Research Article - (2023) Volume 14, Issue 5
Abstract
Background: COVID-19 infection has become a global pandemic, and there is no specific treatment for it. To fight against the virus, a plethora of medicines are repurposed without clinical data which includes paracetamol to Steroids. During the pandemic many antibiotics, antiviral and steroidal drugs along with multivitamins were prescribed, it is crucial to identify trends of prescribing and determine rational medication use.
Methodology: It’s a retrospective observational study conducted at ESIC Medical College Hospital for a period of 3 months (August-October 2020). The data was collected from the medical records department. Demographic details of the patient, clinical information and information of drug usage was collected. Rationality of prescription assessed by using WHO core drug prescribing indicators. Data analysis was done in descriptive method by using Mean, standard deviation and percentages.
Results: Out of 100 case sheets 83 patients’ data was analyzed among which 64% were males, average age was 45 ± 11.8. Average number of drugs prescribed per encounter was eight. Drug prescribed by generic name was 65%, 78% patients received antibiotics followed by 62% injectable steroids and 42% of injectable anticoagulants, 72% of patients received injections and 71% patients received oral antibiotics. Total 14.8% of prescriptions have fixed dose combinations and the average duration of hospital stay is 8 days.
Conclusion: Due to sudden emergence of COVID-19 and lack of preparedness many drugs were irrationally utilized leading to poly pharmacy to overcome life threatening conditions. Now when evidence is available from multicentre international trails unnecessary use of drug should be avoided.
Keywords
COVID-19, Poly pharmacy, Antibiotics, Anticoagulants
Abbreviations
COVID-19: Corona Virus Disease-2019; WHO: World Health Organization; NSAIDS: Non-steroidal Anti-inflammatory Drugs; HCQ: Hydroxychloroquine; BD: Bis in Die; ECG: Electrocardiogram; SpO2: Saturation of peripheral Oxygen; UFH: Unfractionated Heparin; LMWH: Low Molecular Weight Heparin; ATC/ DDD: Anatomical Therapeutic Clinical Classification/Defined Daily Dose; RTPCR: Reverse Transcription Polymerase Chain Reaction; PDD: Prescribed Daily Dose; PDD/DDD: Prescribed Daily Dose/Defined Daily Dose ratio; EML: Essential Medicine List; mg: milli grams; TU: Thousand Units; CKD: Chronic Kidney Disease; DM: Diabetes Mellitus; HTN: Hypertension; PTB: Pulmonary Tuberculosis, CAD: Coronary Artery Disease
Introduction
Corona virus disease has led to 6,873,477 mortalities globally and 530,789 deaths in India, till March 2023. COVID-19 (Corona Virus Disease 2019) hit the globe by challenging the healthcare system. This newly discovered corona virus, was initially identified in China (Wuhan) in late December 2019 (Tarai A, et al., 2021). Since January 2020 COVID-19 has spread out to too many countries worldwide (Ke R, 2020). In March 11, 2020 WHO declared it as a global pandemic (Cucinotta D and Vanelli M, 2020). The entire world has struggled to handle this medical emergency with the pre-existing drugs. Every healthcare system at international, national level and even individual hospital level developed the protocols to prescribe the drugs based on the severity of the disease and change in the laboratory parameters (Gutiérrez-Abejón E, 2020). This led to prescribe multiple drugs ranging from simple NSAIDS like Paracetamol to Immunomodulators like Tocilizumab (Tuccori M, et al., 2020; Khiali S, 2020). Even though all classes of drugs were used to treat the disease, death tolls increased. After using these drugs patients developed adverse effects and multiple drug interactions. To combat the disease, healthcare system has used drugs irrationally especially the antimicrobials. This polypharmacy increased the economic burden on the healthcare system (Potempski F and Bilimoria K, 2021).
In India the government Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division) has published guidelines for clinical management of COVID-19 protocol in the month of June 2020. According to the guidelines cases were divided into mild, moderate and severe and based on this category, treatment protocol has been given. According to guidelines no specific antivirals have been proven to be effective based on the available data but few drugs like Hydroxychloroquine and Azithromycin were approved for off label use in patients with severe disease and requiring ICU management (Clinical Management Protocol, 2020).
Mild COVID-19 cases may be given symptomatic treatment such as antipyretic (Paracetamol) for fever and pain, adequate nutrition and appropriate rehydration. Tab. Hydroxychloroquine (HCQ) may be considered for any of those having high risk features for severe disease (such as age>60; Hypertension, Diabetes, chronic lung/kidney/liver disease, Cerebrovascular disease and obesity) under strict medical supervision. Moderate cases were managed by administering Tab. Hydroxychloroquine (400 mg) BD on 1st day followed by 200 mg BD for 4 days (after ECG Assessment), intravenous Methylprednisolone 0.5 to 1 mg/kg (or) Dexametha- sone 0.1 to 0.2 mg/kg for 3 days, Prophylactic dose of UFH or LMWH (e.g., Enoxaparin 40 mg per day SC) and for severe cases along with oxygen therapy, Methylprednisolone 1-2 mg/kg/day OR Dexamethasone 0.2-0.4 mg/kg/day for a maximum period of 5-7 days and the larger doses of glucocorticoid will delay the removal of coronavirus due to immunosuppressive effects and Prophylactic dose of UFH or LMWH (e.g., Enoxaparin 40 mg per day SC) should be given for anti-coagulation and the guidelines also mentioned that antibiotics should not be prescribed routinely unless there is clinical suspicion of a bacterial infection (Revised Guidelines on Clinical Management of COVID-19, 2020).
Investigational new drugs like Remdesivir, convalescent plasma and Tocilizumab were considered in case of moderate cases which were progressing to the severe disease (Singh AK, et al., 2020). The main goal of drug utilization study is to promote rational use of drugs. The WHO prescribing indicators and ATC/DDD methodology are standards for drug utilization. With this study it is aimed to analyze the prescribing trends and drug use pattern according to COVID-19 National Management Guidelines.
Materials and Methods
This is a Retrospective, cross sectional study conducted in COVID-19 patients admitted to ESIC Medical college and hospital, Hyderabad for a duration of 3 months during the second wave of COVID-19 pandemic in Telangana (March 2021 to May 2021). Ethical approval was obtained from the Institutional Ethics Committee, ESICMCH (ESICMC/SNR/ IEC-F0253/01-2021). Total of 100 case sheet were collected from medical records department from Aug 2020 to Oct 2020 out of them 83 case sheets were met the inclusion criteria were considered for the study.
Inclusion criteria
1. Patients above 18 years of age, diagnosed as COVID-19 RTPCR positive of both the gender admitted in the hospital.
2. Patients with comorbid conditions: Diabetes, hypertension, cardiac disease, chronic lung disease, cerebro-vascular disease, chronic kidney disease, immune-suppression and cancer.
3. Patient with mild, moderate and severe COVID-19 diseases.
According to National Clinical Management Protocol: COVID-19, Based on the clinical symptoms and oxygen saturation levels cases were defined as mild, moderate and severe.
Mild cases: Patients with symptoms of fever, cough, expectoration, fatigue, myalgia, rhinorrhea, sore throat, diarrhea, malaise, loss of smell or taste without dyspnea.
Moderate cases: Adults with presence of clinical features of dyspnea and or hypoxia, fever, cough, including SpO2<94% (range 90%-94%) on room air, respiratory rate more or equal to 24 per minute.
Severe cases: Severe pneumonia in adults: With clinical signs of Pneumonia plus one of the following; respiratory rate>30 breaths/min, severe respiratory distress, SpO2<90% on room air.
Drug utilization data were assessed using the WHO Anatomical Therapeutic Chemical/Defined Daily Dose (ATC-DDD) methodology and the rationality of drug use was assessed by WHO Core Prescribing Indicators and also by using the National Clinical Management 2020 guidelines given by the Government of India. PDD calculation done by (total dose divided by the number of days) and expressed them as the PDD: DDD ratio (amount of DDD per day and person). Data were entered into Microsoft excel and descriptive statistics like Mean, Percentage and Frequency were calculated. Findings were presented as Graphs and Tables.
Results
According to the clinical symptoms and SpO2, out of 83 patients medical records Mild cases were 62 (74.7%), Moderate were 13 (15.6%) and Severe cases were 8 (9.6%).
Demographic data
The mean age of the patients was 45 ± 11.8 years, females were 36% and males were 64%. Age-wise distributions of cases were given in Table 1.
| Age Group | Females (%) | Males (%) | Total (%) |
|---|---|---|---|
| 20-30 | 4(5%) | 9(11%) | 13(16%) |
| 31-40 | 10(12%) | 17(21%) | 27(33%) |
| 41-50 | 6(7%) | 10(12%) | 16(19%) |
| 51-60 | 9(11%) | 7(8%) | 16(19%) |
| 61-70 | 1(1%) | 6(7%) | 7(8%) |
| 71-80 | 1(1%) | 2(3%) | 3(4%) |
| >80 | - | 1 (1%) | 1(1%) |
Table 1: Age-wise distribution of the patients (n=83)
Among 83, 37.3% of patients had comorbidities. Among these 10, 12% had both hypertension and diabetes (Figure 1).
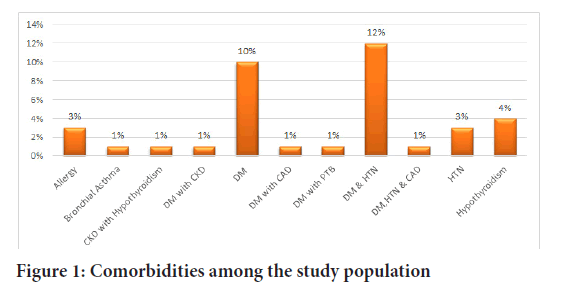
Figure 1: Comorbidities among the study population
All the five parameters of WHO Core Prescribing Indicators and the National guidelines for COVID-19 management were used to assess the rationality of the prescription, of which 67.5% of patients received injections and 77% of patients received antibiotics (Table 2).
| WHO core indicators | Sample size (%) |
|---|---|
| Encounter with injectables | 56(67.5%) |
| Average number of drugs prescribed per encounter | 8 |
| Percentage encounter with antibiotics | 64 (77%) |
| Percentage of drugs with Generic Name | 15(53.5%) |
| Percentage of drugs prescribed according to Revised /National guideline for COVID-19 management | 22% |
Table 2: WHO core prescribing indicators
According to ATC classification twenty-seven drugs were prescribed for the treatment of COVID-19, among this 16 Injectable and 11 Oral drugs were used. Among 16 injectable drugs 7 (46.6%) were antibiotics, the remaining drugs were anticoagulants, steroids, antiviral, proton pump inhibitors and bronchodilators. Among eleven Oral drugs, three were antibiotics and three were corticosteroids, vitamins, and ivermectin.
Antibiotics
The most commonly used injectable antibiotic was Amoxicillin and Clavulanic acid (19.2%) with an average duration of 5.3 days. The second drug was an injection Ceftriaxone (Monocef) (16%) with an average duration of 5 days. The most commonly used oral antibiotics were Doxycycline, Azithromycin, Amoxicillin and Clavulanic acid. Among all injectable and oral antibiotics, the capsule Doxycycline is the most commonly used with 34% prescriptions containing this drug, followed by Azithromycin. Among 83 patients 77% of the patients received at least one antibiotic (Figure 2). ATC classification, DDD, PDD, number of patients received the individual antibiotic and average number of days are mentioned in Tables 3 and 4.
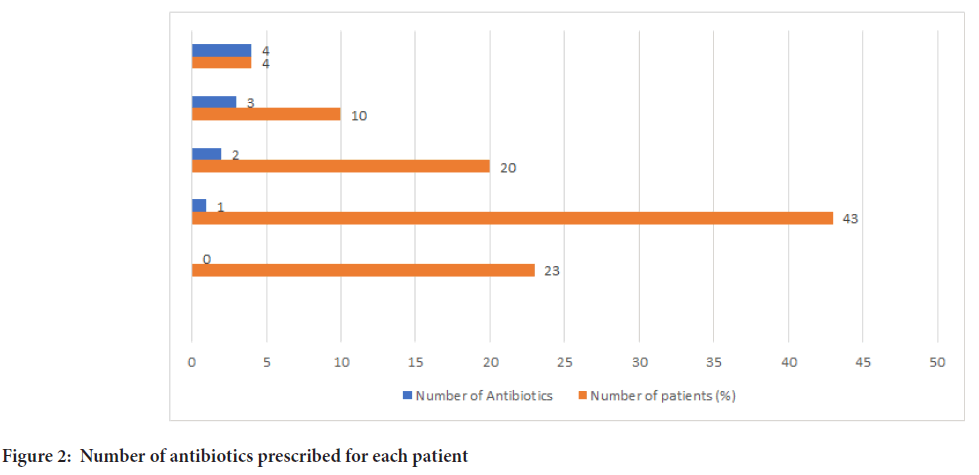
Figure 2: Number of antibiotics prescribed for each patient
| S. no | ATC Code | Drug name and Dose | PDD | DDD | PDD/DDD | Number of patients (%) | Average no. Days |
|---|---|---|---|---|---|---|---|
| 1. | J01AA02 | Inj. Doxycycline 100 mg | 0.2 gm | 0.1 g | 0.1 g | 4 (5%) | 3.25 |
| 2. | J01CA04 | Inj. Amoxicillin and Clavulanic acid (Augmentin)1.2 gm | 2.4 gm | 3 g | 0.6 g | 16 (19%) | 5.3 |
| 3. | J01FA10 | Inj. Cefoperazone and Sulbactam (Magnex forte )1.5 gm | 3 gm | 4 g | 1 g | 1 (1%) | 10 |
| 4. | J01FA10 | Inj. Azithromycin 500 mg | 0.5 gm | 0.5 g | - | 1 (1%) | 6 |
| 5. | J01DD04 | Inj. Ceftriaxone (Monocef )1 gm | 2 g | 2 g | - | 13 (16%) | 5 |
| 6. | J01CR05 | Inj. Piperacillin and Tazobactam (PIPTAZ) 4.5 gm | 13.5 g | 14 g | 0.5 g | 8 (10%) | 5.3 |
| 7. | J01DH02 | Inj. Meropenem 1gm | 3 gm | 3 g | - | 6 (7%) | 6.6 |
| 8. | J05AB16 | Inj. Remdesivir 100mg | 0.1 g | 0.1 g | - | 10(12%) | 5 |
Table 3: Injectable antbiotics and antiviral drugs prescribed for COVID-19 patients
| S. no | ATC Code | Drug name and Dose | PDD | DDD | PDD/DDD | Number of patients (%) | Average no. Days |
|---|---|---|---|---|---|---|---|
| 1. | J01FA10 | T. Azithromycin 500 mg | 0.5 g | 0.3 g | 0.2 g | 22 (26%) | 5.3 |
| 2. | J01AA02 | Cap. Doxycycline 100 mg | 0.2 g | 0.1 g | 0.1 g | 29 (35%) | 5.4 |
| 3. | J01CA04 | T. Amoxicillin and clavulanic acid (Augmentin) 625 mg | 1.3 g | 1.5 g | 0.2 g | 8 (10%) | 5.25 |
Table 4: Oral antibiotics prescribed for COVID-19 patients
Corticosteroids
The most commonly prescribed steroids were Inj. Dexamethasone, Inj. Methylprednisolone and Inj. Hydrocortisone and the majority of the patients (30%) received Dexamethasone in three different doses with an average duration of 6.6 days. 12% of the patients received more than 1 injectable Corticosteroid (Table 5).
| S.no | ATC Code | Drug name | DDD | PDD | PDD/DDD | Number of patients (%) | Average number of days |
|---|---|---|---|---|---|---|---|
| 1 | HO2AB09 | Inj. Hydrocortisone 100 mg | 30 mg | 200 mg | 170 mg | 9 (10%) | 2 |
| 2 | H02AB02 | Inj. Dexamethasone 8 mg | 1.5 mg | 16 mg | 14.5 mg | 11 (13%) | 3.5 |
| Inj. Dexamethasone 6 mg | 1.5 mg | 12 mg | 10.5 mg | 5 (6%) | 4.4 | ||
| Inj. Dexamethasone 4 mg | 1.5 mg | 4 mg | 2.5 mg | 3(4%) | 6 | ||
| Inj. Dexamethasone 4 mg | 1.5 mg | 8 mg | 6.5 mg | 6 (7%) | 6.6 | ||
| T. Dexamethasone | 1.5 mg | 8 mg | 6.5 mg | 4 (5%) | 4 | ||
| 3 | HO2AB04 | Inj. Methylprednisolone 40 Mg | 20 mg | 80 mg | 60 mg | 11 (13%) | 4.4 |
| Inj. Methylprednisolone 40 Mg | 20 mg | 120 mg | 100 mg | 6 (7%) | 7.5 | ||
| 4 | S02BA03 | T. Prednisolone (Omnacortil) | 10 mg | 20 mg | 10 mg | 3 (4%) | 3 |
| T. Methyl Prednisolone (Predmet) |
7.5 mg | 10 mg | 2.5 mg | 1 (1%) | 2 |
Table 5: Corticosteroids prescribed for COVID-19 patients
Anticoagulants
The only anticoagulant prescribed was Enoxaparin, and 42% of the patients received it (Table 6). Other groups of drugs utilized in COVID-19 and their duration of administration are presented in Table 7. Among this the most commonly used were Vitamin C (84%), Multivitamins (73%), and Ivermectin (25%) respectively.
| S.no | ATC Code | Drug name | DDD | PDD | PDD/DDD | Number of patients | Average number of days |
|---|---|---|---|---|---|---|---|
| 1 | B011AB05 | Inj. Enoxaparin (Clexane) 60 mg | 2 TU | 6 TU | 4 TU | 8 (10%) | 7 |
| Inj. Enoxaparin (Clexane) 40 mg | 2 TU | 4 TU | 2 TU | 27 (32%) | 5.25 |
Table 6: Anticoagulant prescribed for COVID-19 patients
| S. no | ATC code | Drug name | DDD | PDD | PDD/DDD | No. of Patients | Average number of Day |
|---|---|---|---|---|---|---|---|
| 1. | A11EA | Multivitamins | - | - | - | 61 (73%) | 8 |
| 2. | A11GA01 | Vitamin C | 0.2 g | 0.5 g | 0.3 g | 70 (84%) | 8 |
| 3. | A12AX | Vitamin D andCalcium | - | - | - | 13 (16%) | 5 |
| 4. | P02CFO1 | Ivermectin | 12 mg | 12 mg | - | 21(25%) | 3 |
| 5. | N02BE01 | Inj. Paracetamol | 3.0 g | 2.0 g | 1.0 g | 4 (5%) | 3 |
| 6. | A02BC02 | Inj. Pantoprazole | 40 mg | 40 mg | - | 25 (30%) | 6.45 |
| 7. | C03CA01 | Inj. Furosemide (Lasix )40 mg | 40 mg | 80 mg | 40 mg | 3 (4%) | 2.6 |
| 8. | A04AA02 | Inj. Ondansetron (Zofer) 4 mg |
16 mg | 8 mg | 8 mg | 4 (5%) | 3.75 |
Table 7: Other drugs prescribed for COVID-19 patients
About 12% of patients received Inj. Remdesivir for 5 days along with injection Enoxaparin and one injectable corticosteroid. The average duration of hospital stay was 8.4 days. About 80% of the patients received more than 5 drugs (Table 8). All the patients were discharged from the hospital.
| Number of Drugs | Number of patients (%) |
|---|---|
| 1-4 | 17(20.5%) |
| 5-10 | 40(48%) |
| >10 | 26 (31.5%) |
Table 8: Number of drugs prescribed for each patient
Discussion
During the first wave of COVID-19 without the evidence of efficacy of drugs, the healthcare system has repurposed all groups of drugs. In this study, the most commonly used drugs for COVID-19 were antibiotics, corticosteroids, anticoagulants, multivitamins, and anti-helminthics. A study was conducted on antimicrobial use in COVID-19 hospitalized patients, according to this study majority of the patients (92%) received antibiotics, third-generation Cephalosporins (60%) were highest followed by Azithromycin (40%) (Tarai A, et al., 2021; Syeda Mah-E-Muneer, et al., 2021). Eduardo study showed that 90% of people received antibiotics (Gutiérrez- Abejón E, et al., 2020). In our study we found that a total of 77% of patients received at least one antibiotic, the most commonly used oral antibiotic is Doxycycline (35%) followed by Azithromycin (26%) and the injectable antibiotic is Amoxicillin and clavulanic acid (19%).
In our study Inj. Dexamethasone was prescribed for 30% of patients and 20% of patients received Inj. Methylprednisolone. All moderate and severe cases received at least one injectable steroid. Awadhesh Kumar Singh have done systemic review on corticosteroids in COVID-19, the principal corticosteroids used in most of the studies were Methylprednisolone and Dexamethasone because of their high bioavailability in the lungs (Singh AK, et al., 2020; El Mezzeoui S, et al., 2021). Recovery trial (2021) found a significantly better outcome with Dexamethasone, mostly in severe cases (Horby P, et al., 2021). About 20% of mild cases also received injectable steroids, which was not indicated by COVID-19 National clinical management.
The only anticoagulant utilized in this study was Enoxaparin and about 42% of patients received the drug. All moderate and severe cases received Enoxaparin. Among 42% of patients, 17% were mild cases that received prophylactically injection Enoxaparin which is not indicated according to National COVID-19 guidelines. Ning Tang, et al., 2020 study concluded that low molecular weight heparin appears to be associated with better prognosis in severe COVID-19 patients. According to a study on Enoxaparin for outpatients with COVID-19; 90-day results from the randomized trial has shown early thromboprophylaxis with enoxaparin, did not improve the course of COVID-19 either in terms of hospitalization or death considering COVID-19-related symptoms (Davide Voci et al., 2023).
Vitamin C was prescribed for about 84% of patients followed by multivitamins which was 73%. The average number of drugs prescribed for each patient was 8 indicating polypharmacy.
According to Revised National Clinical Management Guidelines mild cases should be given symptomatic treatment with antipyretics (Paracetamol) for fever and pain, adequate nutrition and appropriate rehydration. Tab Hydroxychloroquine (HCQ) may be considered for any of those having high-risk features for severe disease (such as age>60; hypertension, diabetes, chronic lung/kidney/liver disease, cerebrovascular disease and obesity) under strict medical supervision, but in our study, Hydroxychloroquine was not prescribed for any patients.
In this study, we have calculated the PDD of each class of drugs and compared it with specific DDD of the drugs as defined by the WHO; PDD/ DDD ratio was calculated. It was found that parenteral and oral Amoxicillin and the Clavulanic acid fixed dose combination were given 20% less than its DDD. Both parenteral and oral Doxycycline was given two times higher; Tablet Azithromycin was given 1.6 times higher than their respective DDD. Dexamethasone was given ten times higher than its DDD. Methylprednisolone was given 4-6 times higher than its DDD. Inj. Enoxaparin was given two to three times higher than its DDD. According to Indian national clinical management guidelines, the doses of Dexamethasone and Methylprednisolone were higher than the WHO DDD and the duration of steroid administration was longer for moderate cases.
Conclusion
In our study, we have concluded that almost all classes of drugs ranging from antipyretics to immuno suppressants were prescribed. Even though it is viral infection antimicrobials were irrationally utilized irrespective of the severity of the disease. Most of the patients received multiple oral and injectable antibiotics which is the leading cause of antimicrobial resistance. To prevent the emergence of antimicrobial resistance, antibiotics have to be cautiously used and multiple antibiotics prescription should be avoided. The doses of steroids prescribed were about ten times higher than the DDD and the duration of administration was longer for moderate cases, and about 17% of mild cases received parenteral steroids and anticoagulation therapy which can lead to immunosuppression, delay in recovery from COVID-19 infection, and increase of burden on the healthcare system.
Limitations
Our study is a single-centre study with a small sample size, so our findings may not be generalizable to other settings. The data were collected from medical records department. Prescription errors cannot be avoided. Further studies need to be done for external validity of the study. Adverse effects of the drugs were not assessed because of lack of documentation.
References
- Tarai A, Beshra S, Pattnaik KP, Misra KH, Giri S. Study of drug utilization pattern of COVID-19 positive cases in isolation ward of PRM medical college and hospital, Baripada, Mayurbhanj, Odisha. Panacea J Med Sci. 2021; 11(3): 491-493.
- Ke R, Sanche S, Romero-Severson E, Hengartner N. Fast spread of COVID-19 in Europe and the US suggests the necessity of early, strong and comprehensive interventions. MedRxiv. 2020: 20050427.
[Crossref] [Google Scholar] [PubMed]
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomedica. 2020; 91(1): 157-160.
[Crossref] [Google Scholar] [PubMed]
- Gutiérrez-Abejón E, Tamayo E, MartÃn-GarcÃa D, Ãlvarez FJ, Herrera-Gómez F. Clinical profile, treatment and predictors during the first COVID-19 wave: A population-based registry analysis from castile and leon hospitals. Int J Environ Res Public Health. 2020; 17(24): 9360.
[Crossref] [Google Scholar] [PubMed]
- Tuccori M, Convertino I, Ferraro S, Cappello E, Valdiserra G, Focosi D, et al. The impact of the COVID-19 "infodemic" on drug-utilization behaviors: Implications for pharmacovigilance. Drug Safety. 2020; 43(8): 699-709.
[Crossref] [Google Scholar] [PubMed]
- Khiali S, Khani E, Entezariâ?Maleki T. A comprehensive review of tocilizumab in covid-19 acute respiratory distress syndrome. J Clin Pharmacol. 2020; 60(9): 1131-1146.
[Crossref] [Google Scholar] [PubMed]
- Potempski F, Bilimoria K. Polypharmacy in the age of COVID-19: Medication management during a pandemic. Univ Tor Med J. 2021; 98(1): 73-75.
- Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division). Clinical management protocol: COVID-19. 2020: 1-22.
- Government of India Ministry of Health and Family Welfare Directorate General of Health Services. Revised guidelines on clinical management of COVID-19. 2020: 1-20.
- Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a clinician's perspective. Diabetes Metab Syndr 2020; 14(5): 971-978.
[Crossref] [Google Scholar] [PubMed]
- Merbouh M, El Kaouini A, Aftiss FZ, Berrajaa S, Bkiyer H, Abda N, et al. Dexamethasone or methylprednisolone therapy in COVID-19 pneumonia: A retrospective and comparative study of 513 cases. Ann Med Surg (Lond). 2021; 70: 102858.
[Crossref] [Google Scholar] [PubMed]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021; 384(8): 693-704.
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020; 18(5): 1094-1099.
[Crossref] [Google Scholar] [PubMed]
- Voci D, Götschi A, Held U, Bingisser R, Colucci G, Duerschmied D, et al. Enoxaparin for outpatients with COVID-19: 90-day results from the randomized, open-label, parallel-group, multinational, phase III OVID trial. Thromb Res. 2023; 221: 157-163.
[Crossref] [Google Scholar] [PubMed]
Author Info
Roja Rani1*, Karuna Sree P2 and Usha Sree TS32Department of Pharmacology, All India Institute of Medical Sciences, Kalyani, West Bengal, India
3Department of Pharmacology, ESIC Medical College and Hospital, Hyderabad, Telangana, India
Received: 25-May-2023 Accepted: 08-Jun-2023 Published: 15-Jun-2023, DOI: 10.31858/0975-8453.14.5.330-335
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






