Wiseman SA, et al., 1997). It is both PH and temperature-dependent. The melting point of myricetin is 357°C (Perkin AG, 1902). Myricetin is a nutraceutical that exhibits a potent antioxidant property (Umadevi I, et al., 1988). Recent evidence claims that myricetin produces a different curative effect in Cancer, diabetes, cardiac effects, and acts against the microbes (Lin GB, et al., 2012). It is a potent anti-oxidant and pro-oxidant (Büchter C, et al., 2013). The average daily consumption of myricetin is 2.2 ± 2.5 mg (Mullie P, et al., 2007). The main intention of this review is to impart new insights into the preclinical pharmacological activities of myricetin (Park KS, et al., 2016) (Figures 1-7).
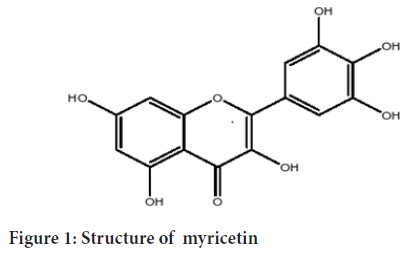
Figure 1: Structure of myricetin
Figure 2: Structure of flavone
Figure 3: Structure of flavonol
Figure 4: Structure of flavanone
Figure 5: Structure of isoflavone
Figure 6: Structure of flavonal
Figure 7: Structure of anthocyanadin
Myricetin occurs in both free and bound glycoside forms include-Myricetin-3-O-(4-acetyl)-α-L-arabinopyranoside, myricetin-3-O-(311-acetyl)-α-L-arabinopyranoside, myricetin-3-O-β-D-galactopyranoside, myricetin-3-O-α-L-rhamnopyranoside, myricetin-3-O-α-L-arabino-furanoside, myricetin-3-O-β-D-xylopyranoside, myricetin-3-O-α-L-rhamnoside, myricetin-3-O-(3-O-galloyl)-β-D-glactactopyranoside and myricetin-3-O-(211-O-galloyl)-α-L-rhamnoside (Büchter C, et al., 2013; Mullie P, et al., 2007; Park KS, et al., 2016). Idiomatic expressions of myricetin are namely kaempferol, quercetin, morin, and fisetin (Yang ZJ, et al., 2019).
Myricetin possesses multiple pharmacological activities: antioxidant, anti-platelet aggregation activity, anti-cancer, anti-photo-aging, anti-inflammatory, antihypertensive, analgesic anti-diabetic, cardio-protective, and neuroprotective.
Literature Review
Source of myricetin
Lettuce, Swedish turnip, parsley, celery leaves and dill (Lugast A and Hovari J, 2000). Flavonol compounds quercetin, kaempferol and Myricetin are widely prevalent in vegetables.
Good source is onions, hot peppers, kale, broccoli, Rutabagas, spinach and Tomatoes.
Diary and eggs: Milk, chocolate, reduced fat, oregano(fresh),Apple juice, apples with skin, Apricots, arctic bramble Berries, avocados, raw Bananas, bayberries, black berries, blue berries, cherries, coconut (Immature flesh), dates, figs raw, grape juice, Guava, jaboticaba, lemon pomegranate, Kiwi fruit, mangoes (Mangifera indica) oranges, papayas, peaches, pears, pineapple, strawberries, watermelon.
Annual saw: Thistle leaves, bitter gourd, bay leaves, beans, cabbage, carrots, cauliflower (raw), drumstick (raw), eggplant, fennel, garlic (raw) and ginger. Mushrooms, mustard Greens, pumpkin radish, seaweed, soyabean, spinach sweet potato and turnip greens.
Nuts: Cashew, badam, pistachio, walnut and peanut.
Beverages: Beer, red wine, coffee and tea (Bhagwat S, etal., 2011).
Chemical synthesis
In 1896, myricetin was isolated first by AG Perkin and Hummel from the Myrica nagi bark (Kim ME, et al., 2014).
Dean HF and Nierenstein M was tried to synthesize myricetin from Kostanecki and Auwers procedure was not successful (Dean HF and Nierenstein M, 1925).
Kalf J and Robinson R, synthesize myricetin from w-methoxy phloroacetophenone. Starting material was trimethylgallic anhydride and sodium trimethylgallate. It undergoes hydrolysis to form 5,7-dihydroxy-3,3`,4`,5`-tetramethoxy flavonr was formed. Demethylation of 5,7-dihydroxy-3,3`,4`,5`-tetramethoxy Flavone produce myricetin (Kalff J and Robinson R, 1925; Rao KV and Seshadri TR, 1948).
By ortho-oxidation reaction, Rao KV and Sheshadri TR synthesized myricetin from Quercetin (Chen YH, et al., 2012). Hibiscetin was synthesized by using myricetin as starting material.
Toxicity studies
According to Organisation for Economic Co-operation and Development (OECD) guidelines, female rats or mice were used for acute toxicity studies. The animals were accommodated in the lab, 7 days before the experiments, to adopt laboratory conditions i.e., temperature 25 ± 3°C with 12 hr dark/light cycle. Animals are supplied with food and water. In vitro and in vivo toxicity studies of myricetin mark-up extremely few unwanted or untoward effects. I.P administration of myricetin at 1000 mg/kg body weight to mice does not show any untoward effects or death. In zebrafish, the myricetin compound does not cause any toxicity at 100 mg/kg (Kim JD, et al., 2006).
Human umbilical vein endothelial cells were not affected by myricetin due to the protective effect of hydroxy groups present on the B-ring (Canada AT, et al., 1989). Myricetin at 450 μM leads to cell impairment to isolated erythrocytes of swine.
Pharmacokinetic Properties of Myricetin
Myricetin is absorbed by Gastrointestinal Tract (GIT) the remaining drug metabolized by Gastrointestinal microflora. The absorbed drug is metabolized by liver metabolites excreted through urine. The major metabolite is 3,5-dihydroxy phenylacetic acid (Dang Y, et al., 2014). Myricetin exhibits low bioavailability after the oral Route of Administration due to poor aqueous solubility. To enhance the bioavailability, inhibit the Cyt P-450 enzymes and P-glycoproteins. Myricetin is a weak acid and exhibited low aqueous solubility, slow dissolution rate, and fast degradation at high PH (Dang Y, et al., 2014; Li C, et al., 2011).
PHARMACOLOGICAL ACTIVITIES
Antioxidant effect
Myricetin is a powerful antioxidant that exhibits free radical scavenging activity (Husain SR, et al., 1987). Myricetin retards the production of 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) and Diphenylpicryl Hydrazine (DPPH) radicals which was polyphenol oxidase-dependent (Jiménez M and García‐Carmona F, 1999). Menadione-stressed HL-60 cells do not produce an effect against Reactive Oxygen Species. But the compounds show a powerful scavenging activity against DPPH radicals (Rusak G, et al., 2005). At 500 μM (160 μg/ml) concentration of myricetin, inhibits the radical at 81.5%. Myricetin defends cells against H2O2 induced cell damage (Kang KA, et al., 2010). Myricetin exhibits an impecunious effect in SOD (Superoxide Dismutase) assay. Compound stops oxidative stress causes cell death through regulation of PI3K and signalling mechanisms (Wang ZH, et al., 2010). Researchers reported that compound at a dose of 100 μM limits H2O2 induced DNA breakage in human lymphocytes (Duthie SJ and Dobson VL, 1999).
The research group states that compound has a restrictive effect on liposomal peroxidation (Miyajima Y, et al., 2004). Nuclear DNA degeneration was influenced by myricetin compound with simultaneous lipid peroxidation, and it is stimulated by Fe (III) or Cu (II) (Sahu SC and Gray GC, 1993). Myricetin-induced Lipid peroxidation was inhibited by Superoxide Dismutase in presence of Cu (II), but it was enhanced by Catalase (CAT) and Sodium Azide in presence of Fe (III) (Yang ZJ, et al., 2019).
Bennett and co-workers revealed that myricetin antioxidant property was surpassed that of Vitamin E (Sahu SC and Gray GC, 1993). At 30, 50 and 100 uM concentration, myricetin act against the production of Reactive Oxygen Species (ROS) are in the Red Blood Cell (RBC) of normal and sickle cell anaemia individuals (Abalea V, et al., 1999). The compound inhibits Malondialdehyde (MDA) formation by microsomes of the rat liver (Henneberg R, et al., 2013; Robak J and Gryglewski RJ, 1988). Zhao and Zhang reported the cytoprotective effect of Myricetin at concentrations of 20 uM, 40 uM, and 60 uM, against H2O2 and Carbon tetrachloride (CCL4) induced oxidative damage in human liver cells (Costantino L, et al., 1992). Laughton and co-workers in 1989 reported that myricetin in presence of antiviral and antibiotic drug bleomycin leads to DNA damage. These results suggested that at low doses myricetin acts as an antioxidant but at high doses, it acts as a pro-oxidant (Zhao X and Zhang X, 2009). Dietary supplement of Myricetin rich food useful in all diseased States. Oyama and his researchers stated that myricetin decreases Ca2+ induced oxidative metabolism without act on intracellular calcium ions (Laughton MJ, et al., 1989). Myricetin compound obstructs Low-Density Lipoprotein (LDL) oxidation which depended on the hydroxy group numbers in the ring-B (Oyama Y, et al., 1994). Sadasivam and Kumaresan in 2011, found that the B-ring in Myricetin structure mainly responsible for antioxidant activity (Teissedre PL, et al., 1996). DPPH scavenging activity was mainly due to the presence of 3`,4`-catechol moiety in the ring-B (Xie HJ, et al., 2013). The reduction of xanthine oxidase is due to the hydroxyl moiety at position C-4 (Wang L, et al., 2006).
Anti-inflammatory activity
Myricetin exhibits anti-inflammatory activity and it was demonstrated by different in vitro assays and in vivo models. Byoung Okcho and co-researchers stated that myricetin inhibits nitric oxide and Prostaglandin E2 (PGE2) production by acting on inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase (COX)-2 (Grenier D, et al., 2015). They reported that pro-inflammatory mediator's inhibition takes place by Myricetin. Interleukins (IL)-6, IL-8, and Matrix Metalloproteinase-3 (MMP-3) production were inhibited by the Myricetin compound. Greiner and Co worked in 2015, stated that Myricetin is used for the treatment of periodontitis i.e., gum infection (Takano-Ishikawa Y, et al., 2006). LPS-induced prostaglandins production was inhibited by this compound. SAR of myricetin suggests that double bonds at C2-C3 and keto groups at C4 are mainly responsible for the inhibitory effects on COX-2.
Myricetin acts against rheumatoid arthritis at various concentrations of 1 uM, 5 uM,10 uM and 20 uM (Kuo PL, 2005). It also produces anti-arthritic activity by inhibiting IL-1B. Induced production of matrix metalloproteinase and Interleukin in synovial cells (SW982) (Lee YS, Choi EM, 2010). Screening activities like carrageenan-induced carrageenan-induced hind paw edema, Xylene-induced ear edema, and acetic acid-induced vascular permeability were inhibited in the human body by Myricetin (Song X, et al., 2021). Zhao J, et al., 2013 and Co-workers reported that the anti-inflammatory activity of the compound might be due to its antioxidant effect. Myricetin at very low doses i.e., 0.1 mg/kg and 0.3 mg/kg body weight through Intravenous (I.V) Route of Administration markedly enhances the PGE2 levels in plasma in a time-dependent manner. Myricetin also enhances the levels of PGD2 and PGF2 α. Hyoung-woo Bai and Co-workers reported that bioflavonoids act as a stimulator of COX-1 and COX-2 mediated formation of prostaglandins in humans (Bai HW and Zhu BT, 2010).
Bai HW and Zhu BT reported that myricetin shows inhibitory activity on IL-1b and IL-6 and inhibits the production of NO, myeloperoxidase, and MDA (Bai HW and Zhu BT, 2010). As well as increases the activities of Superoxide Dismutase and GSH-Px. Myricetin enhances the formation of PGE2 at a dose below 0.3 mg/kg body weight, at higher doses decreases the enhancing effect (Semwal DK, et al., 2016).
Anti-cancer activity
Myricetin exhibits cytotoxicity towards human cancer cell lines, mainly skin, colon, liver, and pancreatic cancer cells (Semwal DK, et al., 2016). Myricetin inhibits cancer initiation and growth by inhibiting main enzymes (Taheri Y, et al., 2020). The Structure-Activity Relationship (SAR) of Myricetin revealed that aromatic ring-B at C2 and C2-C3 and hydroxy groups in the ring-B mainly exhibits cytotoxicity (Romanouskaya TV and Grinev VV, 2009). Myricetin shows action against human colon cancer Ring-A of Myricetin compound, including 4-keto and 5-OH are responsible for human Flap Endonuclease-1(hFEN-1) activity during human colon cancer (Gutiérrez-Venegas G, et al., 2014).
Haizhi H, et al. 2015 reported that myricetin inhibits Human Umbilical Vein Endothelial Cells (HUVEC) angiogenesis in an in-vitro model. The in-vivo model states that the compound inhibits Chorioallantoic Membrane (CAM) angiogenesis. Angiogenesis inhibition mainly responsible for the prevention of ovarian cancer.
Janus Kinase 1 (JAK1), Mitogen-activated protein Kinase (MEK), and Mitogen-activated protein Kinase Kinase (MKK-4) activity reduction take place by the inhibition of tumor promoter induced neoplastic cell transformation, which shows a protective effect against melanoma i.e., skin cancer (Kang NJ, et al., 2011).
Myricetin act against leukemia by antiproliferative activity (Labbé D, et al., 2009). The compound exhibits potent action on Prostate Cancer-3 (PC-3) cells, which has a strong inhibitory action on human prostate cancer. Myricetin alone or with the combination of myricetin enhances PC-3 cell death (Shih YW, et al., 2009). Anti-proliferative action on the human hepatoma cancer cell that is Hep-G2 and arrest of G2/M phase took place by this compound. Myricetin enhances the protein levels in p53/p21 cascade and decreases cell division control (cdc2) and cyclin B protein levels in hepatoma cancer cells (Androutsopoulos VP, et al., 2011). Myricetin shows a modest cytotoxic effect on Oesophageal adenocarcinoma (OE33) cells, which results in human oesophageal adenocarcinoma (Shiomi K, et al., 2013).
Myricetin acts against gastric cancer by targeting Ribosomal protein S6 kinase (Rsk2), Mitotic arrest deficiency 1 (Mad1) proteins which lead to decreased proliferation (Maroufi NF, et al., 2020). Compound targets B-cell lymphoma 2 (Bcl-2), Pr-caspase-3, BCL2 associated X (Bax), caspase-3 enzymes to increase apoptosis in gastric cancer. The compound exerts action on medulloblastoma (brain tumor) which occurs mainly in children. It inhibits Mesenchymal-epithelial transition (Met)/Hepatocyte Growth Factor (HGF) signaling Pathways (Pasetto S, et al., 2014). Metastasis of human A549 cells in vitro by decreasing migration invasion of carcinoma cells. Cells without affecting normal cells (Ortega JT, et al., 2013). It is a potent cytotoxic agent against prostate cancer (Ghassemi‐Rad J, et al., 2018). It was found to induce apoptosis and cytotoxicity in the 231 breast cancer cells. G1-Cell arrest takes place by Myricetin-loaded NLCs (Nanostructured Lipid Carriers) and DXT (Docetaxel) (Gendaram O, et al., 2011). Myricetin exhibits an inhibitory effect on mammalian polymerases α, β, and K (Jiménez R, et al., 1999). Myricetin exhibits antiproliferative, pro-apoptotic effects and reduces the release of several proteins which are involved in cell cycle progression.
Myricetin acts as a suppressor of the topoisomerase-II enzyme by directly interacting with the enzyme. Effect of Myricetin on leukemia, prostate, Pancreas, brain, lungs, skin, uterus, colon, and breast cancer cells by a different mechanism of apoptosis, anti-proliferator, angiogenesis, migration, and metastasis.
Anti-HIV
Myricetin exerts anti-HIV activity. Silvana P reported that myricetin shows the highest anti-HIV activity than quercetin and pinocembrin. Myricetin shows less toxicity and had anti-HIV activity on b1 cells. Myricetin inhibits the HIV-1 reverse transcriptase enzyme (Kanaan NM, et al., 2015). In vitro anti-HIV activity was studied by different concentrations of the Myricetin. This compound inhibits HIV-1 reverse transcriptase glycosyl moiety of Myricetin i.e., myricetin3-rhamnoside shows more effect than myricetin-3-(6-rhamnosyl-galactoside) (Duyckaerts C, et al., 2009). The anti-viral activity of myricetin is mainly due to the inhibitory action of the HIV-1 reverse transcriptase enzyme.
Immunomodulatory activity
Myricetin exhibits immunomodulatory effects in various in-vitro and in-vivo models. The compound exerts a potent inhibitory action on T-Lymphocyte’s activation (Jones JR, et al., 2011). In the preclinical model, myricetin exerts stimulatory action on antibody formation or inhibitory effect on white blood cells. At a concentration of 10 μg/ml, myricetin modulates LPS stimulated activation of bone-marrow-derived dendritic cells of mice. It does not induce any toxic effect towards dendritic cells at 10 μg/ml concentration (Choi YJ, et al., 2008). A preclinical study in a mouse model, on anti-cluster of Differentiation 3 (CD3) and anti-CD-28 monoclonal antibodies show inhibitory action on activation of T-lymphocytes (Jones JR, et al., 2011). Lipopolysaccharides (LPS) induced Interleukin-12 (IL-12) production was inhibited by myricetin through the downregulation of Nuclear Factor kappa-B (NF-kB) binding activity (Shimmyo Y, et al., 2008). At 50 um concentration, myricetin enhances endothelium-dependent contractile responses in isolated rat aortic rings. In-vitro studies reported that myricetin compound affects Interleukin-2 expression (Taheri Y, et al., 2020).
At 5 uM-100 uM concentration, compounds inhibit expression of CD69 and proliferation of lymphocytes in a mouse model.
Neurological effects
Recent research of myricetin pointed to its neuroprotective activity by showing action on brain receptors. Myricetin exhibits a protective effect against neurodegenerative diseases like Alzheimer's disease and Parkinson's disease. Myricetin shows action on specific proteins in brain-like amyloid-beta (β) and Tau proteins, which are more in axons distal part (Chang Y, et al., 2015).
Mechanism of neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s Disease (PD) by the accumulation of amyloid and Senile plaques. Amyloid-beta plaques lead to neuronal loss in different brain regions. Formation of neurofibrillary tangles by the hyper-phosphorylation of Tau proteins (Zhu X and Jia YH, 2014). The target protein for Alzheimer's disease is Tau protein. Compound at a concentration of 50 uM produces an anti-tau effect in HeLa-C3 cells (Laabich A, et al., 2007). Amyloid-Beta 42 aggregates fibril formation. Docking studies, prove that the myricetin can interfere with amyloid-beta fibril formation, therefore it indicates strong evidence of anti-amyloidogenic activity (Lei Y, et al., 2012). In rat cortical neurons, myricetin at 300 uM concentration potentially inhibits amyloid beta-induced cell injury (Liu RN, et al., 2007). At 10 uM concentration, the compound inhibits structural changes in amyloid-beta. Glutamate excessive release leads to serious toxicity which induces brain disorders. Chang H, et al. stated that myricetin inhibits glutamate release, which prevents glutamate-induced toxicity (Mohan M, et al., 2009). Myricetin inhibits COMT (Catechol Ortho Methyltransferase enzyme) which metabolizes levodopa. Levodopa is used to increase dopamine concentration in the brain treats Parkinson's disease (Moonrungsee N, et al., 2014). Myricetin was found to be protective against A2E induced photoreceptors and bipolar cell death (Ma ZG, et al., 2007). Myricetin reduces oxidative stress and increases the activity of the Na+ K+ Adenosine Triphosphate (ATPase) pump, which acts against D-galactose-induced memory loss in mice (Franco JL, et al., 2010). Liu RN, and co-workers (Zhang K, et al., 2011). He stated that in cultured rat neurons myricetin inhibits sodium-dithionite-induced ischemic injury. Compound at a dose of 30 mg/ Kg and 100 mg/Kg body wt. through route, reduces anxiety i.e., anxiolytic activity in mice (Zhang XH, et al., 2012). Myricetin inhibits the tyramine oxidase enzyme, which plays a role in the inactivation of neurotransmitters (Yang Y, et al., 2012). Myricetin reduces dopamine in substantia nigra striatum, by reducing 6-hydroxy dopamine toxicity (Tong Y, et al., 2009). In vitro studies on myricetin reported that it decreases methyl-mercury-induced oxidative stress and mitochondrial dysfunction of the mouse brain (Joshi V, et al., 2019). Myricetin exerts a neuroprotective effect towards MPP+ induced oxidative stress by reducing cell loss and nuclear condensation. Also, it decreases the Mitogen-activated protein Kinase (MAPK) kinase-4 phosphorylation and Janus kinase phosphorylation caused by MPP+ (Hagenacker T, et al., 2010). Myricetin enhances Gamma-Aminobutyric Acid (GABAergic) activity in PVN (Paraventricular Nucleus). Myricetin induced muscle paralysis by inhibiting acetylcholine release at Neuromuscular Junction (NMJ). But the myricetin shows less and shorter effect than Botulinum Neurotoxin (BONT) (Ong KC and Khoo HE, 1996).
Aggregation of misfolded proteins leads to aging and neurodegenerative diseases. Myricetin shows an inhibitory effect on the aggregation of misfolded proteins. Joshi and Co-researchers found that myricetin decreases aggregation of misfolded luciferase proteins which was identified by immunofluorescence staining. It results in decreased accumulation of heat-denatured luciferase Perinuclear bodies. Myricetin also decreases the aggregation of misfolded Superoxide Dismutase 1. Myricetin reduces the aggregation of mutant α-Synuclein. These results vigorously recommended that myricetin May set out as a helpful compound against misfolded protein aggregative neurodegenerative diseases (Ong KC and Khoo HE, 2000).
Analgesic activity
Myricetin acts as a potent analgesic. It acts as peripheral analgesia in screening activities like Hot-plate test, formalin-induced paw licking, and acetic acid-induced writhing response. It is not showing the effect on the opioid system (Zelus C, et al., 2012). Hagenacket T and co-workers reported that myricetin acts as an analgesic in a neuropathic pain rat model, by inhibiting Spinal nerve Ligation-induced and thermal hyperalgesia (Karunakaran U, et al., 2019). In-vitro studies of myricetin reported that it inhibits PGE2 production, platelet aggregation (Li Y, et al., 2017). Myricetin at a dose of 10-1000 mg/kg I.P Route of Administration, decreases the bradykinin-induced nociception.
Antidiabetic activity
Myricetin has a potent antidiabetic activity that mainly acts against NIDDM (Non-Insulin-Dependent Diabetes Mellitus), by enhancing the glucose uptake mechanism (Tzeng TF, et al., 2011). The compound exerts stimulatory action on D-glucose and D-3-O-methyl glucose uptake in adipocytes of rats. Ong KC and Khan reported that Intraperitoneal (I.P) administration of myricetin at a dose of 3 mg/kg/12 hrs for a 2 days to Streptozotocin (STZ) induced diabetic rat’s results in a decrease in half of the increased glucose levels and increases the hepatic glycogen and Glucose-6-phosphate (Liu IM, et al., 2007).
Myricetin at 20 uM concentration exerts action against high glucose-induced B-cell apoptosis by the inhibition of Cyclin Dependent kinase 5 (CDk-5) (Ding Y, et al., 2012). Myricetin exerts antihyperglycemic activity without causing any effect to the liver. Zelus C and co-workers reported that myricetin inhibits the Islet Amyloid Polypeptide (IAPP) aggregation. IAPP is the main cause for cell death of Beta islets of the pancreas in NIDDM (Landolfi R, et al., 1984). Researchers found that myricetin at 100 mg/Kg/ day orally twice for 6 months, inhibits the NF-kB pathway. It also stimulates GLP-1R (Glucagon-like Peptide-1 Receptor) (Robak J, et al., 1988). In fructose-induced insulin-resistant rats myricetin at a dose of 1 mg/kg, 3 times a day for 14 days by I.V Route of Administration, results in reducing high blood glucose levels and enhance insulin resistance by stimulating β-endorphin release. It also acts on the phosphorylation of insulin receptors (Ozcan F, et al., 2012). A study on obese Zucker rats, reported that I.V administration of compound at 1 mg/kg 3 times a day for 1-week decrease blood glucose levels and insulin-glucose index value (Kandasamy N and Ashokkumar N, 2013). Recent studies reported that myricetin stimulates Akt and AMP-activated protein kinase (AMPK) protein activity, increased glucose uptake, and decreased insulin resistance (Gebhardt R, 2003). In-vivo studies of myricetin on rats result in increasing the stimulatory effect of insulin and stimulated lipogenesis in adipocytes (Chang CJ, et al., 2012). In-vivo model on rats reported a 50% decrease in Diabetes by the treatment of myricetin dose at 3 mg/12 hours for 2 days (Tzeng SH, et al., 1991). Myricetin shows an effect in Diabetic nephropathy. I.P administration of myricetin at 1 mg/kg body weight in rats produces antihyperglycemic and renal protective effect in STZ-CD induced diabetic nephrotoxicity (Zang BX, et al., 2003). At 10 uM concentration, myricetin increases hepatocellular cholesterol biosynthesis in rat hepatocytes. But at higher concentrations, it was found to inhibit cholesterol biosynthesis (Melzig MF and Henke K, 2005). Recent studies revealed that myricetin acts against obesity, by decreasing the accumulation of intracellular triglycerides in adipocytes of high-fat diet-fed rats, at a dose of 300 mg/Kg per day for 8 weeks. In reducing body weight and visceral fat depots, myricetin at 300 mg/Kg/day effects were almost found similar to Fenofibrate at a dose of 100 mg/Kg/ day (Liu L, et al., 2010).
Anti-platelet aggregation activity
Myricetin at 150 uM concentration inhibits platelet aggregation and thrombus formation induced by collagen, Arachidonic acid, and platelet-activating factor. Renato SG, et al., in 2020 reported that myricetin is a novel inhibitor of thiol isomerases Endoplasmic Reticulum Protein 5 (ERP5) and Protein Disulfide Isomerase (PDI) with potent anti-thrombotic and anti-platelet functions (Borde P, et al., 2011). Myricetin inhibits thromboxane B2 formation in platelets. This study shows that antiplatelet activity of myricetin may be due to inhibition of thromboxane formation (Godse S, et al., 2010). This compound inhibits the binding of the receptor to Platelet-Activating Factor (PAF) in rabbit platelets which leads to anti-platelet aggregation activity (Borde P, et al., 2011; Godse S, et al., 2010). Melzing MF, et al. reported that myricetin is active against thrombin and neutrophil elastase (Chen SY, et al., 2019). Liu L, et al. found that potential inhibition of thrombin takes place by myricetin which was initially indicated by docking studies. Therefore, this compound is used as anti-thrombotic drugs (Matić S, et al., 2013).
Myricetin stimulates platelet adenosine 3,5-cyclic monophosphate levels. Anti-Platelet aggregation activity takes place by the inhibition of phosphodiesterase activity, which modifies the platelet cyclic Adenosine Monophosphate (AMP) metabolism (Chaabi M, et al., 2008). Myricetin at a dose of 3.6 ug/Kg body weight by I.V Route of Administration inhibits platelet aggregation in cat blood.
The overall effect of myricetin on platelets revealed that it is a potent COX-1 inhibitor with an anti-platelet aggregation effect.
Anti-hypertensive activity
Antihypertensive activity of myricetin was studied in both in-vitro and in-vivo. Myricetin decreases the high blood pressure and oxidative stress produced by DOCA (Deoxycorticosterone Acetate) salt in the rat at a dose of 100 mg and 300 mg/kg body weight (Lee SE and Park YS, 2013).
In recent studies on rats reported that a high Fructose diet fed for 6 weeks exhibits an increase in Blood Pressure (BP), blood glucose, triglycerides, cholesterol, and insulin. But in myricetin-treated rats, we observe a significant decrease in blood pressure and cholesterol in fructose-fed rats. The anti-hypertensive effect of myricetin was produced at a dose of 300 mg/kg. In vitro studies reported that myricetin inhibits Angiotensin-Converting Enzyme (ACE) activity and removing Reactive Oxygen Species generation which leads to vasodilation in vascular endothelial cells. Myricetin exerts vasodilation also by inducing the release of Nitric Oxide (NO) and PGI2 levels. Myricetin exerts antihypertensive activity by inducing NO and PGI2 release, a decrease in the production of ROS, and by inhibiting the Angiotensin-Converting Enzyme.
Hepatoprotective activity
Myricetin produces a hepatoprotective effect by promoting the restoration of hepatic function in pyrogallol-induced toxicity in mice. Myricetin reduces the elevated serum enzymes like Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), Alanine Aminotransferase (ALT), and total bilirubin. It also reduces DNA damage in the liver (Tiwari R, et al., 2009).
Cardioprotective activity
Recent studies on myricetin reported that it produces a protective effect on the human vascular system (Gan CL, et al., 2007). Myricetin produces a protective effect on the human vascular system through transcriptional changes in human endothelial cells, which was measured by gene expression profiling (Liwei W, et al., 2007). This compound also prevents athero-sclerosis by inhibiting CD36 cell surface proteins and mRNA expression.
Myricetin exerts vasodilation effect in isolated and Langendroff-perfused rat hearts, without effect contractions and relaxations of heart muscle (Jung SK, et al., 2010). Myricetin has lipid-lowering activity so, it is used to treat cardiovascular diseases. Tiwari R, et al. reported that myricetin specifically inhibits cardiac marker enzymes mainly Lactate Dehydrogenase (LDH), SOD, AST, Carbonic Anhydrase (CA) and Creatine Kinase (CK) as well as it shows some histopathological changes which indicate the protective effect of myricetin on rat heart (Huang JH, et al., 2010).
Myricetin shows an inhibitory effect on the Signal Transducer and Activator of Transcription 1 (STAT1) mechanism and also protects the heart from injury or ischemia. Myricetin inhibits voltage-gated calcium channels, which was studied in cardiomyocytes of rats (Jung SK, et al., 2008). It does not produce any toxic effects even at a dose of 30 um concentration was observed in cultured neonatal rat cardiac cells (Huang JH, et al., 2010; Jung SK, et al., 2008). It also produces a protective effect towards hydrogen peroxide (H2O2) induced cell death of cardiomyocytes, by inhibiting the Caspase-3-protein activation.
Myricetin at 100 mg/kg and 300 mg/kg dose to Wistar rats through oral Route of Administration, results in decreased heart rate and cardiac markers like LDH, CK, SOD, and CAT as well as Electrocardiogram (ECG) patterns caused by isoproterenol. Myricetin also exerts anti-arrhythmic activity by inhibiting high-speed delayed potassium current in Human Embryonic Kidney 293 (HEK293) cells by time and dose-dependent manner (Kumamoto T, et al., 2009).
Anti-photo aging activity
Myricetin exerts anti-photo aging activity by the act against the free radical generation in the skin. Skin aging is mainly due to chronic exposure of human skin to UV-B light, which was characterized by dark color patches, wrinkles, and loss of flexibility and elasticity of skin.
Myricetin prepared as topical preparation exhibits an inhibitory effect on ultraviolet-B radiations induced wrinkles in mouse skin. The mechanism involved in this is, by suppressing ultraviolet-B induced Raf-kinase activity and phosphorylation of MEK and Extracellular-signal-regulated Kinase (ERK) (Cai L and Wu CD, 1996).
Huang J, et al. revealed that myricetin inhibits UV-B induced generation of H2O2 in keratinocytes which leads to antioxidant activity, enhances the free radical scavenging activity (Górniak I, et al., 2019).
In mouse skin cells compound able to inhibit ultraviolet-B induced COX-2 expression (Salehi B, et al., 2018).
In both ex-vivo and in-vitro models is capable to inhibit the phosphorylation and kinase activity of Akt. Recently researchers reported that myricetin directly targets Akt to limit its action on cell transformation (Ono K, et al., 1990). A recent study revealed that myricetin blocks damage to the basement membrane of the skin by inhibiting the MMP-9 expression which leads to preventing ultraviolet-B induced wrinkle formation (AritaMorioka KI, et al., 2015).
Antimicrobial activity
Myricetin exerts effect against virus and bacteria. 20 ug/L of Myricetin (MYR) act against gram negatives peri-dental oral pathogens. Flavonoids mechanism against the microbial activity involves disruption of the cell membrane, inhibition of cell membrane synthesis, nucleic acid synthesis inhibition, inhibition of bacterial virulence, impairs the formation of biofilms, efflux pump inhibition, Nicotinamide Adenine Dinucleotide Hydrogen (NADH)-cytochrome C reductase activity inhibition, and inhibition of ATP synthase (Yadav AK, et al., 2013; Wu T, et al., 2013).
On E.coli, myricetin shows effect by inhibiting DNA gyrase, DNA helicase, and DNA and RNA polymerases (Jayaraman P, et al., 2010). MYR shows action against E. coli, Salmonella paratyphi, Salmonella enteritidis, and Salmonellacholerasuis.
It also shows a lethal effect against Staphylococcus aureus (Naz S, et al., 2007). Myricetin reported in-vitro anti-HIV activity at 100 uM concentration. It also exerts action against Mycobacterium tuberculosis, due to the presence of hydroxyl group in the MYR structure (Chu SC, et al., 1992). The structure-activity relationship of myricetin reported that hydroxyl group substitution in the Ring A and B position is important for inhibitory action (Yu MS, et al., 2012). Myricetin also exerts a strong inhibitory effect in Pseudomonas aeruginosa (Keum YS and Jeong YJ, 2012).
Myricetin at a concentration of 0.5 mg/ml, produces inhibitory zone against Corynebacterium diphtheria, Bacillus subtilis, Enterococcus Faecium, Enterococcus Faecalis, Streptococcus pyogenes, S. pneumonia, E. coli, Klebsiella, Pneumonia, Pseudomonas aeruginosa, Pseudomonas mirabilis, Salmonellatyphi,S.dysenteriae,S.paratyphi,S.sonneieand Salmonellaflexneriae(Nitulescu G, etal., 2017).
Myricetin reported a potent inhibitory effect on reverse transcriptase. It also inhibits DNA polymerase and DNA polymerase I. SAR suggested that due to the presence of hydroxyl groups at C3 and C4 positions, exhibit potent inhibitory effect (Alvesalo J, et al., 2006; Elshamy AI, et al., 2020).
Myricetin acts against the SARS virus i.e., a Severe Acute Respiratory Syndrome. This compound inhibits coronavirus Helicase by the act against ATPase activity. But it is failed to inhibit ATPase activity of Hepatitis C virus helicase (Moghadam SE, et al., 2017). The compound also exerts an inhibitory effect on Moloney murine leukemia virus. Jayaraman P and Co-workers studied in-vitro antimicrobial activity of 6 natural flavonoids along with myricetin, against 5 strains of Pseudomonas aeruginosa (Mohan M, et al., 1988). The anti-chlamydial effect was produced by Myricetin which shows action against Chlamydia pneumoniae (Hodges LC, et al., 1999).
Wound healing activity
Abdel I, et al. reported that myricetin isolated from Tecomaria capensis exerts wound healing activity in rats by improving cellular re-epithelialization, cell deposition, anti-inflammatory, and cell proliferation effect (Chen R, et al., 2014). Recent studies revealed that myricetin-3-O-B-rhamnoside acts against skin injuries or wounds, by promoting capillary tube formation, closure of wounds, and accelerated fibroblast migration. Myricetin exhibited wound healing property at 10 ug/ml.
Activity against eye disorders
Myricetin exhibits anti-cataract activity by the inhibition of aldose reductase which was studied in galactosemic rats at 1% concentration (Jo S, et al., 2020). IV administration of myricetin at a dose of 1 mg per kg in rabbits lowered the intra-occular pressure. In-vitro study on myricetin at different concentrations i.e., 10 uM, 20 uM, 50 uM, and 100 uM decreases cell proliferation and migration of human retinal pigment as well as secretion of vascular endothelial growth factor.
A novel chemical inhibitor of SARS coronavirus helicase
Mi-sunyu and his co-workers found that myricetin and scutellarein inhibit the SARS Cov helicase by showing an effect on ATPase activity without inhibition of helicase activity. In this research, they observed that myricetin and scutellarein did not produce any toxicity towards healthy breast epithelial cells. Myricetin directly interacts with N265, R443g Y269 residues of ATPase domain (Meotti FC, et al., 2007).
Inhibition of African swine fever virus
African swine fever was caused by the African swine fever virus, which is the most dangerous swine disease or pandemic situation because it shows a large soci-economic impact. Mainly it leads to death within 10 days due to bleeding of internal organs. It was first reported in Kenya and Africa.
Serijo and Co-researchers found that the three hydroxyl groups of 3,4,5-tri-hydroxyphenyl ring of myricetin is mainly responsible for its inhibitory effect against an protease of African Swine flu virus.
Flavonol shows more inhibitory activity than the flavone because the 3-hydroxyl group of flavonol interacts with Protease (Shin JC, et al., 2013).
Other effects
Myricetin mediated inhibition of glutamate release: Recent studies on myricetin found that it is used to treat neurological disorders (Barlas N, et al., 2014). 100 mg/kg dose of myricetin in rats, decreases the D-galactose induced learning and memory impairment and also exerts anti-anxiety activity (Yamaguchi M, et al., 2007). In-vitro studies of myricetin at 50 uM dose reported that it protects neurons against neurotoxins.
In rat cortical nerve terminals myricetin decreases glutamate release by inhibiting the presynaptic influx of calcium ions. Myricetin prevents neuronal injury which was associated with calcium overload.
Shin JC, et al. reported that myricetin is used to treat disorders of circadian rhythm by changing the serum melatonin and locomotor activity.
It decreases the melatonin level by inhibiting the rate-limiting enzyme arylalkylamine N-acetyltransferase in rats. Myricetin also exerts its effect on sex hormones. In rats, myricetin at 100 mg/kg dose per day through oral Route of Administration causes oestrogenic effect by increasing weight of the uterus and height in immature wistar rats.
Myricetin exhibits action on bone. It prevents parathyroid hormone-induced osteoclast and decreases the release of diaphyseal calcium content.
It stimulates osteoblast differentiation from maturation to terminal differentiation BMP-2 (Bone Morphogenetic Protein-2) and P38 production was increased by myricetin which leads to differentiation.
Conclusion
Myricetin is a natural flavonoid with significant pharmacological effects and encouraging therapeutic capability. It is a lead molecule present in many foods i.e., mainly in fruits and vegetables, which exerts potent antioxidant activity by the act against oxidative damage. The compound used as nutraceutical which exhibits potential protective effect towards neurodegenerative diseases like Alzheimer's disease and Parkinson's disease. Preclinical studies revealed, Anti-oxidant activity of myricetin mainly due to the presence of B-ring in its structure. Myricetin exhibits anti-arthritic activity by inhibiting IL-1 β induced production of matrix metalloproteinase and IL-6 in SW892 (synovial cells). Myricetin is a potent cytotoxic agent. It shows anti-cancer activity by different mechanisms of apoptosis, Angiogenesis, and anti-proliferative action. Compound at 1 mg/kg body weight in rats through I.P ROA produces antihyperglycemic activity. The anti-platelet activity of myricetin is due to the inhibition of thromboxane B2 formation. Myricetin protects the heart from injury or ischemia by showing inhibitory action on STAT1 activation. Myricetin induces the release of NO and PGI2 levels which leads to vasodilation. The anti-photoaging effect of myricetin was studied in mouse skin cells (JB6PH), which inhibits UV-B-induced COX-2 expression. It also acts against the SARS virus, showing action on coronavirus helicase by the act against ATPase activity. It acts against the African swine fever virus. Myricetin protects neurons by the act against neurotoxins. Due to its non-toxic nature, which results in various in-vivo studies. Myricetin at higher concentrations exhibits a Pro-oxidant effect.
Myricetin and its derivatives are multifaceted molecules. More in-depth work has to be performed for their wider applications on humans.
References
- Yao Y, Lin G, Xie Y, Ma P, Li G, Meng Q, et al. Preformulation studies of myricetin: A natural antioxidant flavonoid. Pharmazie. 2014; 69(1): 19-26.
- Temple NJ, Gladwin KK. Fruit, vegetables, and the prevention of cancer: Research challenges. Nutrition. 2003; 19(5): 467-470.
- Rive-Evans CA, Miller NJ. Structure-antioxidant activity relationships of flavonoids and isoflavonoids. Flavonoids in Health and Disease. 1998; 199-219.
- Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Crit Rev Food Sci Nutr. 1997; 37(8): 705-718.
- Perkin AG. XXI-Myricetin Part II. J Chem Soc Trans. 1902; 81: 203-210.
- Umadevi I, Daniel M, Sabnis SD. Chemotaxonomic studies on some members of Anacardiaceae. Proc Plant Sci. 1988; 98(3): 205-208.
- Lin GB, Xie Y, Li GW. Research advances of myricetin. J Int Pharm Res. 2012; 39: 483-487.
- Büchter C, Ackermann D, Havermann S, Honnen S, Chovolou Y, Fritz G, et al. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int J Mol Sci. 2013; 14(6): 11895-11914.
- Mullie P, Clarys P, Deriemaeker P, Hebbelinck M. Estimation of daily human intake of food flavonoids. Plant Foods Hum Nutr. 2007; 62(3): 93-98.
- Park KS, Chong Y, Kim MK. Myricetin: Biological activity related to human health. Appl Biol Chem. 2016; 59(2): 259-269.
- Yang ZJ, Wang HR, Wang YI, Zhai ZH, Wang LW, Li L, et al. Myricetin attenuated diabetes-associated kidney injuries and dysfunction via regulating nuclear factor (erythroid derived 2)-like 2 and nuclear factor-κB signaling. Front Pharmacol. 2019; 10: 647.
- Lugast A, Hovari J. Flavonoid aglycons in foods of plant origin I. Vegetables. Acta Aliment. 2000; 29(4): 345-352.
- Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods, release 3. US Department of Agriculture. 2011; 159.
- Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res. 2014; 34(2): 701-706.
- Dean HF, Nierenstein M. Attempts to synthesize myricetin. J Am Chem Soc. 1925; 47(6): 1676-1684.
- Kalff J, Robinson R. XXVIII-A synthesis of myricetin and of a galangin monomethyl ether occurring in galanga root. J Chem Soc Trans. 1925; 127: 181-184.
- Rao KV, Seshadri TR. Nuclear oxidation in flavones and related compounds. Proc Indian Acad Sci A. 1948; 27(5): 375-390.
- Chen YH, Yang ZS, Wen CC, Chang YS, Wang BC, Hsiao CA, et al. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012; 134(2): 717-724.
- Kim JD, Liu L, Guo W, Meydani M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem. 2006; 17(3): 165-176.
- Canada AT, Watkins WD, Nguyen TD. The toxicity of flavonoids to guinea pig enterocytes. Toxicol Appl Pharmacol. 1989; 99(2): 357-361.
- Dang Y, Lin G, Xie Y, Duan J, Ma P, Li G, et al. Quantitative determination of myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability. Drug Res. 2014; 64(10): 516-522.
- Li C, Lim SC, Kim J, Choi JS. Effects of myricetin, an anticancer compound, on the bioavailability and pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats. Eur J Drug Metab Pharmacokinet. 2011; 36(3): 175-182.
- Husain SR, Cillard J, Cillard P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry. 1987; 26(9): 2489-2491.
- Jiménez M, García‐Carmona F. Myricetin, an antioxidant flavonol, is a substrate of polyphenol oxidase. J Sci Food Agric. 1999; 79(14): 1993-2000.
- Rusak G, Gutzeit HO, Müller JL. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr Res. 2005; 25(2): 143-155.
- Kang KA, Wang ZH, Zhang R, Piao MJ, Kim KC, Kang SS, et al. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 2010; 11(11): 4348-4360.
- Kang KA, Zhang R, Piao MJ, Jo SH, Kim JS. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ Toxicol Pharmacol. 2010; 29(1): 12-18.
- Duthie SJ, Dobson VL. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr. 1999; 38(1): 28-34.
- Miyajima Y, Kikuzaki H, Hisamoto M, Nakatani N. Antioxidative polyphenols from berries of Pimenta dioica. Biofactors. 2004; 22(1-4): 301-303.
- Sahu SC, Gray GC. Interactions of flavonoids, trace metals, and oxygen: Nuclear DNA damage and lipid peroxidation induced by myricetin. Cancer Lett. 1993; 70(1-2): 73-79.
- Abalea V, Cillard J, Dubos MP, Sergent O, Cillard P, Morel I. Repair of iron-induced DNA oxidation by the flavonoid myricetin in primary rat hepatocyte cultures. Free Radic Biol Med. 1999; 26(11-12): 1457-1466.
- Henneberg R, Otuki MF, Furman AE, Hermann P, Nascimento AJ, Leonart MS. Protective effect of flavonoids against reactive oxygen species production in sickle cell anemia patients treated with hydroxyurea. Rev Bras Hematol Hemoter. 2013; 35: 52-55.
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988; 37(5): 837-841.
- Costantino L, Rastelli G, Albasini A. Inhibitory activity of flavonols towards the xanthine oxidase enzyme. Int J Pharm. 1992; 86(1): 17-23.
- Zhao X, Zhang X. Comparisons of cytoprotective effects of three flavonoids against human hepatocytes oxidative injury induced by hydrogen peroxide or carbon tetrachloride in vitro. J Med Plants Res. 2009; 3(10): 776-784.
- Laughton MJ, Halliwell B, Evans PJ, Robin J, Hoult S. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin: Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem Pharmacol. 1989; 38(17): 2859-2865.
- Oyama Y, Fuchs PA, Katayama N, Noda K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca2+-loaded brain neurons. Brain Res. 1994; 635(1-2): 125-129.
- Teissedre PL, Frankel EN, Waterhouse AL, Peleg H, German JB. Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wines. J Sci Food Agric. 1996; 70(1): 55-61.
- Xie HJ, Mou WS, Lin FR, Xu JH, Lei QF. Radical scavenging activity of myricetin. Acta Physico Chim Sin. 2013; 29(7): 1421-1432.
- Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem. 2006; 54(26): 9798-9804.
- Grenier D, Chen H, Ben Lagha A, Fournier-Larente J, Morin MP. Dual action of myricetin on Porphyromonas gingivalis and the inflammatory response of host cells: A promising therapeutic molecule for periodontal diseases. PloS One. 2015; 10(6): 131758.
- Takano-Ishikawa Y, Goto M, Yamaki K. Structure-activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine. 2006; 13(5): 310-317.
- Kuo PL. Myricetin inhibits the induction of anti-Fas IgM-, tumor necrosis factor-α-and interleukin-1 β-mediated apoptosis by Fas pathway inhibition in human osteoblastic cell line MG-63. Life Sci. 2005; 77(23): 2964-2976.
- Lee YS, Choi EM. Myricetin inhibits IL-1 β-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int Immunopharmacol. 2010; 10(7): 812-814.
- Song X, Tan L, Wang M, Ren C, Guo C, Yang B, et al. Myricetin: A review of the most recent research. Biomed Pharmacother. 2021; 134: 111017.
- Zhao J, Hong T, Dong M, Meng Y, Mu J. Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol Med Rep. 2013; 7(2): 565-570.
- Bai HW, Zhu BT. Myricetin and quercetin are naturally occurring co-substrates of cyclooxygenases in vivo. Prostaglandins Leukot Essent. 2010; 82(1): 45-50.
- Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: A dietary molecule with diverse biological activities. Nutrients. 2016; 8(2): 90.
- Taheri Y, Suleria HA, Martins N, Sytar O, Beyatli A, Yeskaliyeva B, et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement Altern Med. 2020; 20(1): 1-4.
- Romanouskaya TV, Grinev VV. Cytotoxic effect of flavonoids on leukemia cells and normal cells of human blood. Bull Exp Biol Med. 2009; 148(1): 57.
- Gutiérrez-Venegas G, Luna OA, Arreguín-Cano JA, Hernández-Bermúdez C. Myricetin blocks lipoteichoic acid-induced COX-2 expression in human gingival fibroblasts. Cell Mol Biol Lett. 2014; 19(1): 126-139.
- Huang H, Chen AY, Ye X, Li B, Rojanasakul Y, Rankin GO, et al. Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int J Oncol. 2015; 47(4): 1494-1502.
- Kang NJ, Jung SK, Lee KW, Lee HJ. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann N Y Acad Sci. 2011; 1229(1): 124-132.
- Labbé D, Provençal M, Lamy S, Boivin D, Gingras D, Béliveau R. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J Nutr. 2009; 139(4): 646-652.
- Shih YW, Wu PF, Lee YC, Shi MD, Chiang TA. Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: Possible mediation by blocking the ERK signaling pathway. J Agric Food Chem. 2009; 57(9): 3490-3499.
- Androutsopoulos VP, Papakyriakou A, Vourloumis D, Spandidos DA. Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. Bioorg Med Chem. 2011; 19(9): 2842-2849.
- Shiomi K, Kuriyama I, Yoshida H, Mizushina Y. Inhibitory effects of myricetin on mammalian DNA polymerase, topoisomerase and human cancer cell proliferation. Food Chem. 2013; 139(1-4): 910-918.
- Maroufi NF, Vahedian V, Mazrakhondi SA, Kooti W, Khiavy HA, Bazzaz R, et al. Sensitization of MDA-MBA231 breast cancer cell to docetaxel by myricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn Schmiedebergs Arch. 2020; 393(1): 1.
- Pasetto S, Pardi V, Murata RM. Anti-HIV-1 activity of flavonoid myricetin on HIV-1 infection in a dual-chamber in vitro model. PLoS One. 2014; 9(12): 115323.
- Ortega JT, Suárez AI, Serrano ML, Baptista J, Pujol FH, Rangel HR. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res Ther. 2017; 14(1): 1-6.
- Ghassemi‐Rad J, Maleki M, Knickle AF, Hoskin DW. Myricetin‐induced oxidative stress suppresses murine T lymphocyte activation. Cell Biol Int. 2018; 42(8): 1069-1075.
- Jiménez R, Andriambeloson E, Duarte J, Andriantsitohaina R, Jiménez J, Pérez‐Vizcaino F, et al. Involvement of thromboxane A2 in the endothelium‐dependent contractions induced by myricetin in rat isolated aorta. Br J Pharmacol. 1999; 127(7): 1539-1544.
- Gendaram O, Choi YH, Kim YS, Ryu SY. Anti-oxidative and antibacterial constituents from Sedum hybridum. Nat Prod Sci. 2011; 17(4): 279-284.
- Kanaan NM, Himmelstein DS, Ward SM, Combs B, Binder LI. Tau protein: Biology and pathobiology. Movement Disorders. 2015; 857-874.
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009; 118(1): 5-36.
- Jones JR, Lebar MD, Jinwal UK, Abisambra JF, Koren III J, Blair L, et al. The diarylheptanoid (+)-a R, 11 S-myricanol and two flavones from bayberry (Myrica cerifera) destabilize the microtubule-associated protein Tau. J Nat Prod. 2011; 74(1): 38-44.
- Choi YJ, Kim TD, Paik SR, Jeong KJ, Jung SH. Molecular simulations for anti-amyloidogenic effect of flavonoid myricetin exerted against Alzheimer’s β-amyloid fibrils formation. Bull Korean Chem Soc. 2008; 29(8): 1505-1509.
- Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Multifunction of myricetin on Aβ: Neuroprotection via a conformational change of A β and reduction of A β via the interference of secretases. J Neurosci Res. 2008; 86(2): 368-377.
- Chang Y, Chang CY, Wang SJ, Huang SK. Myricetin inhibits the release of glutamate in rat cerebrocortical nerve terminals. J Med Food. 2015; 18(5): 516-523.
- Zhu X, Jia YH. Inhibition of Catechol-o-methyltransferase (COMT) by myricetin, dihydromyricetin, and myricitrin. Int J Pharm Pharm Sci. 2014; 69(3): 183-186.
- Laabich A, Manmoto CC, Kuksa V, Leung DW, Vissvesvaran GP, Karliga I, et al. Protective effects of myricetin and related flavonols against A2E and light mediated-cell death in bovine retinal primary cell culture. Exp Eye Res. 2007; 85(1): 154-165.
- Lei Y, Chen J, Zhang W, Fu W, Wu G, Wei H, et al. In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 2012; 135(4): 2702-2707.
- Liu RN, Wang W, Ding Y, Xie WD, Ma C, Du LJ. A new flavonol glycoside and activity of compounds from the flower of Nymphaea candida. J Asian Nat Prod Res. 2007; 9(4): 333-338.
- Mohan M, Jadhav SS, Kasture VS, Kasture SB. Effect of myricetin on behavioral paradigms of anxiety. Pharm Biol. 2009; 47(10): 927-931.
- Moonrungsee N, Shimamura T, Kashiwagi T, Jakmunee J, Higuchi K, Ukeda H. An automated sequential injection spectrophotometric method for evaluation of tyramine oxidase inhibitory activity of some flavonoids. Talanta. 2014; 122: 257-263.
- Ma ZG, Wang J, Jiang H, Liu TW, Xie JX. Myricetin reduces 6-hydroxydopamine-induced dopamine neuron degeneration in rats. Neuroreport. 2007; 18(11): 1181-1185.
- Franco JL, Posser T, Missau F, Pizzolatti MG, Santos AR, Souza DO, et al. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ Toxicol Pharmacol. 2010; 30(3): 272-278.
- Zhang K, Ma Z, Wang J, Xie A, Xie J. Myricetin attenuated MPP+-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23. 5 cells. Neuropharmacology. 2011; 61(1-2): 329-335.
- Zhang XH, Ma ZG, Rowlands DK, Gou YL, Fok KL, Wong HY, et al. Flavonoid myricetin modulates receptor activity through activation of channels and CaMK-II pathway. Evid Based Complement Altern Med. 2012; 2012.
- Yang Y, Choi JK, Jung CH, Koh HJ, Heo P, Shin JY, et al. SNARE-wedging polyphenols as small molecular botox. Planta Med. 2012; 78(03): 233-236.
- Tong Y, Zhou XM, Wang SJ, Yang Y, Cao YL. Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Arch Pharm Res. 2009; 32(4): 527-533.
- Joshi V, Mishra R, Upadhyay A, Amanullah A, Poluri KM, Singh S, et al. Polyphenolic flavonoid (Myricetin) upregulated proteasomal degradation mechanisms: Eliminates neurodegenerative proteins aggregation. J Cell Physiol. 2019; 234(11): 20900-20914.
- Hagenacker T, Hillebrand I, Wissmann A, Büsselberg D, Schäfers M. Anti-allodynic effect of the flavonoid myricetin in a rat model of neuropathic pain: Involvement of p38 and protein kinase C mediated modulation of Ca2+ channels. Eur J Pain. 2010; 14(10): 992-998.
- Ong KC, Khoo HE. Insulinomimetic effects of myricetin on lipogenesis and glucose transport in rat adipocytes but not glucose transporter translocation. Biochem Pharmacol. 1996; 51(4): 423-429.
- Ong KC, Khoo HE. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000; 67(14): 1695-1705.
- Zelus C, Fox A, Calciano A, Faridian BS, Nogaj LA, Moffet DA. Myricetin inhibits Islet Amyloid Polypeptide (IAPP) aggregation and rescues living mammalian cells from IAPP toxicity. Open Biochem J. 2012; 6: 66.
- Karunakaran U, Elumalai S, Moon JS, Jeon JH, Kim ND, Park KG, et al. Myricetin protects against high glucose-induced β-cell apoptosis by attenuating endoplasmic reticulum stress via inactivation of cyclin-dependent kinase 5. Diabetes Metab J. 2019; 43(2): 192-205.
- Li Y, Zheng X, Yi X, Liu C, Kong D, Zhang J, et al. Myricetin: A potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. FASEB J. 2017; 31(6): 2603-2611.
- Tzeng TF, Liou SS, Liu IM. Myricetin ameliorates defective post-receptor insulin signaling via β-endorphin signaling in the skeletal muscles of fructose-fed rats. Evid Based Complement Altern Med. 2011.
- Liu IM, Tzeng TF, Liou SS, Lan TW. Improvement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007; 73(10): 1054-1060.
- Ding Y, Dai XQ, Zhang ZF, Li Y. Myricetin attenuates hyperinsulinemia-induced insulin resistance in skeletal muscle cells. Eur Food Res Technol. 2012; 234(5): 873-881.
- Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids: Structure-activity relations. Biochem Pharmacol. 1984; 33(9): 1525-1530.
- Robak J, Korbut R, Shridi F, Swies J, Rzadkowska-Bodalska H. On the mechanism of antiaggregatory effect of myricetin. Pol J Pharmacol Pharm. 1988; 40(3): 337-340.
- Ozcan F, Ozmen A, Akkaya B, Aliciguzel Y, Aslan M. Beneficial effect of myricetin on renal functions in streptozotocin-induced diabetes. Clin Exp Med. 2012; 12(4): 265-272.
- Kandasamy N, Ashokkumar N. Myricetin modulates streptozotocin-cadmium induced oxidative stress in long term experimental diabetic nephrotoxic rats. J Funct Foods. 2013; 5(3): 1466-1477.
- Gebhardt R. Variable influence of kaempferol and myricetin on in vitro hepatocellular cholesterol biosynthesis. Planta Med. 2003; 69(12): 1071-1074.
- Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM. Myricetin increases hepatic peroxisome proliferator-activated receptor α protein expression and decreases plasma lipids and adiposity in rats. Evid Based Complement Altern Med. 2012.
- Tzeng SH, Ko WC, Ko FN, Teng CM. Inhibition of platelet aggregation by some flavonoids. Thromb Res. 1991; 64(1): 91-100.
- Zang BX, Jin M, Wu W, Chen WM, Piao YZ, Li JR. Antagonistic effect of myricetin on platelet activing factor. Acta Pharm Sin B. 2003; 38(11): 831-833.
- Melzig MF, Henke K. Inhibition of thrombin activity by selected natural products in comparison to neutrophil elastase. Planta Med. 2005; 71(08): 787-789.
- Liu L, Ma H, Yang N, Tang Y, Guo J, Tao W. A series of natural flavonoids as thrombin inhibitors: Structure-activity relationships. Thromb Res. 2010; 126(5): 365-378.
- Borde P, Mohan M, Kasture S. Effect of myricetin on Deoxycorticosterone Acetate (DOCA)-salt-hypertensive rats. Nat Prod Res. 2011; 25(16): 1549-1559.
- Godse S, Mohan M, Kasture V, Kasture S. Effect of myricetin on blood pressure and metabolic alterations in fructose hypertensive rats. Pharm Biol. 2010; 48(5): 494-498.
- Chen SY, Chu CC, Jiang CL, Duh PD. The vasodilating effect and angiotensin converting enzyme inhibition activity of three dietary flavonols: Comparsion between myricetin, quercetin and morin, in vitro. J Food Nutr Res. 2019; 7: 347-354.
- Matić S, Stanić S, Bogojević D, Vidaković M, Grdović N, Dinić S, et al. Methanol extract from the stem of Cotinus coggygria Scop., and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat Res. 2013; 755(2): 81-89.
- Chaabi M, Beghidja N, Benayache S, Lobstein A. Activity-guided isolation of antioxidant principles from Limoniastrum feei (Girard) Batt. Z Naturforsch C. 2008; 63(11-12): 801-807.
- Lee SE, Park YS. Gene expression profiling of human umbilical vein endothelial cells exposed to myricetin. Biochip J. 2013; 7(4): 335-343.
- Tiwari R, Mohan M, Kasture S, Maxia A, Ballero M. Cardioprotective potential of myricetin in isoproterenol‐induced myocardial infarction in Wistar rats. Phytother Res. 2009; 23(10): 1361-1366.
- Gan CL, Liu FZ, Jiang SX, Cao SJ, Dong NW. Effects of five flavonols on [Ca2+]i in cardiomyocytes of rats. Chin J Epidemiol. 2007; 26: 624-626.
- Liwei W, Fengzhi L, Jingkun L, Youchang L, Baofeng Y. Effect of flavonols on caspase-3, Bcl-2 and bax expression in cardiomyocytes apoptosis. Chin Pharm J. 2007; 42: 749-753.
- Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010; 79(10): 1455-1461.
- Huang JH, Huang CC, Fang JY, Yang C, Chan CM, Wu NL, et al. Protective effects of myricetin against ultraviolet-B-induced damage in human keratinocytes. Toxicol Vitro. 2010; 24(1): 21-28.
- Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, Heo YS, et al. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008; 68(14): 6021-6029.
- Kumamoto T, Fujii M, Hou DX. Akt is a direct target for myricetin to inhibit cell transformation. Mol Cell Biochem. 2009; 332(1): 33-41.
- Cai L, Wu CD. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod. 1996; 59(10): 987-990.
- Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019; 18(1): 241-272.
- Salehi B, Sharopov F, Martorell M, Rajkovic J, Ademiluyi AO, Sharifi-Rad M, et al. Phytochemicals in Helicobacter pylori infections: What are we doing now? Int J Mol Sci. 2018; 19(8): 2361.
- Ono K, Nakane H, Fukushima M, Chermann JC, Barré‐Sinoussi F. Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. Eur J Biochem. 1990; 190(3): 469-476.
- Arita-Morioka KI, Yamanaka K, Mizunoe Y, Ogura T, Sugimoto S. Novel strategy for biofilm inhibition by using small molecules targeting molecular chaperone DnaK. Antimicrob Agents Chemother. 2015; 59(1): 633-641.
- Yadav AK, Thakur J, Prakash OM, Khan F, Saikia D, Gupta MM. Screening of flavonoids for antitubercular activity and their structure-activity relationships. Med Chem Res. 2013; 22(6): 2706-2716.
- Wu T, Zang X, He M, Pan S, Xu X. Structure-activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J Agric Food Chem. 2013; 61(34): 8185-8190.
- Jayaraman P, Sakharkar MK, Lim CS, Tang TH, Sakharkar KR. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int J Biol Sci. 2010; 6(6): 556.
- Naz S, Siddiqi R, Ahmad S, Rasool SA, Sayeed SA. Antibacterial activity directed isolation of compounds from Punica granatum. J Food Sci. 2007; 72(9): 341-345.
- Chu SC, Hsieh YS, Lin JY. Inhibitory effects of flavonoids on Moloney murine leukemia virus reverse transcriptase activity. J Nat Prod. 1992; 55(2): 179-183.
- Yu MS, Lee J, Lee JM, Kim Y, Chin YW, Jee JG, et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett. 2012; 22(12): 4049-4054.
- Keum YS, Jeong YJ. Development of chemical inhibitors of the SARS coronavirus: Viral helicase as a potential target. Biochem Pharmacol. 2012; 84(10): 1351-1358.
- Nitulescu G, Nicorescu IM, Olaru OT, Ungurianu A, Mihai DP, Zanfirescu A, et al. Molecular docking and screening studies of new natural sortase A inhibitors. Int J Mol Sci. 2017; 18(10): 2217.
- Alvesalo J, Vuorela H, Tammela P, Leinonen M, Saikku P, Vuorela P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem Pharmacol. 2006; 71(6): 735-741.
- Elshamy AI, Ammar NM, Hassan HA, El-Kashak WA, Al-Rejaie SS, Abd-Elgawad AM, et al. Topical wound healing activity of myricetin isolated from Tecomaria capensis V. aurea. Molecules. 2020; 25(21): 4870.
- Moghadam SE, Ebrahimi SN, Salehi P, Moridi FM, Hamburger M, Jabbarzadeh E. Wound healing potential of chlorogenic acid and Myricetin-3-O-β-Rhamnoside isolated from Parrotia persica. Molecules. 2017; 22(9): 1501.
- Mohan M, Gupta SK, Agnihotri S, Joshi S, Uppal AK. Anticataract effect of topical quercetin and myricein in galactose cataracts. Med Sci Res. 1988; 16: 685.
- Hodges LC, Kearse EC, Green K. Intraocular pressure-lowering activity of phenolic antioxidants in normotensive rabbits. Curr Eye Res. 1999; 19(3): 234-240.
- Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, et al. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol Vis. 2014; 20: 242.
- Jo S, Kim S, Shin DH, Kim MS. Inhibition of African swine fever virus protease by myricetin and myricitrin. J Enzyme Inhib Med Chem. 2020; 35(1): 1045-1049.
- Meotti FC, Fachinetto R, Maffi LC, Missau FC, Pizzolatti MG, Rocha JB, et al. Antinociceptive action of myricitrin: Involvement of the K+ and Ca2+ channels. Eur J Pharmacol. 2007; 567(3): 198-205.
- Shin JC, Jung HY, Harikishore A, Kwon OD, Yoon HS, Kim KT, et al. The flavonoid myricetin reduces nocturnal melatonin levels in the blood through the inhibition of serotonin N-acetyltransferase. Biochem Biophys Res Commun. 2013; 440(2): 312-316.
- Barlas N, Özer S, Karabulut G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol Lett. 2014; 226(1): 35-42.
- Yamaguchi M, Hamamoto R, Uchiyama S, Ishiyama K. Effects of flavonoid on calcium content in femoral tissue culture and parathyroid hormone-stimulated osteoclastogenesis in bone marrow culture in vitro. Mol Cell Biochem. 2007; 303(1): 83-88.
Author Info
Swathi Nalla1* and Ganta Suhasin22Department of Pharmacology, Gitam Institute of Pharmacy, Visakhapatnam, India
Received: 20-Sep-2021 Accepted: 04-Oct-2021 Published: 11-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3