Research Article - (2024) Volume 15, Issue 1
Abstract
Background and objectives: A 1 cm Distal Resection Margin (DRM) was recommended. Controversy still exists if we can obtain DRM less than 1 cm who had preoperative Chemoradiotherarpy (CRT). Our study aimed to determine the clinical outcomes who had subcentimeter DRM.
Methods: 740 patients with rectal cancer were assessed. 145 patients with rectal cancer underwent curative resection after preoperative CRT were included. The median follow-up time was 28 months.
Results: 36 (24.8%) patients had DRM <1 cm. Local recurrence occurred in 27 patients (18.6%). Overall recurrence was significantly higher in patients with DRM ≤ 0.4 cm than DRM >0.4 cm (50% vs. 16.1%; p=0.015). Moreover, patients who had DRM ≤ 0.4 cm were significantly associated with higher local recurrence than systemic recurrence (p=0.037). Disease Free Survival (DFS) of patients with DRM >0.4 cm was significantly higher than that of patients with DRM ≤ 0.4 cm (p=0.002). In patients with DRM <0.5 cm and >0.5 cm, there were no statistically significant differences in recurrence (p=0.068) and DFS (p=0.107).
Conclusion: The results suggest that cutoff point at 0.5 cm was a minimally acceptable DRM. Minimizing DRM to <1 cm to increase chance of sphincter-saving. Intense adjuvant therapy should be used in these patients to reduce recurrence.
Keywords
Rectal cancer, distal resection margin, surgery, preoperative chemoradiotherapy
Introduction
According to the Global Cancer Observatory (GLOBOCAN) 2018 (Bray F, et al., 2018), Rectal cancer is the 8th most incidence. The mortality rate is 3.2%. Overall, it is apparent that the oncologic resection of rectal cancer depend on Circumferential Resection Margin (CRM) and Distal Resection Margin (DRM). A negative CRM is defined as tumor more than 1 millimeter from the margin (Benson AB, et al., 2021). Total Mesorectal Excision (TME) in rectal cancer surgery reduces local recurrence rate from 14%-40% down to 6.5% (Ahuja V, 2010). The distal rectal margin is defined as the distance from the lowest mesorectal cancer spread from TME and intramural spread to the distal dissection line. Therefore, DRM is important factor for the decision of function outcome if sphincter preserving procedure is feasible. From the literature reviews (Krishnamurty MD and Wise PE, 2016) of the cut point of DRM in rectal cancer patient treated without neoadjuvant therapy, DRM of 1 cm-2 cm was found to be oncologic all sufficient. Therefore, the current guidelines (Benson AB, et al., 2021; Hashiguchi Y, et al., 2020) supported with previous evidence show that 2 cm distal mural margin combined with TME in middle and lower rectal cancer is acceptable (Figure 1).

Figure 1: These pictures illustrate the specimens of middle and lower rectal cancer after TME
In case of rectal cancer patient treated with neoadjuvant therapy, preoperative CCRT induces regression in most rectal cancers (Manegold P, et al., 2019). However rectal cancer regression in response to CCRT follows a scattered approach. Hayden DM, et al., 2012 reported 49.1% of patients with tumor scatter had tumor cells scatter distally from the inferior edge of the visible ulcer. The distance of distal scatter found 0.1 cm to 3 cm. The reason why the optimal distal rectal margin after preoperative CCRT from the literature reviews (Krishnamurty MD and Wise PE, 2016; Manegold P, et al., 2019) of the cut point of DRM in rectal cancer patient treated with neoadjuvant therapy, adequate DRM is still controversial. A 1 cm DRM was recommended as the shortest clearance margin in clinical guidelines. Controversy still exists if we can obtain DRM less than 1 cm in order to achieve goal of sphincter-saving, especially in patients who had preoperative CRT. So, this study aimed to determine the clinical outcomes of rectal cancer patients who had subcentimeter DRM and the cutoff point that may affect the oncological outcomes.
Material and Methods
This retrospective study was approved by the human research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (COA. MURA2021/427). We reviewed the data of locally advanced rectal cancer underwent curative resection after preoperative CCRT from a prospective cancer database institution between 2015 and 2019 in Ramathibodi hospital.
A total of 740 patients with rectal cancer were enrolled. Then, 595 patients were excluded by less than 6 months of follow up (n=96), no radiotherapy (n=190), post-operative RT (n=129), no surgery (n=68), stage 4 (n=69), synchronous lesion (n=15), colon cancer (n=21), benign disease (n=1), watch and wait management after preoperative CCRT (n=1), R2 resection (n=2) and local excision (n=3). Finally, there were 145 patients with locally advanced rectal cancer underwent curative resection after preoperative CCRT for data collection and analysis.
All patients had preoperative clinical assessment and staging, including medical history, digital rectal examination, colonoscopy, sigmoidoscopy, Computerized Tomography (CT) and magnetic resonance imaging. All patients underwent preoperative CCRT. Data of preoperative CCRT were collected including chemosensitization regimen, waiting time to surgery, post CCRT Carcinoembryonic Antigen (CEA) and clinical response. All patients had radical resection of rectal cancer after neoadjuvant treatment. Operation included low anterior resection, ultra-low anterior resection, intersphincteric resection and coloanal anastomosis, abdominoperineal resection, low anterior resection and end colostomy surgery.
Final pathology with DRM, CRM, number of examined Lymph Nodes (LNs), number of positive lymph node, Perineural Invasion (PNI), Lymphovascular Invasion (LVI), tumor deposit, postoperative CEA, postoperative complication, adjuvant chemotherapy, postoperative CEA were collected. The following clinical outcomes were analyzed for recurrence and disease free survival.
Primary outcomes were clinical outcomes of subcentimeter DRM and the cutoff point of DRM. Secondary outcome were other prognosis factors related recurrence.
Deflnition of variables
Measurement of distal rectal margin by the pathologist from lowest edge of tumor or ulcer to distal rectal margin after tissue fixation was carried out. Complete response to CCRT, the lowest edge from scar tissue to the distal resection was measured.
Circumferential Resection Margin (CRM): A clear CRM was defined as the absence of tumor involvement of the margin on microscopic examination of the pathologic specimen.
Preoperative chemoradiotherapy: In general, T3/T4 or nodal metastasis are indications for preoperative chemoradiotherapy at our institution. Preoperative CCRT consisted of a radiation dose of 50.4 to 55.8 Gy in 28 to 33 fractions, administered five times a week. Concurrent chemotherapy consisted of 5-fluorouracil, 1,000 mg/m2/day continuous drip for 5 days per cycle for the first and last weeks of RT, or capecitabine, 850 mg/m2/day for 5 days per week for 5 weeks.
Technique of total mesorectal excision: In case of low and middle rectal cancer, surgical dissection (Figure 2) along embryonic plane comprising avascular areolar tissue between the mesorectal fascia and the fascia of pelvic sidewall while upper rectal cancer, the mesorectum was divided at 5 cm distal to the mucosal edge of tumor. All the surgeons were experienced in colorectal surgery. Intravenous antibiotics were administered 30 minutes before operation and continued for 24-48 hours after surgery. Anastomosis was performed by circular stapling devices or handsewn. According to tumor location and the intraoperative situation, a temporary ileostomy was performed at the surgeon’s preference.
Figure 2: Specimen of middle rectal cancer after preoperative CCRT
Adjuvant therapy: Indications for adjuvant therapy included positive lymph nodes, unfavorable prognostic factors, pT3 or T4, pathological stage 3. Moreover, for patient with a microscopically positive DRM (0 mm<DRM ≤ 1 mm), the decision for adjuvant chemotherapy was made by multidisciplinary team.
Follow up
Patients were followed with history, DRE and CEA 3 month interval within the first 2 years and 6 months interval thereafter. Patients were recommended to CT abdomen and pelvis at 6-month interval and colonoscopy every 1-2 years. If recurrence was suspected, MRI ± PET/CT were performed.
Recurrence
Local recurrence was defined as recurrence in the intrapelvic area, including the anastomotic area and/or regional lymphatics. Systemic recurrence was defined as recurrence beyond the local recurrence, such as in the liver, lung, and/or non-regional lymphatics and other extra pelvic sites. DFS and OS were defined as the interval to the date of the first recurrence and to the date of death from any cause, respectively.
Statistical analysis
Patients with a distal margin of ≤ 0.4 cm (group A) were compared with those with a distal margin >0.4 cm (group B). Categorical variables were summarized as counts and percentages and compared with the use of Chisquare test. Continuous variables compared with the use of two-sample independent T-test. Univariate and multivariate analysis compared with the use of Cox proportional hazards model. Survival and recurrence were calculated by the Kaplan-Meier method. STATA version 14 was used for statistical analysis. The level of significance was set at p<0.05.
Results
Study population
One hundred, 45 patients were included in the study. 93 (64.13%) were male with a mean age at surgery 61.4 years. Preoperative clinical staging included T2 (n=6), T3 (n=120), T4 (n=19) and N stage included N0 (n=47), N1 (n=78), N2 (n=19), and N3 (n=1). The median distance of the tumor from the anal verge on preoperative assessment was 6.7 cm. The median tumor length was 4 cm. Histology confirmed rectal cancer including 30 (20.7%) well differentiated adenocarcinoma, 108 (74.5%) moderately differentiated adenocarcinoma, 5 (3.5%) poorly differentiated adenocarcinoma, 2 (1.4%) dysplasia cell (Table 1).
| Characteristics | n=145 |
|---|---|
| Distal margin (cm), median (IQR) | 2 (1.1 to 3.9) |
| Age; mean ± SD | 61.4 ± 10.9 |
| Gender, n (%) | |
| Male | 93 (64.1) |
| Female | 52 (35.9) |
| Location (cm from AV), median (IQR) | 6.7 (4.8 to 9.0) |
| Preoperative biopsy, n (%) | |
| Well differentiation | 30 (20.7) |
| Moderate differentiation | 108 (74.5) |
| Poor differentiation | 5 (3.5) |
| Fragment of dysplastic cell | 2 (1.4) |
| Tumor length (cm), median (IQR) | 4 (3 to 5.2) |
| Clinical T stage, n (%) | |
| T2 | 6 (4.1) |
| T3 | 120 (82.8) |
| T4 | 19 (13.10) |
| Clinical N stage, n (%) | |
| N0 | 47 (32.4) |
| N1 | 78 (53.8) |
| N2 | 19 (13.1) |
| N3 | 1 (0.7) |
| Concurrent chemotherapy regimen, n (%) | |
| 5 FU/LV | 67 (46.2) |
| Xeloda | 76 (52.4) |
| FOLFOX | 2 (1.4) |
| Waiting time from last RT (days), median (IQR) | 69 (60 to 88) |
| Pre CRT CEA, median (IQR) | 5.6 (2.9 to 13.9) |
| Post CRT CEA, median (IQR) | 3.2 (2.2 to 5.2) |
| Postoperative CEA, median (IQR) | 2.6 (1.7 to 3.9) |
| Clinical response, n (%) | |
| No | 3 (2.1) |
| Partial (by scope) | 119 (82.1) |
| complete Clinical Response (cCR) | 14 (9.7) |
| Not assessed | 9 (6.2) |
| Operation; n (%) | |
| LAR | 68 (46.9) |
| Ultra LAR | 5 (3.5) |
| APR | 22 (15.2) |
| LAR with end colostomy | 18 (12.4) |
| Diversion then LAR | 7 (4.8) |
| Diversion then APR | 3 (2.1) |
| Laparoscopic LAR | 16 (11.0) |
| ISR with CCA | 2 (1.4) |
| Laparoscopic APR | 4 (2.8) |
| Complication, n (%) | |
| Surgical site infection (SSI) | 4 (2.8) |
| Perineal SSI | 3 (2.1) |
| Presacral collection | 8 (5.5) |
| Urine retention | 1 (0.7) |
| Ureteric injury | 1 (0.7) |
| Specimen, n (%) | |
| No residual tumor | 20 (13.8) |
| Well differentiation | 13 (8.9) |
| Moderate differentiation | 102 (70.3) |
| Poorly differentiation | 3 (2.1) |
| Residual tumor (could not classify) | 7 (4.8) |
| T down staging, n (%) | 76 (52.4) |
| ypT staging, n (%) | |
| 0 | 23 (15.9) |
| 1 | 4 (2.8) |
| 2 | 39 (26.9) |
| 3 | 74 (51.0) |
| 4 | 5 (3.5) |
| N down staging; n (%) | 91 (62.8) |
| Positive(number) LN, median (IQR) | 0 (0 to 1) |
| LN all (number), median (IQR) | 13 (8 to 19) |
| LN ratio group, n (%) | |
| 0 | 97 (67.8) |
| 0.1-0.2 | 30 (20.9) |
| 0.2 | 16 (11.2) |
| ALI, n (%) | 33 (23.2) |
| PNI, n (%) | 27 (19.4) |
| CRM, median (IQR) | 1 (0.5 to 2) |
| Tumor deposit, n (%) | 7 (46.7) |
| Timing adjuvant CMT (days), median (IQR) | 43 (32 to 56) |
| Adjuvant chemotherapy regimen, n (%) | |
| FOLFOX | 20 (19.4) |
| XELOX | 31 (30.1) |
| 5FU/LV | 25 (24.3) |
| Xeloda | 27 (26.2) |
| Cycle, median (IQR) | 6 (6 to 8) |
| Follow-up time (months); median (IQR) | 28 (19 to 44) |
Table 1: Characteristics of patients
Preoperative chemoradiotherapy
Preoperative CRT consisted of a median radiation dose of 50.4 Gy and concurrent intravenous fluorouracil in 67 (46.2%) patients or oral capecitabine in 76 (52.4%) patients. 2 (1.38%) patients received a combination of fluoropyrimidine based chemotherapy and oxaliplatin. Median of CEA level before and after CRT were 5.6 and 3.2, respectively.
Surgery
Surgery was performed 69 weeks (range 60 to 88 weeks) after completion of CRT. Operative procedure performed included 68 (46.9%) Low Anterior Resection (LAR), 5 (3.5%) ultraLAR, 22 (15.2%) abdominoperineal resection, 18 (12.4%) LAR with end colostomy, 7 (4.8%) diversion colostomy in case obstruction follow by LAR, 3 (2.1%) diversion colostomy in case obstruction follow by APR, 16 (11.0%) laparoscopic LAR, 4 (2.8%) laparoscopic APR, and 2 (1.4%) intersphincteric resection with coloanal anastomosis. Median of CEA level after operation was 2.6.
Postoperative morbidity
Post-operative complication occurred in 17 (11.72%) out of 145 patients. Surgical Site Infection (SSI), perineal SSI, persacral collection, anastomosis stricture, urinary retention and ureteric injury developed in 4 (2.8%), 3 (2.1%), 8 (5.5%), 1 (0.7%), 1 (0.7%) patients, respectively.
Tumor characteristics
Distal margin of resection ranged from 0 to 11.3 cm, with a median of 2 cm. Thirty-six (24.8%) patients had DRM <1 cm. Circumferential rectal margin range 0.5-2, with median of 1 cm. Complete and partial clinical response were resulted in 14 (9.7%), 119 (82.1%) patients, respectively. No clinical response was found in 3 (2.1%) patients. Final pathology reported 20 (13.8%) no residual tumor or complete pathological response, 13 (8.9%) well differentiated adenocarcinoma, 102 (70.3%) moderately differentiated adenocarcinoma, 3 (2.1%) poorly differentiated adenocarcinoma, 7 (4.8%) unclassified residual tumor. There were 33 (23.2%) Angiolymphatic In-vasion (ALI), 27 (19.4%) Perineural Invasion (PNI) and 7 (46.7%) tumor deposit. Pathological staging included T0 (n=23), T1 (n=4), T2 (n=39), T3 (n=74) and T4 (n=3.5). Pathological T and N down staging were found in 76 (52.4%) and 91 (62.8%). Median of total lymph nodes were 13, ranged 8 to 19.
Adjuvant chemotherapy
Patients were received adjuvant chemotherapy on median 43 days after surgery with 20 (19.4%) FOLFOX, 31 (30.1%) XELOX, 25 (24.3%) 5FU/ LV and 27 (26.2%) capecitabine. A median cycles of adjuvant chemotherapy were 6 (range 6 to 8). There were 8 patients in group A and 137 patients in group B. Comparison of patient characteristics between the 2 groups is shown in Table 2.
| Variables | ≤ 0.4 cm (n=8) | >0.4 cm (n=137) | p-value |
|---|---|---|---|
| Distal margin (cm), median (IQR) | 0.2 (0.0-0.3) | 2.2 (1.5-4.0) | <0.001 |
| Age, mean ± SD | 55 (43-58.5) | 62 (54-69) | 0.006 |
| Gender, n (%) | |||
| Male | 5 (62.5) | 88 (64.2) | 0.921 |
| Female | 3 (37.5) | 49 (35.8) | |
| Location (cm from AV), median (IQR) | 3.7 (2.3-4.1) | 7.0 (5.0-9.0) | 0.0005 |
| Preoperative biopsy, n (%) | |||
| Well differentiation | 3 (37.5) | 27 (19.7) | 0.631 |
| Moderate differentiation | 5 (62.5) | 103 (75.2) | |
| Poorly differentiation | 0 (0) | 5 (3.7) | |
| Fragment of dysplastic cell | 0 (0) | 2 (1.5) | |
| Tumor length (cm), median (IQR) | 4.1 (3.1-5.4) | 4.0 (3.0-5.2) | 0.758 |
| Clinical T stage, n (%) | |||
| T2 | 0 (0) | 6 (4.4) | 0.934 |
| T3 | 7 (87.5) | 113 (82.5) | |
| T4 | 1 (12.5) | 18 (13.13) | |
| Clinical N stage, n (%) | |||
| N0 | 0 (0) | 47 (34.3) | 0.219 |
| N1 | 6 (75) | 72 (52.6) | |
| N2 | 2 (25) | 17 (12.4) | |
| Concurrent chemotherapy regimen, n (%) | |||
| 5 FU/LV | 2 (25) | 65 (47.5) | 0.014 |
| Xeloda | 5 (62.5) | 71 (51.8) | |
| FOLFOX | 1 (12.5) | 1 (0.7) | |
| Waiting time from RT (days), median (IQR) | 70.5 (61.5-78.5) | 69.0 (60.0-89.0) | 0.883 |
| Pre CRT CEA, median (IQR) | 2.5 (1.7-4.6) | 5.8 (3.1-14.1) | 0.071 |
| Post CRT CEA, median (IQR) | 2.0 (1.5-5.9) | 3.2 (2.2-5.2) | 0.272 |
| Post-operative CEA, median (IQR) | 2.0 (1.5-3.5) | 2.6 (1.7-3.9) | 0.547 |
| Clinical response, n (%) | |||
| No | 1 (12.5) | 2 (1.5) | 0.059 |
| Partial (by scope) | 5 (62.5) | 114 (83.2) | |
| cCR (complete Clinical Response) | 2 (25.0) | 12 (8.8) | |
| Not assessed | 0 (0) | 9 (6.6) | |
| Operation, n (%) | |||
| LAR | 3 (37.5) | 65 (47.45) | 0.192 |
| Ultra LAR | 1 (12.5) | 4 (2.9) | |
| APR | 1 (12.5) | 21 (15.3) | |
| LAR with end colostomy | 0 (0) | 18 (13.1) | |
| Diversion then LAR | 0 (0) | 7 (5.1) | |
| Diversion then APR | 1 (12.5) | 2 (1.5) | |
| Laparoscopic LAR | 1 (12.5) | 15 (10.9) | |
| ISR with CCA | 0 (0) | 2 (1.5) | |
| Laparoscopic APR | 1 (12.5) | 3 (2.2) | |
| Complication, n (%) | |||
| Surgical site infection (SSI) | 0 (0) | 4 (2.9) | 0.624 |
| Perineal SSI | 0 (0) | 3 (2.2) | 0.672 |
| Presacral collection | 0 (0) | 8 (5.8) | 0.482 |
| Urine retention | 0 (0) | 1 (0.7) | 0.808 |
| Ureteric injury | 0 (0) | 1 (0.7) | 0.808 |
| Specimen, n (%) | |||
| no residual tumor | 2 (25) | 18 (13.1) | 0.71 |
| Well differentiation | 0 (0) | 13 (9.5) | |
| Moderate differentiation | 6 (75) | 96 (70.1) | |
| Poorly differentiation | 0 (0) | 3 (2.2) | |
| Residual tumor (could not classify) | 0 (0) | 7 (5.1) | |
| T down staging, n (%) | 5 (62.5) | 71 (51.8) | 0.557 |
| ypT staging, n (%) | |||
| 0 | 2 (25) | 21 (15.3) | 0.384 |
| 1 | 0 (0) | 4 (2.9) | |
| 2 | 3 (37.5) | 36 (26.3) | |
| 3 | 2 (25) | 72 (52.6) | |
| 4 | 1 (12.5) | 4 (2.9) | |
| N down staging, n (%) | 5 (62.5) | 86 (62.8) | 0.988 |
| Pos LN, median (IQR) | 0 (0, 2) | 0 (0, 1) | 0.885 |
| LN all (number), median (IQR) | 18.5 (15, 27) | 13 (8, 18.5) | 0.022 |
| LN ratio group, n (%) | |||
| 0 | 6 (75) | 91 (67.4) | 0.193 |
| 0.1-0.2 | 0 (0) | 30 (22.2) | |
| 0.2 | 2 (25) | 14 (10.4) | |
| ALI, n (%) | 1 (12.5) | 32 (23.9) | 0.459 |
| PNI, n (%) | 3 (37.5) | 24 (18.3) | 0.183 |
| CRM, median (IQR) | 1.3 (0.5, 2) | 1 (0.5, 2) | 0.962 |
| Adjuvant chemotherapy regimen, n (%) | |||
| FOLFOX | 1 (16.7) | 19 (19.6) | 0.586 |
| XELOX | 1 (16.7) | 30 (30.9) | |
| 5FU/LV | 1 (16.7) | 24 (24.7) | |
| Xeloda | 3 (50) | 24 (24.7) | |
| Cycles, median (IQR) | 7 (6, 8) | 6 (6, 8) | 0.261 |
| Tumor deposit, n (%) | 1 (20) | 6 (60) | 0.143 |
Table 2: Characteristics of patients in different subgroups
Clinical and pathologic characteristics
Characteristics of each group are described in Table 2. The location of tumor and age were significantly higher in group B than group A (7 cm vs.3.7 cm, p=0.0005, 62 years vs.55 years, p=0.006, respectively). Other characteristics including gender, preoperative histology, tumor length and clinical stage were no significant difference between 2 groups. According to CCRT regimen, xeloda regimen was significantly higher than 5FU/LV regimen in group A (p=0.014). Most of patients in 2 groups had partial clinical response. Other characteristics including waiting time from last RT to surgery, CEA level before and after CCRT and postoperative CEA were no significant difference between 2 groups. The kind of procedure included non-diversion, diversion first and laparoscopic surgery. There was no significant difference between 2 groups (p=0.192). Pathological characteristics between 2 groups, overall LN was significantly higher in group A than group B (18.5 vs.13, p=0.022). Other characteristics including final histology, pathological staging, angiolymphatic invasion, perineural invasion, tumor deposit, circumferential rectal margin were no significant difference between 2 groups. Post-operative complication, there was no significant difference between 2 groups. There was no significant difference for adjuvant chemotherapy regimen between 2 groups. Most patients received a combination of fluoropyrimidine based chemotherapy and oxaliplatin.
Local recurrence rate and disease free survival
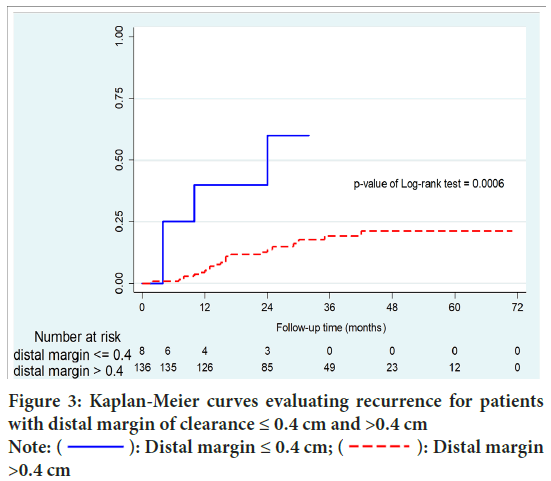
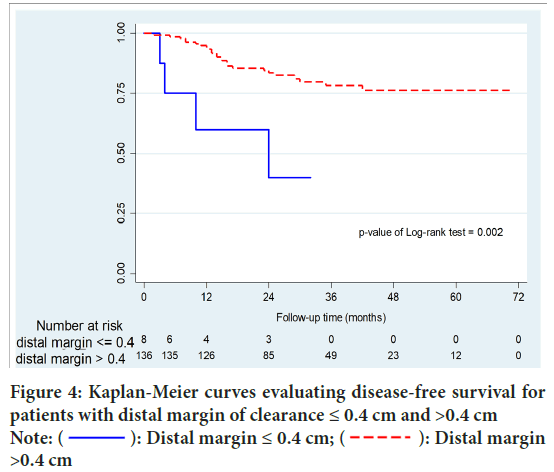
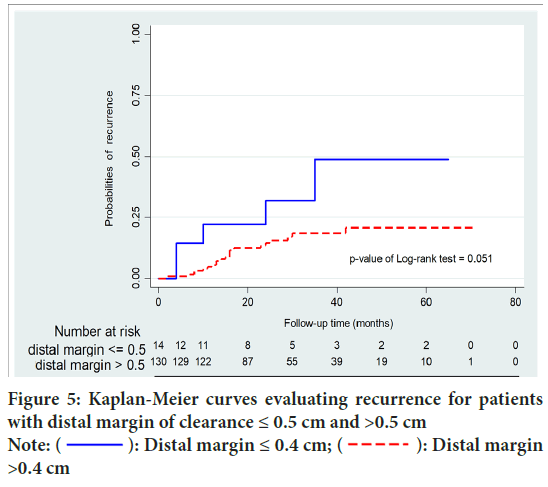
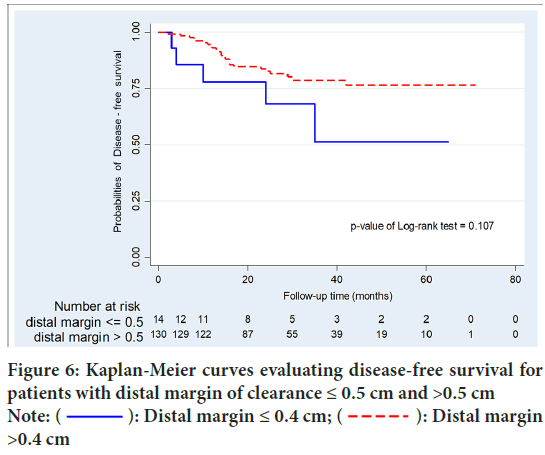
After a median length of follow-up of 28 months (range 19-44), Local Recurrence (LR) occurred in 26 patients (17.9%). Local recurrence occurred in 27 patients (18.6%). Overall recurrence was significantly higher in group A than group B (50% vs.16.1%; p=0.015). Moreover, group A were significantly associated with higher local recurrence than systemic recurrence (p=0.037). Disease Free Survival (DFS) of group B was significantly higher than that of patients with group A (p=0.002). In patients with DRM <0.5 cm and >0.5 cm, there were no statistically significant differences in recurrence (p=0.068) and DFS (p=0.107). No significant difference of survival between local and systemic recurrence group (p=0.146) (Figures 3-6 and Table 3).
| Variables | ≤ 0.4 cm (n=8) | >0.4 cm (n=137) | p-value |
|---|---|---|---|
| Recurrence, n (%) | 4 (50) | 22 (16.1) | 0.015 |
| Type of recurrence, n (%) | |||
| Local recurrence | 3 (75) | 5 (22.7) | 0.037 |
| Systemic recurrence | 1 (25) | 17 (77.3) | |
| Recurrence and death, n (%) | 4 (50) | 26 (19) | 0.035 |
| Death, n(%) | 1 (12.5) | 16 (11.7) | 0.944 |
Table 3: Recurrence and death between margin ≤ 0.4 cm and >0.4 cm
Figure 3: Kaplan-Meier curves evaluating recurrence for patients
with distal margin of clearance ≤ 0.4 cm and >0.4 cm Note:  : Distal margin ≤ 0.4 cm;
: Distal margin ≤ 0.4 cm;  : Distal margin
>0.4 cm
: Distal margin
>0.4 cm
Figure 4: Kaplan-Meier curves evaluating disease-free survival for
patients with distal margin of clearance ≤ 0.4 cm and >0.4 cm Note:  : Distal margin ≤ 0.4 cm;
: Distal margin ≤ 0.4 cm;  : Distal margin
>0.4 cm
: Distal margin
>0.4 cm
Figure 5: Kaplan-Meier curves evaluating recurrence for patients with distal margin of clearance ≤ 0.5 cm and >0.5 cm Note:  : Distal margin ≤ 0.4 cm;
: Distal margin ≤ 0.4 cm;  : Distal margin
>0.4 cm
: Distal margin
>0.4 cm
Figure 6: Kaplan-Meier curves evaluating disease-free survival for
patients with distal margin of clearance ≤ 0.5 cm and >0.5 cm Note:  : Distal margin ≤ 0.4 cm;
: Distal margin ≤ 0.4 cm;  : Distal margin
>0.4 cm
: Distal margin
>0.4 cm
A univariable analysis (Table 4) of unfavorable factors associated with LR and DFS including N staging, waiting time from last RT to surgery, postoperative CEA, post-operative complication (surgical site infection, presacral collection), ypT staging, Lymph Node (LN) ratio (positive LN/ total LN), perineural invasion. A multivariable analysis (Table 5) of unfavorable factors associated with LR and DFS including positive lymph node.
| Variables | Recurrence | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Distal margin >0.4 cm | 0.19 (0.06-0.55) | 0.002 | 0.22 (0.08-0.64) | 0.005 |
| Age | 1.00 (0.97-1.05) | 0.804 | 0.99 (0.96-1.03) | 0.61 |
| Gender | 0.631 | 0.814 | ||
| Male | Baseline | - | Baseline | - |
| Female | 0.82 (0.35-1.88) | 0.91 (0.43-1.95) | ||
| Location (cm from AV) | 0.97 (0.85-1.10) | 0.63 | 0.97 (0.86-1.09) | 0.638 |
| Preoperative biopsy | 0.57 (0.27-1.21) | 0.144 | 0.65 (0.33-1.31) | 0.229 |
| Tumor length (cm) | 0.97 (0.80-1.17) | 0.724 | 0.98 (0.83-1.16) | 0.829 |
| Clinical T stage | 0.77 (0.30-1.98) | 0.581 | 0.74 (0.31-1.80) | 0.512 |
| Clinical N stage | 2.03 (1.14-3.60) | 0.015 | 1.84 (1.08-3.13) | 0.025 |
| Concurrent chemotherapy regimen, n (%) | ||||
| 5 FU/LV | Baseline | - | Baseline | - |
| Xeloda | 0.76 (0.34-1.69) | 0.504 | 0.78 (0.38-1.64) | 0.517 |
| XELOX | - | - | - | - |
| FOLFOX | 3.27 (0.41-25.85) | 0.261 | 3.34 (0.43-25.67) | 0.247 |
| Waiting time from last RT (days) | 1.01 (0.99-1.01) | 0.076 | 1.01 (1.00-1.01) | 0.03 |
| Pre CRT CEA | 0.99 (0.99-1.01) | 0.857 | 0.99 (0.99-1.00) | 0.789 |
| Post CRT CEA | 1.02 (0.99-1.04) | 0.197 | 1.02 (1.00-1.04) | 0.043 |
| Post-operative CEA | 1.02 (1.01-1.02) | <0.001 | 1.02 (1.01-1.02) | <0.001 |
| Clinical response | ||||
| No | Baseline | - | Baseline | - |
| Partial (by scope) | 0.14 (0.03-0.67) | 0.013 | 0.09 (0.03-0.33) | <0.001 |
| cCR (complete Clinical Response | 0.04 (0.003-0.42) | 0.008 | 0.02 (0.002-0.22) | 0.001 |
| Not assessed | 0.08 (0.007-1.00) | 0.05 | 0.10 (0.02-0.65) | 0.016 |
| Operation | ||||
| LAR | Baseline | - | Baseline | - |
| Ultra LAR | 16.01 (4.18-61.35) | <0.001 | 14.30 (4.43-46.14) | <0.001 |
| APR | 2.78 (0.80-9.65) | 0.106 | 2.42 (0.81-7.23) | 0.112 |
| LAR with end colostomy | 1.46 (0.28-7.62) | 0.654 | 1.04 (0.21-5.04) | 0.965 |
| Diversion then LAR | 1.87 (0.22-16.16) | 0.569 | 1.32 (0.16-10.82) | 0.795 |
| Diversion then APR | 40.65 (9.06-182.42) | <0.001 | 30.04 (7.41-121.73) | <0.001 |
| Laparoscopic LAR | 3.96 (1.05-14.91) | 0.042 | 2.77 (0.81-9.56) | 0.106 |
| Laparoscopic APR | 10.86 (2.08-56.75) | 0.005 | 7.74 (1.59-37.63) | 0.011 |
| Complication | ||||
| Surgical Site Infection (SSI) | 4.06 (0.94-17.43) | 0.06 | 3.30 (0.78-14.02) | 0.105 |
| Presacral collection | 3.89 (1.14-13.24) | 0.03 | 3.16 (0.94-10.57) | 0.062 |
| Specimen | 1.08 (0.71-1.65) | 0.706 | 1.02 (0.69-1.52) | 0.903 |
| T down staging | 0.39 (0.16-0.93) | 0.035 | 0.38 (0.17-0.85) | 0.018 |
| ypT stage | 1.63 (1.02-2.60) | 0.041 | 1.58 (1.03-2.44) | 0.038 |
| N down staging | 0.61 (0.28-1.32) | 0.211 | 0.53 (0.26-1.10) | 0.088 |
| Positive LN | 1.44 (1.23-1.68) | <0.001 | 1.38 (1.18-1.60) | <0.001 |
| LN all | 1.00 (0.96-1.05) | 0.857 | 1.01 (0.96-1.05) | 0.794 |
| LN ratio group | ||||
| 0 | Baseline | - | Baseline | - |
| 0.1-0.2 | 3.29 (1.24-8.69) | 0.016 | 2.42 (0.97-6.03) | 0.057 |
| >0.2 | 6.66 (2.47-17.95) | <0.001 | 5.66 (2.30-13.95) | <0.001 |
| ALI | 2.05 (0.88-4.74) | 0.095 | 1.90 (0.86-4.17) | 0.11 |
| PNI | 4.38 (2.00-9.58) | <0.001 | 4.48 (2.17-9.27) | <0.001 |
| CRM | ||||
| <0.1 | Baseline | - | Baseline | - |
| ≥ 0.1 | 0.18 (0.04-0.82) | 0.026 | 0.23 (0.05-0.99) | 0.049 |
| Waiting time adjuvant CMT (days) | 1.00 (0.99-1.01) | 0.778 | 1.00 (0.99-1.01) | 0.631 |
| Adjuvant chemotherapy regimen | ||||
| FOLFOX | Baseline | - | Baseline | - |
| XELOX | 0.13 (0.03-0.58) | 0.008 | 0.18 (0.05-0.66) | 0.01 |
| 5FU/LV | 0.26 (0.08-0.84) | 0.024 | 0.25 (0.08-0.80) | 0.02 |
| Xeloda | 0.04 (0.004-0.35) | 0.004 | 0.03 (0.004-0.32) | 0.003 |
| Cycles | 0.73 (0.53-0.99) | 0.046 | 0.68 (0.51-0.91) | 0.01 |
| Tumor deposit | 1.30 (0.17-10.12) | 0.8 | 1.75 (0.28-11.04) | 0.552 |
| FOLFOX | Baseline | - | Baseline | - |
| XELOX | 0.13 (0.03-0.58) | 0.008 | 0.18 (0.05-0.66) | 0.01 |
| 5FU/LV | 0.26 (0.08-0.84) | 0.024 | 0.25 (0.08-0.80) | 0.02 |
| Xeloda | 0.04 (0.004-0.35) | 0.004 | 0.03 (0.004-0.32) | 0.003 |
| Cycles | 0.73 (0.53-0.99) | 0.046 | 0.68 (0.51-0.91) | 0.01 |
| Tumor deposit | 1.30 (0.17-10.12) | 0.8 | 1.75 (0.28-11.04) | 0.552 |
Table 4: Factors associated with recurrence and DFS after operations between distal margin ≤ 0.4 with >0.4 cm
| Variables | Recurrence | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Distal margin >0.4 cm | 0.02 (0.001-0.63) | 0.026 | 0.08 (0.003-2.36) | 0.144 |
| Operation | ||||
| LAR | Baseline | - | Baseline | - |
| Ultra LAR | 143.98 (0.86-24206.39) | 0.057 | 87.18 (0.93-8191.73) | 0.054 |
| APR | 14.01 (0.85-231.43) | 0.065 | 11.33 (0.87-148.28) | 0.064 |
| LAR with end colostomy | 0.79 (0.008-77.73) | 0.92 | 0.58 (0.007-49.15) | 0.811 |
| Diversion then LAR | 3.97 (0.16-97.19) | 0.398 | 2.08 (0.13-34.36) | 0.608 |
| Diversion then APR | 2573.25 (9.54-694227.70) | 0.006 | 679.08 (6.12-75310.44) | 0.007 |
| Laparoscopic LAR | 2.93 (0.12-69.34) | 0.505 | 0.52 (0.03-9.25) | 0.658 |
| Laparoscopic APR | 49.08 (1.27-1896.19) | 0.037 | 5.91 (0.34-103.73) | 0.224 |
| T down staging | - | - | 1.47 (0.45- 4.81) | 0.526 |
| ypT stage | - | - | 1.47 (0.45-4.81) | 0.526 |
| Pos LN | 6.63 (2.13-20.58) | 0.001 | 3.97 (1.57-10.06) | 0.004 |
| LN ratio group | ||||
| 0 | Baseline | - | Baseline | - |
| 0.1-0.2 | 0.14 (0.006-3.58) | 0.237 | 0.28 (0.02-3.70) | 0.334 |
| >0.2 | - | - | 0.02 (0.00-2.32) | 0.103 |
| PNI | 3.73 (0.30-45.77) | 0.304 | 1.28 (0.16-10.45) | 0.817 |
| CRM | ||||
| <0.1 | Baseline | - | Baseline | - |
| ≥ 0.1 | 0.96 (0.02-60.44) | 0.985 | 6.18 (0.20-191.17) | 0.299 |
| Adjuvant chemotherapy regimen | ||||
| FOLFOX | Baseline | - | Baseline | - |
| XELOX | 0.02 (0.001-0.56) | 0.021 | 0.09 (0.01-1.28) | 0.076 |
| 5FU/LV | 1.79 (0.06-49.13) | 0.732 | 0.44 (0.03-7.03) | 0.56 |
| Xeloda | 0.50 (0.004-59.65) | 0.775 | 0.48 (0.01-18.29) | 0.691 |
| Cycles | - | - | 0.38 (0.20-0.73) | 0.004 |
Table 5: Multivariable analysis of factors associated with recurrence and DFS after operation between distal margin ≤ 0.4 with >0.4 cm
A univariable analysis (Table 4) of favorable factors associated with LR and DFS including DRM >0.4 cm, clinical response (partial or complete clinical response), CRM ≥ 0.1 cm. A multivariable analysis (Table 5) of favorable factors associated with LR and DFS including DRM >0.4 cm, adjuvant XELOX regimen.
Discussion
Preoperative CRT may increase the rate of sphincter saving rectal cancer surgery due to shrinkage of the primary tumor. However, rectal cancer shows scattered regression after preoperative CRT (Smith FM, et al., 2014; Kim TG, et al., 2010). Hayden DM, et al., 2012 demonstrated distal intramural spread of the primary cancer was not observed beyond 3 cm in 49.1% of patients. Several studies have evaluated oncologic outcomes after curative resection for patients who had preoperative CRT with a distal margin shorter than 1 cm. Kim TG, et al., 2010 found 66.7% 5 year pelvic control rate in group of patients with distal margin less than 0.3 cm compared with 86.2% 5 year pelvic control rate in group of patients with distal margin more than 0.3 cm, (p=0.049). Rutkowski A, et al., 2012 evaluated the cut off 5 mm DRM in 412 rectal cancer patients. In this study, 63% of patients received preoperative radiotherapy. The risk for local cancer recurrence was only slightly increased with a DRM of ≤ 5 mm (5.4%) compared to a DRM of >5 mm (4.1%). Nash GM, et al., 2010 showed no significant difference of local recurrence at cut point 0.8 cm, (6% vs.4%). Some studies 13-16 found no difference in LR for patients who underwent curative resection after preoperative CRT with a distal margin less or greater than 1 cm. However, these studies had small numbers of patients or other associated factors of recurrence. Kuvshinoff B, et al., 2001 studied 28 patients with rectal cancer within 8 cm from the anal verge who received a sphincter preserving procedure after preoperative CRT, no significant difference at cut off 1 cm distal margin (Moore HG, et al., 2003). Rutkowski A, et al., 2008 included 94 rectal cancer patients post curative resection after preoperative CRT. Similarly, no significant difference at cut off 1 cm was seen (Leo E, et al., 2009).
Whereas, Kiran RP, et al., 2011 analyzed 784 patients with rectal cancer receiving preoperative CRT in 40% of patients. They found 5 year LR rate of 4.4% for patients with DRM ≤ 1 cm compared to 4.3% for a DRM >1 cm. A DRM ≤ 5 mm was associated with a 5-year LR of 6.4% compared to 4.1% for a DRM >5 mm. Thus a DRM of <1 cm might not compromise oncological outcome. Moreover, Zeng WG, et al., 2017 analyzed 6,574 patients with rectal cancer 20% of patients received preoperative radiotherapy. This study found 24.1% LR in a group of patients with a distal margin less than 1 cm compared with 12% in patients with a distal margin >1 cm. In the current study suggest that a 1 cm distal margin is adequate in most patients who had preoperative CRT. Controversy still exists if we can obtain DRM less than 1 cm in order to achieve goal of sphincter-saving, especially in patients who had preoperative Chemoradiotherarpy (CRT).
In this study, there were 145 patients who were analyzed with median time follow up 28 months. DRM ranged from 0 cm-11.3 cm (median 2 cm). There were 36 patients (24.8%) who had DRM less than 1 cm. Recurrence was found in 27 (18.6%) patients. Local and systemic recurrence was found in 9 and 24 patients, respectively. In the local recurrence group, two patients had lymph node recurrence and other patients had anastomosis recurrence. There was statistically significant differences in recurrence and DFS at cut off 0.4 cm, p=0.0006 and 0.002, respectively. Moreover, no statistically significant differences in recurrence and DFS were found at cut off 0.5 cm, p=0.051 and 0.107, respectively. Similarly, no significant difference in recurrence and DFS at cut off 0.6 cm were seen, p=0.061 and 0.107, respectively. On univariable and multivariable analysis of others factors, unfavorable recurrence and DFS were associated with N staging, waiting time from last RT to surgery, postoperative CEA, post-operative complication (surgical site infection, presacral collection), ypT staging, LN ratio (positive LN/total LN), perineural invasion and positive lymph node. In addition, favorable factors were associated with DRM >0.4 cm, clinical response (partial or complete clinical response), CRM ≥ 0.1 cm. and adjuvant XELOX regimen. It is important to consider these factors that who was good candidates for subcentimeter DRM.
Our study is a retrospective review conducted at a single institution; thus, it has some limitations. First, the study is statistically underpowered for a valid statistical analysis with small sample size in subcentimeter group. Second, measuring of DRM after tissue fixation may retract the specimen. Third, including R1 resection (DRM 3 patients, CRM 4 patients) might affect oncologic outcome. Finally, our study lacked pathological assessment other prognosis factors including extramural vascular invasion and tumor deposit in some patients. Therefore, we were not able to analyse these factors. Further study should be considered in rectal cancer patients who received total neoadjuant therapy with benefit of higher pCR which improve prognosis.
Conclusion
In conclusion, the results suggest that cutoff point at 0.5 cm was a minimally acceptable DRM in rectal cancer after preoperative CCRT. Minimizing DRM to less than 1 cm to increase chance of sphincter-saving procedures should be balanced with individual patient and tumor characteristics including tumor staging, response to preoperative CRT and tumor differentiation. Intense adjuvant therapy should be used in these patients to reduce recurrence.
Ethical Approval
The study was approved by Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University COA, MURA2021/427.
Acknowledgement
We would like to acknowledge the surgical research unit, faculty of medicine Ramathibodi Hospital, Mahidol University, Thailand
Author Contribution
Miss Yada Phengsalae Yada collected and analyzed the data.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394-424.
[Crossref] [Google Scholar] [Pubmed]
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021; 19(3): 329-359.
[Crossref] [Google Scholar] [Pubmed]
- Ahuja V. 14 in colorectal cancer. Early diagnosis and treatment of cancer series: Colorectal cancer e-book: Expert consult. 2010.
- Krishnamurty MD, Wise PE. Importance of surgical margins in rectal cancer. J Surg Oncol. 2016; 113(3): 323-332.
[Crossref] [Google Scholar] [Pubmed]
- Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020; 25: 1-42.
[Crossref] [Google Scholar] [Pubmed]
- Manegold P, Taukert J, Neeff H, Fichtner-Feigl S, Thomusch O. The minimum distal resection margin in rectal cancer surgery and its impact on local recurrence-a retrospective cohort analysis. Int J Surg. 2019; 69: 77-83.
[Crossref] [Google Scholar] [Pubmed]
- Hayden DM, Jakate S, Pinzon MC, Giusto D, Francescatti AB, Brand MI, et al. Tumor scatter after neoadjuvant therapy for rectal cancer: Are we dealing with an invisible margin? Dis Colon Rectum. 2012; 55(12): 1206-1212.
[Crossref] [Google Scholar] [Pubmed]
- Smith FM, Wiland H, Mace A, Pai RK, Kalady MF. Depth and lateral spread of microscopic residual rectal cancer after neoadjuvant chemoradiation: Implications for treatment decisions. Colorectal Dis. 2014; 16(8): 610-615.
[Crossref] [Google Scholar] [Pubmed]
- Kim TG, Park W, Choi DH, Kim SH, Kim HC, Lee WY, et al. The adequacy of the distal resection margin after preoperative chemoradiotherapy for rectal cancer. J Gastrointest Surg. 2010; 14(8): 1331-1337.
[Crossref] [Google Scholar] [Pubmed]
- Rutkowski A, Nowacki MP, Chwalinski M, Oledzki J, Bednarczyk M, Liszka‐Dalecki P, et al. Acceptance of a 5‐mm distal bowel resection margin for rectal cancer: Is it safe? Colorectal Dis. 2012; 14(1): 71-78.
[Crossref] [Google Scholar] [Pubmed]
- Nash GM, Weiss A, Dasgupta R, Gonen M, Guillem JG, Wong WD. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon Rectum. 2010; 53(10): 1365-1373.
[Crossref] [Google Scholar] [Pubmed]
- Kuvshinoff B, Maghfoor I, Miedema B, Bryer M, Westgate S, Wilkes J, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: Are ≤ 1 cm distal margins sufficient? Ann Surg Oncol. 2001; 8: 163-169.
[Crossref] [Google Scholar] [Pubmed]
- Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, et al. Adequacy of 1 cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003; 10: 80-85.
[Crossref] [Google Scholar] [Pubmed]
- Rutkowski A, Bujko K, Nowacki MP, Chmielik E, Nasierowska-Guttmejer A, Wojnar A, et al. Distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: Is it safe? Ann Surg Oncol. 2008; 15: 3124-3131.
[Crossref] [Google Scholar] [Pubmed]
- Leo E, Belli F, Miceli R, Mariani L, Gallino G, Battaglia L, et al. Distal clearance margin of 1 cm or less: A safe distance in lower rectum cancer surgery. Int J Colorectal Dis. 2009; 24: 317-322.
[Crossref] [Google Scholar] [Pubmed]
- Kiran RP, Lian L, Lavery IC. Does a subcentimeter distal resection margin adversely influence oncologic outcomes in patients with rectal cancer undergoing restorative proctectomy? Dis Colon Rectum. 2011; 54(2): 157-163.
[Crossref] [Google Scholar] [Pubmed]
- Zeng WG, Liu MJ, Zhou ZX, Wang ZJ. A distal resection margin of ≤ 1 mm and rectal cancer recurrence after sphincter-preserving surgery: The role of a positive distal margin in rectal cancer surgery. Dis Colon Rectum. 2017; 60(11): 1175-1183.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Puttiporn Naovaset1* and Weerapat Suwanthanm22Surgical Research Unit, Faculty of medicine Ramathibodi hospital, Mahidol University, Thailand
Citation: A Subcentimeter Distal Rectal Margin and its Impact on Local Recurrence in Rectal Cancer Patients Undergoing Curative Surgery after Preoperative Chemoradiotherapy
Received: 11-Dec-2023 Accepted: 25-Dec-2023 Published: 03-Jan-2024, DOI: 10.31858/0975-8453.15.1.15-23
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3