Review Article - (2023) Volume 14, Issue 9
Abstract
A recent innovation in new drug delivery systems aims to improve the safety and efficacy of the therapeutic molecule by developing a dosage form that is easy to administer. Swallowing is more difficult for geriatrics, pediatrics, bedridden, crippled (dysphagia), and the mentally ill patients. The oral route is the most preferred route for drug administration, mainly because it has the greatest compliance factor in pediatrics and geriatrics. It is believed to be the most efficient and safe method of drug delivery. Tablets, delicate pills, and chewable gums are examples of chewable dose forms. “Chewable squares” is a lengthy piece of drug specialty equipment. Chewable tablets are palatable and should be crushed and chewed between the teeth with little or no water added prior to consumption. The purpose of chewable tablets is to provide a unit dosage form of a pharmaceutical product that can be easily administered to infants, children, or the elderly who have difficulty swallowing whole tablets. Reduced grittiness, pleasant flavor, tongue feel, appropriate bioavailability and stability are the prerequisites. Various factors such as flow ability, lubricity, disintegration, organoleptic properties, compressibility, compatibility and stability influence the formulation of chewable tablets. Advantages of chewable tablet formulations include stability, palatability, precise dosage, portability, and ease of administration. Taste-masking of bitter drug candidates can be achieved through the use of sweeteners, flavoring agents, and the use of taste-masking techniques. Chewable tablets can be formulated using tableting techniques such as dry granulation, wet granulation and direct compression. Chewable dosage forms such as soft pills, tablets, gums and chewing squares have long been part of pharmacy collections.
Keywords
Chewable tablet, Oral route, Bioavailability, Compressibility, Taste-masking, Granulation, Formulation, Palatability
Introduction
Even with the amazing advancements in drug delivery, oral administration of medicinal agents continues to be the preferred method due to its low cost of care, ease of administration of the therapeutic agent, and patient compliance. When compared to parenteral medication administration, oral drug administration is usually more comfortable for patients and less invasive. Due to their many benefits over other distribution methods, including safety, efficacy, cost-effectiveness, and patient compliance, oral dosage forms account for the majority of the drug delivery market. Chewable tablets have benefits over traditional tablets as a dosage form in terms of manufacturing, dosing precision, portability, and long-term stability. Oral dosage forms have the most optimal dosage form characteristics out of all the different pharmaceutical dosage forms (Bhusnure O, et al., 2015).
Advantages of chewable tablets
• Increased patient comfort; no water required to swallow.
• Improves bioavailability by avoiding spoilage.
• Pleasant taste and product differentiation have improved patient acceptance, especially in pediatrics.
• Suitable for bedridden people, disabled people, travelers, busy people, etc. who do not have water every time.
• For physiological and psychological reasons, children by early childhood usually have difficulty swallowing tablets and capsules. In such cases, chewable tablets are preferred due to their superior patient acceptability (palatableness) and stability (Taranum R and Mittapally S, 2018).
• Enables effective taste masking along with pleasant mouth feel.
• The size of the dosage form is difficult to swallow. In such cases, chewable tablets are suitable.
• The efficacy of the therapeutic is enhanced by the size reduction that occurs while chewing the tablet prior to swallowing.
• It serves as an ideal drug delivery method for aphasia patients as it reduces the risk of aspiration.
• Stimulate the flow of saliva in the mouth.
• Can be used as an alternative to liquid dosage forms when fast acting is required.
• The efficacy of the therapeutic is enhanced by the size reduction that occurs while chewing the tablet prior to swallowing.
Disadvantages of chewable tablets
Of course, bad-tasting drugs and very high-dose chewable tablets have some limitations. Other drawbacks of chewable tablets include:
• If not properly formulated, it may leave an unpleasant taste in the mouth.
• Medicines that have a very bad taste cannot be prescribed as chewable tablets.
• Chewing chewable tablets for a long time can lead to facial muscle soreness.
• Proper packaging is essential to keep the product safe and stable.
• Sweeteners like sorbitol can cause diarrhea and sucrose can cause tooth decay.
• The presence of flavorings can cause ulcers in the mouth. Since it has no mechanical strength, care must be taken when handling it.
• Chewable tablets require proper packaging for stable drug safety and stability.
Desirable Properties of Chewable Tablets
• Adequate bioavailability and bioactivity.
• Suitable stability and quality.
• Improved overall palatability (taste and mouth feel).
• Desired size and shape.
• Sufficient mechanical strength.
• Leaves no residue in the mouth after oral administration.
• Ability to dissolve easily to facilitate dissolution.
• Economic formulas and procedures.
Important quality attributes of chewable tablets should include hardness, disintegration, dissolution, and factors that can affect drug bioavailability and bioequivalence. In addition, special attention should be paid to tablet size, thickness, friability and taste. These may affect the patient’s ability or willingness to chew the chewable tablet (i.e., the patient may swallow the chewable tablet whole rather than chewing it) (Thakur RR, et al., 2012; Renu JD, et al., 2015). No single quality attribute is considered sufficient to control the performance of chewable tablets. Instead, the goal should be to develop the right combination of these attributes to ensure performance in the intended use of the chewable tablet (Michele TM, et al., 2002).
Literature Review
Formulation factors
Various factors such as flow, lubrication, disintegration, organoleptic properties, compressibility, compatibility and stability play a role. Tablets must have acceptable flow ability, compressibility and stability.
Taste and flavor: The product should have an acceptable sweetness and aroma. Physiologically, taste is a sensory response resulting from chemical stimulation of the taste buds on the tongue. The four basic tastes are salty, sour, sweet and bitter. The term flavor usually refers to a specific sense that combines taste and smell. For example, sugar has a sweet taste but no aroma, whereas honey has a sweet taste and a distinctive odor.
Mouth feels: This term is related to the type of sensation or touch that a tablet produces in the mouth upon chewing. However, for a formulation to be effective, the overall effect in the mouth is important. In general, gritty (e.g., calcium carbonates) or gummy texture is objectionable, whereas soothing and cooling sensation (e.g., mannitol) with smooth texture is preferred.
Compressibility: When formulating chewable tablets, the powder blend or granules should have the desired flow properties to obtain the final product. Powders/granules should have an optimum compression index to obtain the highest quality final product.
Compatibility: The active pharmaceutical ingredient must be compatible with the excipients in the chewable tablet formulation and compatible with compression.
Taste masking: To achieve patient acceptability and compliance, taste-masking methods are applied to mask the bitter or unpleasant taste of active pharmaceutical ingredients/drugs (Sohi H, et al., 2004; Ayenew Z, et al., 2009). Oral administration of bitter or unpleasant drugs is often the greatest obstacle for patients mainly for pediatrics and geriatrics. Taste-masking effectiveness is often a key factor in enabling specialized dosage forms such as disintegrating tablets, orally disintegrating films and chewable tablets. The mechanism of taste-masking methods often relies on two main approaches. The first is to add sweeteners, flavors and effervescent agents to overcome the unpleasant taste, and the second is to prevent the interaction of bitter/unpleasant drugs with the taste buds.
The following techniques are used for taste masking:
• Coating by wet granulation (Sajal JK, et al., 2008; Joshi S and Petereit HU, 2013)
• Microencapsulation (Tripathi A, et al., 2011; Al-Kasmi B, et al., 2013)
• Solid dispersion (Ozkan G, et al., 2019; Sobel R, et al., 2014)
• Inclusion complexes (Ye Q, et al., 2018; Song H, et al., 2018)
• Ion exchange (Ma X and Williams III RO, 2019; Ni Y and Li D, 2018)
• Spray congealing and spray coating (Abou-Okeil A, et al., 2018; Tan DC, et al., 2018)
• Formation of various derivatives or salts (Ilić I, et al., 2009; Chauhan R, 2017)
• Use of amino acids and protein hydrolysates (Akitomi H, et al., 2013; He W, et al., 2019)
• Molecular complexes (Liu T, et al., 2019; Huang T, et al., 2019)
• Melt extrusion (Tan DC, et al., 2018)
• pH adjustment (Chirag JP, et al., 2013)
• Development of liposomes (Chirag JP, et al., 2013)
• Viscosity adjustment (Chirag JP, et al., 2013)
• Prodrug approach (Chirag JP, et al., 2013)
Excipients used for preparation of chewable tablets
The pharmaceutical industry is constantly striving to meet the therapeutic needs of patients and apart from active ingredients inert excipients play an important role in formulation development. Excipients are substances other than pharmacologically active drugs or prodrugs that are incorporated in the manufacturing process or included in the finished pharmaceutical dosage form (Patel H, et al., 2011; Choudhary A, 2013).
Diluent: A diluent is a type of filler used to fill the tablet volume when the tablet is not sufficient to fill the volume. Examples include mannitol, sorbitol, xylitol, calcium carbonate, magnesium carbonate, calcium sulfate, magnesium trisilicate, lactose and Microcrystalline Cellulose (MCC).
Binder: Provides cohesion to powdered materials and can be added both dry and wet to form granules. Examples include lactose, cellulose derivatives-methylcellulose, ethyl cellulose, hydroxyl propylmethyl cellulose, hydroxyl propyl cellulose, starch, polyvinyl pyrrolidone (povidone), sodium alginate, Carboxy Methyl Cellulose (CMC) and acacia. Examples include crospovidone, MCC, sodium starch glycolate, carboxy methyl cellulose and modified corn starch.
Sweeteners: The sweetness profile is adjusted by adding the desired sweetener. Sweeteners are added to improve the palatability of the formulation, especially for chewable tablets. Sweeteners are commonly included in chewable tablets when the commonly used carriers such as lactose, sucrose, mannitol and dextrose do not completely mask the taste of the active substance or active substance ingredients. Due to the potential carcinogenicity of artificial sweeteners (such as cyclamate and saccharin), pharmaceutical companies are increasingly trying to develop tablet products that do not use such agents. The taste-masking method is the first and simplest method of taste-masking, especially for pediatric definitions, chewable tablets, and liquid indications. Examples of commonly utilized sweetening agents along with their relative sweetness are mentioned in Table 1.
| Materials | Relative sweetness |
|---|---|
| Aspartame | 200 |
| Glycyrrhiza | 50 |
| Saccharin | 500 |
| Fructose(laevulose) | 1.7 |
| Lactose | 0.2 |
| Mannitol | 0.5-0.7 |
| Sorbitol | 0.5-0.6 |
| Sucrose | 1 |
| Cyclamates | 30-50 |
| Dextrose(glucose) | 0.7 |
| Maltose | 0.3 |
Table 1: Sweetening agents and their relative sweetness levels
Lubricants: Lubricants prevent ingredients from agglomerating and sticking to the tablet press. Examples are talcum powder, magnesium stearate, stearic acid, etc. These are used to facilitate powder flow by reducing friction and agglomeration between particles. Lubricants are basically used in combination with lubricants. These include fumed silica, talc, magnesium carbonate, and more.
Flavor: Flavor is an important excipient in chewable tablets. Flavors are used to impart a pleasant taste and can be used in combination with sweeteners to mask the off-taste of the active ingredient and improve the acceptability of the formulation. Flavors are used based on their intended characteristics and requirements (Table 2).
| Flavors | Group for tasting types |
|---|---|
| Sweet | Vanilla, fruits, maple, stone fruits, berries, grape |
| Sour (Acidic) | Raspberry, anise, cherry, root beer, cherry, strawberry |
| Salty | Mixed citrus, butterscotch, maple, nutty, buttery, spice, mixed fruits, butterscotch |
| Bitter | Coffee, cherry, Liquorice, grapefruit, wine fennel, peach, mint |
| Metallic | Grape, burgundy, lemon-lime |
| Alkaline | Chocolate, Mint, cream, vanilla |
Table 2: Flavor groups and its taste types
Colors: Colorants are used to enhance the appearance and organoleptic profile of dosage forms. FD and C, D and C colors are used. The form of colorant used in the manufacture of chewable tablets depends on the manufacturing process. Coloring agents are commonly used in chewable tablets manufactured by wet granulation process.
Manufacturing methods for chewable tablets
Manufacturing of tablet includes formulating the colorants correctly, while maintaining the correct moisture content to achieve the correct tablet hardness and to obtain the desired quality of product. Various methods are used to manufacture chewable tablets (Surbhi G, et al., 2012; Lachman L, et al., 1989; Lachman L, et al., 1976) these are:• Dry granulation
• Wet granulation
• Direct compression
Granulation is the process of making granules from a powder or solid substance. Granulation usually involves agglomeration of fine particles to form larger units of multiple particles, called granules, ranging in size from 0.2 to 4.0 mm.
Dry granulation: This is a new method for semi-automatic production of granules. This method is applicable to all fixed-dose drugs. Dry granulation, also known as pre-compression or double-compression, is a size-enlarging process that has been considered to improve the flow and compression properties of powders that are not suitable for compression. In this process the powder mixture is compacted by applying force. This generally increases the size significantly. Dry granulation is typically used to produce tablets where formulation ingredients are prone to flow problems. Manufacture of tablets by the dry granulation process eliminates many unit operations, but includes milling, weighing, mixing, beating, dry sieving, lubricating, and compressing granules into tablets. Dry granulation refers to the process of granulating without the use of liquids. Forming granules using the dry granulation process is generally accomplished by either impact techniques or roller compaction. Slugging involves compacting primary powder particles into large flat pallets using a tablet press, or more commonly a large, heavy-duty rotary press. The resulting compact is then ground using conventional grinding equipment. The ground slag was then passed through a screen of desired mesh size for sizing. After adding lubricant granules, it is finally compressed into tablets. Roller compaction is a relatively simple, efficient and inexpensive form of dry granulation. In this process, the compounding ingredients are passed between two counter-rotating rollers where they are compressed and bonded into a layer of solid mass. The compact is then further ground, sorted, lubricated and compressed into tablets (Figure 1). Various steps involved in dry granulation. These are:
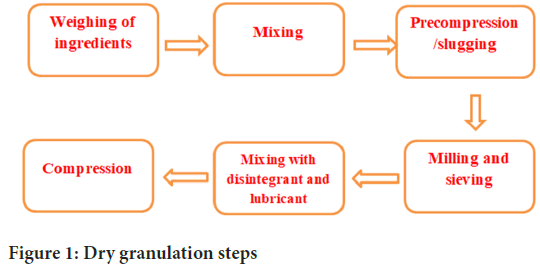
Figure 1: Dry granulation steps
• Weighing of ingredients
• Mixing
• Compression of powder into slugs (Pre-compression)
• Milling and sieving
• Mixing with disintegrant and lubricant
Compression of granules into tablets
Wet granulation: Wet granulation is the most commonly used granulation method. It uses a suitable non-toxic and volatile granulating fluid such as water, isopropanol or ethanol to agglomerate fine powder particles or into larger, stronger and relatively permanent structures called granules. It’s a sizing process. The granulation solution can be used alone or as a solvent with a binder or granulating agent. The choice of granulation fluid is highly dependent on the properties of the material being granulated.
This involves four key mechanism steps:
• Wetting and Nucleation
• Coalescence
• Consolidation
• Attrition or breakage
Mechanism in wet granulation: Steps involved in the wet granulation method for tablet production are:
• Weighing and mixing of ingredients (excluding lubricants)
• Preparation of damp mass by the addition of binder solution
• Screening of damp mass into granules and drying
• Sizing of granules by dry screening
• Lubrication of granules
• Compression of granules into tablets
Direct compression: Direct compression is a method of compressing a powder mixture of drug substance and excipients into tablets without granulating them in a tableting machine. There is no mechanical processing of powders other than the mixing process. This is suitable for moisture and heat sensitive Active Pharmaceutical Ingredients (APIs) as it eliminates the wetting and drying steps and increases drug stability by reducing adverse effects. In this process, the API is mixed with excipients and lubricants, followed by compression to make the product easier to handle. The manufacture of tablets by direct compression involves comparatively few steps and these are:
• Pre-milling of ingredients (API and ingredients)
• Mixing of all ingredients
• Compression
Discussion
Recent advancements in granulation technology
In a support to enhance commercial output of pharmaceutical formulations, the granulation process has witnessed numerous technological innovations (Solanki HK, et al., 2010) which include:
• Thermal adhesion granulation (Lin HL, et al., 2008; Narang AS and Badawy SI, 2019)
• Pneumatic dry granulation (Verma R, et al., 2019)
• Melt/thermoplastic granulation (Kittikunakorn N, et al., 2019; Patel AU, et al., 2018)
• Moisture activated dry granulation (Moravkar KK, et al., 2017; Takasaki H, et al., 2013)
• Spray drying granulation (Kaur G, et al., 2019; Figueroa CE and Bose S, 2013)
• Fluidized bed granulation (Gupta R, 2017)
• Extrusion-spheronization granulation (Muley S, et al., 2016; Sriamornsak P, et al., 2007)
• Freeze granulation (Stuer M, et al., 2012; Chou KS, et al., 2014)
• Steam granulation (Cavallari C, et al., 2002; Suresh P, et al., 2017)
• Foam binder granulation (Tan MX and Hapgood KP, 2011; Tan MX and Hapgood KP, 2011)
Evaluation of chewable tablets
Evaluation of chewable tablets includes various physical and chemical parameters (Jagdale S, et al., 2010; FDA, 2023; Farheen F and Bharadwaj S, 2014). These are:
Physical evaluation: It involves-• Tablet physical appearance and organoleptic characteristics
• Friability
• Hardness
• Weight variation
• Disintegration
• Dissolution
Chemical evaluation: It involves -
• Assay
• Drug content uniformity
• In vitro and in vivo evaluation
Physical appearance and organoleptic characteristics: The general appearance, visual identity and overall elegance of all tablets are essential for consumer acceptance. Chewable tablets are evaluated for sensory characteristics such as size, shape, color, odor and taste. For chewable tablets, taste is an important factor in patient acceptance. Flavors can be attributed to APIs and additives, especially flavorings and sweeteners. One can control the size and state of the tablet by controlling its dimensions. Size and thickness should be consistent from tablet to tablet and batch to batch. Tablet diameter and thickness can be estimated with vernier calliper.
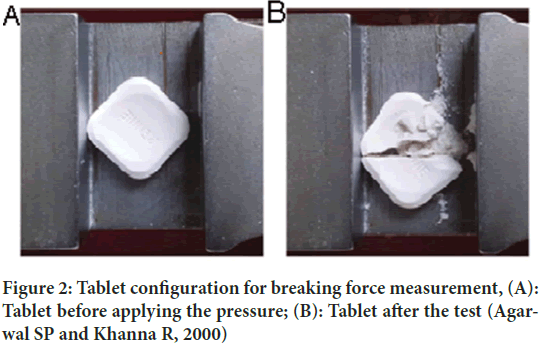
Hardness: Hardness testing is performed to measure the force required to break a tablet on a particular plane. Tablets should be hard enough to withstand the rigors of manufacturing, packaging, transportation, and distribution, but not so hard as to cause chewing problems. It can be measured and expressed in units. An index relating tablet hardness to tablet breaking load was developed to produce a number that can be used to compare the chewability of chewable tablets (Agarwal SP and Khanna R, 2000). Tablet hardness is determined using a hardness analyzer that measures the force required to break a tablet (Figure 2).
Figure 2: Tablet configuration for breaking force measurement, (A): Tablet before applying the pressure; (B): Tablet after the test (Agarwal SP and Khanna R, 2000)
Friability: Tablet friability can be determined using the Roche friabilator. 10 tablets are weighed and placed in a friabilator rotating at 25 rpm for 4 minutes. Then remove the tablet, dust with powder and weigh again. Tablets with less than 0.5%-1.0% weight loss are considered acceptable. Also, discard the tablet if capping occurs during the test. The percentage friability of the tablet is calculated by the formula Percentage friability=[(Initial weight-Final weight)/Initial weight] × 100
Weight variation: According to the United States Pharmacopeia (USP) weight variation study, the weight of 20 tablets is regulated by calculating the standard load and comparing the individual tablet load to normal (Uddin M, et al., 2016). Weight grade test values are given in percent. According to the USP, a tablet passes the test if no more than 2 tablets are outside the percentage limit and no tablets are outside the percentage limit by more than twice (Table 3).
| Average weight tablets (mg) | Maximum % difference limits |
|---|---|
| 130 or less | ± 10.0 |
| 130 to 324 | ± 7.50 |
| More than 324 | ± 5.0 |
Table 3: Weight variation limits for tablets (Uddin M, et al., 2016; Sheaikh SS, et al., 2018)
Weight variation=[(Initial weight-Average weight)/Average weight] × 100
Disintegration: Disintegration time is the time it takes for a tablet to break up into small particles. The presence of the right amount and type of disintegrant usually makes it easier for the patient to disintegrate the tablet quickly and chew it completely. In vitro disintegration testing should be performed on intact tablets in a suitable medium using USP disintegration apparatus and methods (Sheaikh SS, et al., 2018).
Dissolution: Drug absorption from chewable tablets depends on the release of the drug substance from the intact or chewed tablet. In vitro dissolution testing of chewable tablets should follow the principles of conventional Immediate Release (IR) tablet dissolution testing. During dissolution, the active pharmaceutical ingredient of a chewable tablet should leach sufficiently out of the tablet. For characterization of products under development, in vitro dissolution studies should be performed on intact tablets in at least four media which includes water, pH 1.2 aqueous media, pH 4.5 buffered aqueous media and a buffered aqueous medium at pH 6.8.
Consistency of drug content: The drug content of all formulations is assessed by High Performance Liquid Chromatography (HPLC) technique. Powder 20 chewable tablets and accurately weigh 100 mg of powdered drug into a 50 ml volumetric flask. Add 5 ml of methanolic sulfuric acid and shake well. Bring the final volume to 50 ml with methanol. Then filter with filter paper (Whatman) No. 41. The first 10 ml are discarded. 5 ml of the clear filtrate is then pipetted into a 50 ml volumetric flask and made up to 50 ml with methanol. Inject 2 µl of standard solution and sample preparation separately onto the column. The flow rate is maintained at 2 mL/min and estimates are made at 254 nm. Chromatograms are recorded separately for both standard and sample preparations (Table 4).
| Attributes | Recommendations |
|---|---|
| Tablet Hardness | Less than 12 kp, higher hardness values may be considered if justified (e.g., tablet rapidly softens or disintegrates after brief (<30 s) exposure to simulated saliva |
| Disintegration | Typically, the same specifications as immediate-release tablets; important to determine since some individuals may swallow tablets without chewing |
| Dissolution | Typically, the same specifications as immediate-release tablets. Does not apply to chewable modified release products |
| In vitro dissolution testing should be conducted on intact chewable tablets since some individuals may swallow tablets without chewing | |
| Others | Specific to the individual product (e.g., tablet with functionally coated particles should not be adversely affected by chewing) |
| Tablet size, shape, thickness, friability, palatability | |
| Chewing difficulty index is discussed in the guidance: However limits are not provided |
Tablet 4: FDA chewable tablets guidance-critical quality considerations summary
Applications of chewable tablet
Local therapy: Chewable tablets can release controlled amounts of active ingredients over an extended period of time to achieve long-lasting local effects.
Pain: Successful treatment of mild aches, headaches, cold sores, muscle aches, etc. requires rapid absorption of therapeutic doses of active ingredients. A chewable tablet drug delivery system may be beneficial for the management of mild pain where oral absorption provides a rapid onset of action and reduces the risk of gastrointestinal side effects.
Systemic therapy: Chewable Tablets offers advantages for systemic drug delivery, especially when drugs are absorbed through the buccal mucosa.
Quit smoking: Chewing gum formulations containing nicotine, lobeline, and silver acetate have been clinically tested as smoking cessation aids.
Obesity: Several chewing gum formulations are available that contain caffeine, guarana, or chromium. Caffeine and guarana are major appetite suppressants that have been shown to increase metabolic rate. Today, pediatric, geriatric and bedridden patients prefer chewable tablets to traditional dosage forms due to difficulty swallowing, reduced water intake to swallow medications, and intolerance to the bitter taste of certain medications. Chewable tablets are versatile dosage forms that combine the manufacturing and stability advantages of solid products while offering beneficial organoleptic and administration benefits. Formulators can use one or more approaches to arrive at a formulation and process combination that yields a product with acceptable flow, compressibility, and stability.
Marketed products of chewable tablet
Chewable tablet is one of the most popular dosage forms available in market, used for delivering the active components. The available marketed products of chewable tablet are given below in Table 5.
| Name of drug | Category | Method used | Result | Reference |
|---|---|---|---|---|
| Acetaminophen | Anti-pyretic | Direct compression | Good drug release with suppressed bitterness and low sweetness. | Sohi H, et al., 2004 |
| Levamisole | Anthelmintic | Wet granulation | Less disintegration time and complied with all specified parameters. | Jagdale S, et al., 2010 |
| Lamotrigine | Anticonvulsant or Antiepileptic | Melt granulation | Taste masked with 90% drug release within 1 hr. | Solanki HK, et al., 2010 |
| Albendazole | Anthelmintic | Non-aqueous, Aqueous, Direct compression | Product with direct compression had faster dissolution rate | Tripathi A, et al, 2011 |
| Albendazole | Anthelmintic | Wet granulation | All parameters were found acceptable within their limits. | Surbhi G, et al., 2012 |
| Metformin Hydrochloride (HCL) | Hypoglycemic agent | Wet granulation | All the parameters were found to be satisfactory. | Chirag JP, et al., 2013 |
| Paracetamol and Metoclopramide HCL | Analgesic, Antiemetic | Wet granulation | Formulation shown the satisfactory drug release with disintegration time of 56 s. | Sobel R, et al., 2014 |
| Mebendazole | Anthelmintic | Non-aqueous, Aqueous, Direct compression | Product formulated by direct compression had faster dissolution rate. | Farheen F and Bharadwaj S, 2014 |
| Montelukast sodium | Leukotriene receptor antagonists to prevent and manage asthma | Direct compression | All parameters were found acceptable within their limits. | Renu JD, et al., 2015 |
| Albendazole | Anthelmintic | Direct compression | All the parameters were found to be satisfactory. | Muley S, et al., 2016 |
| Multivitamin | To treat or prevent vitamin deficiencies | Direct compression | Pre-compression and post-compression parameters were found satisfactory within their acceptable limits. | Suresh P, et al., 2017 |
Table 5: Available marketed products of chewable tablets
Conclusion
These tablets are intended to disintegrate smoothly in the mouth, whether chewed or not chewed at a moderate rate and the characteristic chewable tablets have a smooth texture when disintegrated and are pleasant to the taste and it doesn’t has unpleasant taste or bitterness. Chewable tablets improve administration of drugs by the oral route, benefiting children, the elderly, traveling patients and patients with swallowing difficulties.
References
- Bhusnure O, Shaikh F, Sugave B, Kavale B, Sayyed R, Hucche B. Formulation strategies for taste-masking of chewable tablets. Am J Pharm Res. 2015; 5: 3836-3849.
- Taranum R, Mittapally S. Soft chewable drug delivery system: Oral medicated jelly and soft chew. J Drug Deliv Ther. 2018; 8(4): 65-72.
- Thakur RR, Rathore DS, Narwal S. Orally disintegrating preparations: Recent advancement in formulation and technology. J Drug Deliv Ther. 2012; 2(3): 87-96.
- Renu JD, Jalwal P, Singh B. Chewable Tablets: A comprehensive review. Pharm Innov J. 2015; 4(5): 100-105.
- Michele TM, Knorr B, Vadas EB, Reiss TF. Safety of chewable tablets for children. J Asthma. 2002; 39(5): 391-403.
[Crossref] [Google Scholar] [Pubmed]
- Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev Ind Pharm. 2004; 30(5): 429-448.
[Crossref] [Google Scholar] [Pubmed]
- Ayenew Z, Puri V, Kumar L, Bansal AK. Trends in pharmaceutical taste masking technologies: A patent review. Recent Pat Drug Deliv Formul. 2009; 3(1): 26-39.
[Crossref] [Google Scholar] [Pubmed]
- Sajal JK, Uday SR, Surendra V. Taste masking in pharmaceuticals: An update. J Pharm Res. 2008; 1(2): 126-130.
- Joshi S, Petereit HU. Film coatings for taste masking and moisture protection. Int J Pharm. 2013; 457(2): 395-406.
[Crossref] [Google Scholar] [Pubmed]
- Tripathi A, Parmar D, Patel U, Patel G, Daslaniya D, Bhimani B. Taste masking: A novel approach for bitter and obnoxious drugs. J Pharma Sci Biosci Res. 2011; 1(3): 36-142.
- Al-Kasmi B, Alsirawan MB, Bashimam M, El-Zein H. Mechanical microencapsulation: The best technique in taste masking for the manufacturing scale-Effect of polymer encapsulation on drug targeting. J Control Release. 2017; 260: 134-141.
[Crossref] [Google Scholar] [Pubmed]
- Ozkan G, Franco P, de Marco I, Xiao J, Capanoglu E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019; 272: 494-506.
[Crossref] [Google Scholar] [Pubmed]
- Sobel R, Gundlach M, Su CP. Novel concepts and challenges of flavor microencapsulation and taste modification. Microencapsulation in the food industry. 2014: 421-442.
- Ye Q, Georges N, Selomulya C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci Technol. 2018; 78: 167-179.
- Song H, Moon C, Lee BJ, Oh E. Mesoporous pravastatin solid dispersion granules incorporable into orally disintegrating tablets. J Pharm Sci. 2018; 107(7): 1886-1895.
[Crossref] [Google Scholar] [Pubmed]
- Ma X, Williams III RO. Characterization of amorphous solid dispersions: An update. J Drug Deliv Sci Technol. 2019; 50: 113-124.
- Ni Y, Li D. Preparation, characterization and evaluation of an inclusion complex of steviolbioside with γ-cyclodextrin. Food Biosci. 2018; 26: 65-72. [Crossref]
- Abou-Okeil A, Rehan M, El-Sawy SM, El-Bisi MK, Ahmed-Farid OA, Abdel-Mohdy FA. Lidocaine/β-cyclodextrin inclusion complex as drug delivery system. Eur Polym J. 2018; 108: 304-310.
- Tan DC, Ong JJ, Gokhale R, Heng PW. Hot melt extrusion of ion-exchange resin for taste masking. Int J Pharm. 2018; 547(1-2): 385-394.
[Crossref] [Google Scholar] [Pubmed]
- Ilić I, Dreu R, Burjak M, Homar M, Kerč J, Srčič S. Microparticle size control and glimepiride microencapsulation using spray congealing technology. Int J Pharm. 2009; 381(2): 176-183.
[Crossref] [Google Scholar] [Pubmed]
- Chauhan R. Taste masking: A unique approach for bitter drugs. J Stem Cell Bio Transplant. 2017; 1(2): 12-20.
- Akitomi H, Tahara Y, Yasuura M, Kobayashi Y, Ikezaki H, Toko K. Quantification of tastes of amino acids using taste sensors. Sens Actuators B: Chem. 2013; 179: 276-281.
- He W, Yang R, Zhao W. Effect of acid deamidation-alcalase hydrolysis induced modification on functional and bitter-masking properties of wheat gluten hydrolysates. Food Chem. 2019; 277: 655-663.
[Crossref] [Google Scholar] [Pubmed]
- Liu T, Wan X, Luo Z, Liu C, Quan P, Cun D, et al. A donepezil/cyclodextrin complexation orodispersible film: Effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Asian J Pharm Sci. 2019; 14(2): 183-192.
[Crossref] [Google Scholar] [Pubmed]
- Huang T, Zhao Q, Su Y, Ouyang D. Investigation of molecular aggregation mechanism of glipizide/cyclodextrin complexation by combined experimental and molecular modeling approaches. Asian J Pharm Sci. 2019; 14(6): 609-620.
[Crossref] [Google Scholar] [Pubmed]
- Chirag JP, Tyagi PS, Dhruv M, Ishita M, Gupta AK, Rageeb M, et al. Pharmaceutical taste masking technologies of bitter drugs: A concise review. J Drug Discov Ther. 2013; 1: 39-46.
- Patel H, Shah V, Upadhyay U. New pharmaceutical excipients in solid dosage forms-A review. Int J Pharm Life Sci. 2011; 2(8).
- Choudhary A. Pharmaceutical excipients and their suggested quantity. Pharmaguidelines. 2013.
- Surbhi G, Seema S, Singh G, Rana AC. Industrial process Validation of tablet Dosage form: An overview. Int Res J Pharm. 2012; 3(3): 49-51.
- Lachman L, Liberman HA, Schwartz JB. Pharmaceutical dosage forms. Marcel Dekkar Inc. 1989; 2(1).
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Lea and Febiger. 1976: 420.
- Solanki HK, Basuri T, Thakkar JH, Patel CA. Recent advances in granulation technology. Int J Pharm Sci Rev Res. 2010; 5(3): 48-54.
- Lin HL, Ho HO, Chen CC, Yeh TS, Sheu MT. Process and formulation characterizations of the Thermal Adhesion Granulation (TAG) process for improving granular properties. Int J Pharm. 2008; 357(1-2): 206-212.
[Crossref] [Google Scholar] [Pubmed]
- Narang AS, Badawy SI. Emerging paradigms in pharmaceutical wet granulation. Handbook of Pharmaceutical Wet Granulation. 2019; 825-840.
- Verma R, Patil M, Paz CO. Current Practices in Wet Granulation-Based Generic Product Development. Handbook of Pharmaceutical Wet Granulation. 2019; 203-259.
- Kittikunakorn N, Koleng III JJ, Listro T, Sun CC, Zhang F. Effects of thermal binders on chemical stabilities and tabletability of gabapentin granules prepared by twin-screw melt granulation. Int J Pharm. 2019; 559: 37-47.
[Crossref] [Google Scholar] [Pubmed]
- Patel AU, Caudhari DV, Shah PJ, Shah SA. Hot melt granulation method for the preparation of floating matrix tablets of Tolperison Hydrochloride. Future J Pharm Sci. 2018; 4(2): 139-149.
- Moravkar KK, Ali TM, Pawar JN, Amin PD. Application of Moisture Activated Dry Granulation (MADG) process to develop high dose Immediate Release (IR) formulations. Adv Powder Technol. 2017; 28(4): 1270-1280.
- Takasaki H, Yonemochi E, Messerschmid R, Ito M, Wada K, Terada K. Importance of excipient wettability on tablet characteristics prepared by Moisture Activated Dry Granulation (MADG). Int J Pharm. 2013; 456(1): 58-64.
[Crossref] [Google Scholar] [Pubmed]
- Kaur G, Singh M, Matsoukas T, Kumar J, de Beer T, Nopens I. Two-compartment modeling and dynamics of top-sprayed fluidized bed granulator. Appl Math Model. 2019; 68: 267-280.
- Figueroa CE, Bose S. Spray granulation: importance of process parameters on in vitro and in vivo behavior of dried nanosuspensions. Eur J Pharm Biopharm. 2013; 85(3): 1046-1055.
[Crossref] [Google Scholar] [Pubmed]
- Gupta R. Fluid bed granulation and drying. Predictive Modeling of Pharmaceutical Unit Operations. 2017; 137-158.
- Muley S, Nandgude T, Poddar S. Extrusion–spheronization a promising pelletization technique: In-depth review. Asian J Pharm Sci. 2016; 11(6): 684-699.
- Sriamornsak P, Nunthanid J, Luangtana-anan M, Puttipipatkhachorn S. Alginate-based pellets prepared by extrusion/spheronization: A preliminary study on the effect of additive in granulating liquid. Eur J Pharm Biopharm. 2007; 67(1): 227-235.
[Crossref] [Google Scholar] [Pubmed]
- Stuer M, Zhao Z, Bowen P. Freeze granulation: Powder processing for transparent alumina applications. J Eur Ceram Soc. 2012; 32(11): 2899-2908.
- Chou KS, Liu HL, Kao LH, Yang CM, Huang SH. A novel granulation technique using a freeze-thaw method. Ceram Int. 2014; 40(6): 8875-8878.
- Cavallari C, Abertini B, González-Rodrı́guez ML, Rodriguez L. Improved dissolution behaviour of steam-granulated piroxicam. Eur J Pharm Biopharm. 2002; 54(1): 65-73.
[Crossref] [Google Scholar] [Pubmed]
- Suresh P, Sreedhar I, Vaidhiswaran R, Venugopal A. A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chem Eng J. 2017; 328: 785-815.
- Tan MX, Hapgood KP. Foam granulation: Binder dispersion and nucleation in mixer-granulators. Chem Eng Res Des. 2011; 89(5): 526-536.
- Tan MX, Hapgood KP. Foam granulation: Liquid penetration or mechanical dispersion?. Chem Eng Sci. 2011; 66(21): 5204-5211.
- Jagdale S, Gattani M, Bhavsar D, Kuchekar B, Chabukswar A. Formulation and evaluation of chewable tablet of levamisole. Int J Res Pharm Sci. 2010; 1(3): 282-289.
- FDA. Guidances, Drugs. US Food and Drug Administration. 2023.
- Farheen F, Bharadwaj S. Formulation and evaluation of chewable tablets of mebendazole by different techniques. PharmaTutor. 2014; 2(6): 183-189.
- Agarwal SP, Khanna R. A review on chewable tablet. CBS Publishers and Distributors. 2000: 2; 247.
- Uddin M, Mamun A, Rashid M, Asaduzzaman M. In-process and finished products quality control tests for pharmaceutical capsules according to pharmacopoeias. Br J Pharm Res. 2016; 9(2): 1-9.
- Sheaikh SS, Gaikwad RP, Shaikh AA, Pawar YD, Gavit BD. Solubility enhancement of etodolac chewable tablet using honey, and evaluation with (Doe) Design of experiment. Acta Sci Pharm Sci. 2018; 2(6): 199-205.
Author Info
Amit Antil1, Monika Dahiya2 and Deepali Tomar3*2Department of Pharmaceutical Sciences, College of Pharmacy, Pt. Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Haryana, India
3Department of Pharmaceutical Chemistry, Lala Birkha Ram College of Pharmacy, Haryana, India
Citation: Antil A: An Overview on Efficacy of Chewable Tablets in Improving Oral Drug Delivery
Received: 17-Aug-2023 Accepted: 11-Sep-2023 Published: 18-Sep-2023, DOI: 10.31858/0975-8453.14.9.571-577
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3