Review Article - (2024) Volume 15, Issue 8
Abstract
Medicinal plants have been used by humans since ancient times to relieve and treat many ailments. Traditional medicine is replacing conventional medicine in many parts of the world. Watercress, which is a nutrient-rich leafy plant, traditionally grown in outdoor aquatic systems is more recognized as being suitable for indoor hydroponic systems. Several civilizations regularly eat the herbal plant “watercress” as food. Moreover, it is used in folk medicine as an aid in the treatment of hypertension, bronchitis, asthma and other illnesses. To provide a comprehensive overview on the traditional uses, phytochemistry and pharmacological activities of Nasturtium officinale (N. officinale) with its toxicity data and make recommendations for further research we carried out the chemical analysis of alkaloids, flavonoids, saponins, terpenoids/steroids, protein, essential and volatile oil, glycosides, tannins, folic acid, vitamins, and elements were all found in the plant. Previously reported pharmacological studies have shown that it has lipid-lowering, anti-inflammatory, hepatoprotective, renal-protective, antidiabetic, antioxidant, anticancer, antibacterial and dermatological effects. Therefore, additional studies on the biomarkers are needed to establish mechanism of action and to validate the traditional use of this drug in clinical practice after proper safety assessment.
Keywords
Nasturtium officinale, Traditional medicine, Phytochemistry, Pharmacological effects, Toxicity profile
Introduction
Medicinal plants have been identified and used throughout the ancient era. Toxic plants have even been used in pharmaceutical development (University of Chicago, 1993). Few plants or their phytochemical constituents have been proven to have medicinal effects by rigorous study or have been approved by regulatory agencies such as the United States Food and Drug Administration (USFDA) or European Food Safety Authority (EFSA) (Berkes F and Turner NJ, 2006). Plants have always been used by humans to relieve and cure many diseases (Ramawat KG and Mérillon JM, 2008). Today, in many parts of the world traditional medicinal systems have replaced conventional medicinal systems. The use of plant sources of remedies for the treatment of many diseases dated back to prehistory and people of all continents have this old tradition. The search for agents to cure infectious diseases began long before people were aware of the existence of microbes. These early attempts used natural substances, usually, native plants of their extracts and many of these herbal remedies proved to be successful (Davies J, 1994). Plants serve as the basis of traditional medicine systems for thousands of years in Nigeria, India, China, Indonesia and several other countries (Akinmoladun AC, et al.., 2007). Medicinal plants are now getting more attention than ever because they have the potential of innumerable benefits to society or indeed to all mankind, especially in the line of medicine and pharmacology. The medicinal value of these plants lies in the bioactive phytochemical action on the human body (Goh SH, 1995).
Herbal medicines have been used since ancient civilizations to maintain health and treat diseases. The World Health Organization (WHO) estimates that about three-quarters of the world’s population currently uses herbs and other forms of traditional medicines to get relief from the illness. Even as we commence the new century with its exciting prospect of gene therapy, herbal medicines remain one of the common forms of therapy available to the world’s population (Hussain F, et al.., 2006). Therefore, the present review intended to summarize the utmost possible information information on its traditional uses, phytochemistry, pharmacology and toxicity of N. officinale.
Literature Review
Botanical description of the plant
N. officinale (family: Brassicaceae), is an herbaceous perennial herb commonly known as halim (watercress) (Table 1). It includes many economically important crops and ornamental species. The two (N. officinale and Nasturtium microphyllum) most common and widespread species of the genus is Nasturtium and both are native to Eurasia and Northern Africa and widely naturalized elsewhere in the world (Chopra RN, et al.., 2006). In India, two species, i.e., N. officinale and Nasturtium microphyllum have been grown wildly. The former has been recorded from the Indian states of Tamil Nadu, Goa, Madhya Pradesh, Punjab, Sikkim and Uttarakhand, while latter has been recorded only from the region of Himachal Pradesh and Uttarakhand (Rasheed S, et al.., 2018).
| Kingdom | Plantae |
|---|---|
| Class | Magnoliopsida |
| Subclass | Dilleniidae |
| Order | Brassicales |
| Family | Brassicaceae |
| Genus | Nasturtium |
| Species | officinale W.T Aiton |
Table 1: Plant taxonomy of Nasturtium officinale
Description
N. officinale is a perennial, growing up to 0.5-1 m, much-branched aquatic herb with creeping or floating stems, pinnate leaves and leaflets (Figure 1); sessile, ovate, oblong or obtuse, flowers are white in short racemes, fruits are siliqua, shortly cylindrical and seeds are minute, ovoid and muriculate (Chaudhary SA, et al.., 2018; Prajapati ND and Purohit SS, 2003). It is found in temperate regions throughout the world; watercress thrives alongside or in fresh running water, while commonly found in the wild. It is propagated by seeds via vegetative methods (Plants for a Future, 2024). The species is hermaphrodite (has both male and female organs) and is pollinated by bees and flies. The plant is self-fertile and usually it attracts wildlife. It is suitable for light (sandy), medium (loamy) and heavy (clayey) soils. The suitable pH for plant growth is acid, neutral, and basic (alkaline) soils. It cannot grow in the shady area. However, it prefers wet soil and can grow in water (Blüthner WD, 2020). Watercress production is threatened by certain diseases and pests, such as crooked roots (Spongospora, Cercospora, Xanthomonas, Fasciola, Lemna and Plutella are the main pests and diseases (Deni B, 200 8).

Figure 1: N. officinale in its habitat
Traditional uses
Watercress has a peculiar odor and a slightly bitter taste which is widely used to treat urinary tract infections in children and as an expectorant to treat bronchitis. It has been traditionally used as a vegetable or salad. The leaf part is mostly used as a depurative, diuretic, expectorant, hypoglycaemic, hypolipidemic, odontalgic and stimulant; it is also used for the treatment of different diseases such as pulmonary diseases, hypertension and cardiovascular diseases (Zargari A, 1990; Zargari F, et al.., 2014; Ozen T, 2009). It can also be administered during abdominal pain, using it as an anti-ulcerogenic, in the treatment of scurvy, tuberculosis, bronchitis, influenza and asthma throughout history (Palaniswamy UR, et al.., 2003; Chen L, et al.., 1996; Zeb A, 2015). The herb is officially listed in Germany for use in phytotherapy. According to the German Commission E Monograph (phytotherapy), Nasturtii herba can be used to treat rhinitis in the form of ground fresh or dried plant parts (Chai TT, et al.., 2015). It also plays a crucial role in the German Commission D Monograph on homeopathic medicines (Zafar R, et al.., 2017). Among the indications for its use is the treatment of urinary tract irritation.
N. officinale is also recognized by the EFSA as a safe edible plant and is included in the leafy vegetables, herbs, and edible flowers monograph (Shahani S, et al.., 2017). In addition, it also received positive recognition as a valuable plant for cosmetic production in the European Commission Cosmetic Ingredients (EC’s CosIng) database (Haro G, et al.., 2018).
Chemical constituents
There are different kinds of active secondary metabolites which have been identified and discovered in plants which include glycosides, flavonoids, terpenoids/steroids, saponins, essential, volatile oils, folic acid, tannins, vitamins A, E, C and K, protein, iodine, iron, potassium, sodium and calcium (Ginting H, et al.., 2016; Gonçalves EM, et al.., 2009; Pandey Y, et al.., 2018) (Figure 2). Different nutritional evaluations of plant showed that it contained calories (18 kcal), protein (2.4 g), fat (0.8 g), fiber (1.2 g), Beta (β)-carotene (2016 mcg), vitamin A (336 mcg), vitamin B1 (0.13 mg), vitamin B6 (0.18 mg), vitamin C (50 mg), vitamin E (1.17 mg), folate (36 mcg), vitamin K (200 mcg), calcium (136 mg), iodine (12 mcg), iron (1.8 mg), magnesium (12 mg), manganese (0.5 mg), phosphorus (42 mg), potassium (184 mg), zinc (0.6 mg) and selenium (1.6 mcg). Additionally, it also contained sodium (68.8 mg/100 g) and copper (0.58 mg/100 g) (Pradhan S, et al.., 2015; Al-Snafi AE, 2015).
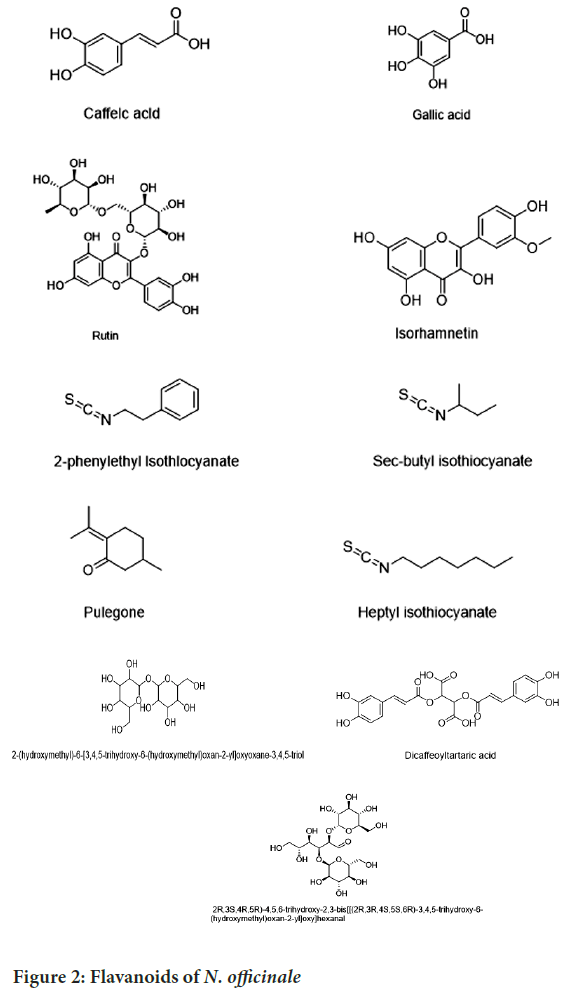
Figure 2: Flavanoids of N. officinale
N. officinale is a rich source of phenyl ethyl glucosinolate (benzyl glucosinolates, the precursors of phenyl ethyl isothiocyanate and benzyl isothiocyanates. Glucosinolate, (gluconasturtin, 5.32 g of gluconasturtin/100 g pneuof defatted seed) was the most abundant glucosinolate in plant, followed by indole glucosinolates (glucobrassicin, 4-methoxy glucobrassicin, 4-hydroxy glucobrassicin and the aliphatic glucosinolate glucoibarin) (Gil V and MacLeod AJ, 1980; Rubin E, et al.., 2018; Lee TH, et al.., 2019). The methanolic extract of the plant included sixteen components, including the following, (6-ohydrocinnamoyl-bis(1-deoxy-1-thio-D-glucopyranosyl)-1,1’-disulfide; ohydrocinnamoyl-bis(1-deoxy-1-thio-D-glucopyranosyl)- 1,1′-disulfide; bis(1-deoxy-1-thio-D-glucopyranosyl)-1 (methylsulfonyl) nonanenitrile, 7-(methylsulfinyl)-heptanenitrile, 8-(methylsulfinyl)-heptanenitrile, and 9-(methylsulfinyl)-heptanenitrile; syringing; sinapic aldehyde 4-O-D-glucopyranoside, 1-sinapoyl-D-glucopyranoside, 1,2-di-O-E-sinapoyl-gentiobiose, and 6-O-D-glucopyranosyl- 1-(3-(4-hydroxy-3,5-dimethoxyphenyl)-2 propanate)-D-glucopyranoside are all examples of glucose and lycibarbarphenylpropanoid C (Aires A, et al.., 2013).
In a study, the total phenolic content and total flavonoid content in the organic baby-leaf watercress was (14.00 ± 27 mg Gallic Acid Equivalents (GAE) kg-1) dry weight and (5600 ± 10 mg GAE kg-1) dry weight, respectively. The main phenolics identified were caffeic acid (University of Chicago, 1993), gallic acid (Berkes F and Turner NJ, 2006), chlorogenic acid, quercetin-3-O-rutinoside (Ramawat KG and Mérillon JM, 2008), isorhamnetin (Davies J, 1994), dicaffeoyltartaric acid (Chaudhary SA, et al.., 2018). Quercetin-3-O-rutinoside (Prajapati ND and Purohit SS, 2003) and isorhamnetin represent almost 70% of the total individual phenolic content and these all compounds are often associated with decreasing cardiovascular and inflammatory risks (Camponogara C, et al.., 2019). The potential anti-inflammatory activity of watercress also proved that the watercress extract could be useful for irritant contact dermatitis (Afsharypuor S and Salehi MS, 2008).
Volatile constituents of the dried leaves and stems of N. officinale collected after hydrolysis were analyzed by Gas Chromatography (GC) and GC/ Mass Spectrometry (GC/MS). Fifteen components in the leaves and 11 in the stems of the flowering plant were identified. 2-phenyl ethyl isothiocyanate (Nasturtiin) constitutes the major volatile component of both the leaves (72.9%) and stems (83.5%) of the plant. The major volatile constituents of the leaves were 2-phenyl ethyl isothiocyanate (72.9%) (Akinmoladun AC, et al.., 2007), pulegone (8.0%), heptyl isothiocyanate (4.9%) (Chopra RN, et al.., 2006) and 4-phenyl butyl isothiocyanate (3.2%), while the main volatile constituents of the stems were 2-phenyl ethyl isothiocyanate (83.5%), 4-phenyl butyl isothiocyanate (6.9%), pulegone (2.2%) (Hussain F, et al.., 2006) and sec-butyl isothiocyanate (1.9%) (Goh SH, 1995) (Bahramikia S and Yazdanparast R, 2010). Various compounds isolated from watercress are demonstrated.
Discussion
Pharmacological effects of the plant (N. officinale) have briefly been discussed.
Cardioprotective activity
Based on the study, it is presumed that the N. officinale Hydroalcoholic Extract (HAE) has distinct cardioprotective potential. Intragastric organization of HAE (500 mg/kg body weight each day) to different groups of hypercholesterolaemic rodents for 10 days decreased their serum Total Cholestrol (TC), Triglycerides (TG) and Low Density Lipoprotein-Cholesterol (LDL-C) by 34.2%, 30.1% and 52.9%, separately, while raised the serum High Density Lipoprotein-Cholesterol (HDL-C) level by 27.0% following 10 days of test administration. Treatment with HAE diminished serum Alanine Transaminase (ALT) and Aspartate Aminotransferase (AST) levels (Zeb A, 2015). Furthermore, the study has shown that extracts of plants have angiotensin-changing over compound inhibitory movement (Esteban R, et al.., 2015).
Antioxidant activity
Watercress has been for quite some time utilized as a home cure or a restorative plant by individuals. Various examinations were done to research the antioxidant activity of N. officinale extract utilizing different in vitro tests, ferric reducing antioxidant power and 2, 2- azinobis (3-ethylbenzothiazoline-6-sulfonate) (ABTS) assays, 1,1-Diphenyl-2-Picrylhydrazyl (DPPH), Hydrogen peroxide (H2O2), Nitric oxide (NO) radical scavenging, and Ferrous (Fe2+) ion chelating activity including the inhibitory effect on Fe2+/ascorbate induced lipid peroxidation, in rat liver. The outcomes have shown that plant extract has significant reducing power in a ferric-reducing antioxidant power assay, concentration-dependent scavenging ability on ABTS, DPPH, NO radicals and H2O2, as well as chelating ability on Fe2+ ions. Besides, plant extract prevented thiobarbituric acid reactive substances formation in Fe2+ ion/ascorbate induced lipid peroxidation in rat liver in a dose-dependent manner. In addition, N. officinale extract had the 96.2 mg GAE/g dried extract of phenolic and 63.2 mg of flavonoid catechin equivalents/g dried extract, respectively (Bahramikia S and Yazdanparast R, 2008). In other findings, phenolic profile, antioxidant potential and pigment contents of wild watercress were studied. Extracts of different parts of plants were investigated which included roots, stems and leaves, for the determination of total phenolic contents, pigment composition and free radical scavenging activity. 14 phenolic compounds were identified in the leaves, where coumaric acid and its derivatives (caftaric acid and quercetin derivatives) were determined significantly. In roots, a total of 20 compounds were identified, with coumaric acid and its derivatives, sinapic acid, caftaric acid and quercetin derivatives being the major phenolic compounds. Watercress has significant antioxidant activity and contains important phenolic compounds, which showed potential biological interest (Yaricsha CA, 2017; Bayrami A, et al.., 2019).
Antidiabetic activity
The leave extract was subjected through an ultrasound-facilitated technique and used for the preparation of Zinc oxide (ZnO) nanoparticles by means of a joint ultrasound-microwave helped technique and controlled by Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-Ray Diffraction (XRD), Energy Dispersive X-ray (EDX), Brunauer-Emmett-Teller (BET), Fourier Transform Infrared (FTIR) spectroscopy, Thermogravimetric Analysis (TGA), and Ultraviolet-Visible Diffuse Reflectance (UV-Vis DR) spectroscopy investigations. The presence of carbon and carbonaceous bonds, changes in the morphology, size, band gap energy and weight-decay percentage were in various concentrations among ZnO and extract/ZnO that affirmed the connection of extract over nanoparticles. Extract/ZnO, watercress leaf extract, ZnO and insulin treatments were administered to treat alloxan-diabetic Wister rats and their healing effectiveness results were compared with each other. The serum levels of the primary diabetic records like insulin, fasting blood glucose and lipid profile (TG, TC and HDL-C) were assessed for healthy, diabetic and the rats restored their health with the studied therapeutic agents. The watercress extract improved ZnO nanoparticles, offered the best execution and suppressed the diabetic status of rats. Besides, both ZnO samples satisfactorily inhibited the microscopic organisms, Staphylococcus aureus and Escherichia coli. As an outcome, the utilization of plant leaf extract can firmly engage ZnO nanoparticles towards prevalent antidiabetic and improved antibacterial activity (Gill CI, et al.., 2007; Zafar R, et al.., 2017).
Antibacterial activity
The methanolic extract of N. officinale was investigated for its antimicrobial action. In this investigation, the antibacterial activity Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the methanolic extract of plant against Escherichia coli, Klebsiella pneuof monia, Enterococcus faecalis and Bacillus cereus were resolved. The extract showed antibacterial action and the most elevated antibacterial movement was recorded for Bacillus cereus. MIC and MBC were likewise decided where the MIC against escherichia coli was 0.06, Klebsiella pneumonia was 0.04, Enterococcus faecalis was 0.08 and Bacillus cereus was 0.06. MBC was additionally decided against Escherichia coli which was 0.10, 0.08 against Klebsiella pneumonia, 0.10 against Enterococcus faecalis and 0.08 against Bacillus cereus was 0.08 (Ek P, et al.., 2018).
Nutraceutical potential
Dehydrated watercress can possibly be a novel item, for food plans or food supplements because of its high nutritional content. Watercress is enriched with supplements like vitamin C, minerals and other bioactive mixtures. For example, phenolic and antioxidants that may diminish cancer occurrence. As per the investigation, the assessment of the drying qualities and the impacts of convective drying temperature on quality credits of watercress were determined with no pretreatment. Drying temperatures of 40°, 55° and 70° brought about drying times of 230 min, 119 min and 92 min, separately. The nutritional parameters were negatively correlated with the drying temperature. Content of vitamin C, total phenolic compounds and total antioxidant capacity were calculated individually. Expanded estimations of Total Color Difference (TCD), Browning Index (BI) and higher chlorophylls losses were likewise seen as the drying temperature expanded. In conclusion, longer drying periods are needed for lower drying temperatures just as more productive regarding watercress nourishing features and overall quality to the consumer (Alibas I, 2010; Kaskey JB and Tindall DR, 1979; Sadeghi H, et al.., 2014).
Anti-inflammatory activity
N. officinale has some time been utilized in Iranian society medication to treat hypertension, hyperglycemia and renal colic. In addition, anticancer, antioxidant and hepatoprotective properties of plants have been accounted for. In this examination, anti-inflammatory activity of the hydro-alcoholic concentrate from aerial parts of plant (250, 500 and 750) mg/kg was examined on two standard animal models of inflammation, including carrageenan-or formalin-induced paw edema in rats. At that point, the effective anti-inflammatory activity of the plant (2 and 5) mg/kg) was concentrated on 12-O-Tetradecanoylphorbol-13-acetate (TPA) induced mouse ear edema. At last, biopsy of the paw or ear was performed for pathological assessment. Acute toxicity trial of watercress in rats proposed an oral Lethal Dose (LD50) of 45 g/kg. The extract (250, 500 and 750) mg/kg significantly restrained carrageenan induced paw edema 1, 2, 3 and 4 h after carrageenan challenge (p ≤ 50.001). The extract (500 mg/kg) likewise showed extensive action against formalin-induced paw edema over a time of 24 h (p ≤ 50.001). Moreover, topical utilization of plant (5 mg/kg) diminished TPA-induced ear edema (p ≤ 50.05). Histopathologically the extract decreased swelling and tissue damage induced by carrageenan or TPA. Investigations indicate significant anti-inflammatory activity of plant in systemic and topical application and suggest its potential as an anti-inflammatory agent for treatment of inflammatory conditions (Akkol EK, et al.., 2008; Shahani S, et al.., 2017). The aim of another study was to assess the part of inflammation and also oxidative damage in nephrotoxic capability of Gentamicin (GM) and defensive impacts of watercress against GM-actuated nephrotoxicity in Wistar rats. Reactive Oxygen Species (ROS), Glutathione (GSH) content, Lipid Peroxidation (LPO), Protein Carbonyl (PCO), NO and Tumor Necrosis Factor-Alpha (TNF-α) were assessed in kidney tissues and furthermore neurotic assessment and estimating of Blood Urea Nitrogen (BUN) and Creatinine (Cr) were finished. The intake of GM for 10 days brought about an increase in kidney markers (BUN and Cr) and neurotic changes in kidney tissue. Additionally, stress was significant in the GM group by expanded ROS, LPO and PCO level and GSH oxidation. Increases in inflammation measure appeared by expansion in NO and TNF-α. Administration of watercress extract had the option to secure against weakening of nephrotoxic markers and stifled the expansion in oxidative pressure and inflammation. The examination showed the critical role of oxidative damage and inflammation in GM initiated nephrotoxicity that was especially restrained by administration of watercress (Zeb A, 2015, Kadkhodaee M, et al.., 2007; Karami M, et al.., 2015).
Protective effects
The examination was done to explore the protective action of the methanolic extract of N. officinale against gamma-radiation-initiated hepatotoxicity as far as histopathological changes. Pre-treatment with 100 mg/kg body weight each day for 15 days and 2 h previously γ-radiation essentially brought down occurrence of inflammation. Besides, liver cells necrosis, edema and congestion were somewhat decreased. Total phenolic and total flavonoid contents of the extract were (11.3 ± 0.4) mg GAE and (9.4 ± 0.7) mg quercetin GAE of dried extract. This significant result can determine the presence of phenols and isothiocyanates in the extract of N. officinale which act as antioxidants and anti-inflammatory agents (Carrasco G, et al.., 2011; Ozen T, 2009; Karami M, et al.., 2018).
Nephroprotective activity
This study was intended to explore the impact of N. officinale Hydro-Alcoholic Extract (HAE) and vitamin E against Vancomycin (VCM) instigated nephrotoxicity in grown-up male Wistar rats. VCM fundamentally increased serum creatinine and urea levels, Malondialdehyde (MDA) levels, kidney weight/100 mg and diminished clearance. HAE (250, 500 mg/kg) and vitamin E (500 mg/kg) pretreatment impressively mitigated these progressions when contrasted and VCM treated alone. Histological assessment of the VCM-treated group showed a significant renal injury with tubular epithelial cell desquamation, swelling, and tubular dilatation. These progressions were moderated with HAE and vitamin E. The information shows that HAE clearly constricted VCM-initiated nephrotoxicity (Abdel-Naim AB, et al.., 1999; Clemente M, et al.., 2019).
Toxicological effects
The aim of this study was to evaluate the safety of the Standardized Extract of N. officinale (SENO) with phenyl ethyl glucosinolate (5.0 mg/ml), utilizing acute and sub-acute oral dosage in Wistar rats. In the acute toxicity study dose assessed was LD50 in the range of 2000-5000 mg/kg, indications of mortality and toxicity in female rats were observed for 14 days, after single doses of 2000 and 5000 mg/kg. In the subacute examination, female and male rats, aged 10 weeks, were enhanced at doses of 250, 500 and 1000 mg/kg for 28 days. No critical changes were accounted for with respect to the acute study, while the sub-acute study showed no toxicity of the hematopoietic and biochemical systems. The outcomes showed that SENO at a dose up to 5000 mg/kg in the acute study was safe and No-Observed-Adverse-Effect Level (NOAEL) in the sub-acute dose was up to 1000 mg/kg. The outcomes, for acute and sub-acute oral toxicity studies in rats, may give strong proof in regards to the safety of SENO to its application in future pharmacological investigations and clinical trials, and its resulting use in the phyto-drug industry as a natural medication item and SENO at measurements up to 5000 mg/kg was safe for acute study (Fuchs TC, et al.., 2012; Ginting H, et al.., 2016). However, the acute toxicity of ethanolic extract (0.5, 5, 50, 500, 1000, 2000, and 4000 mg/kg body weight) of N. officinale was concentrated in mice. The maximal portion caused no death; animals were still in normal circumstances. No huge differences were observed in relative organ weights for the liver, heart and kidneys in mice in all doses. Histopathological study showed that the highest doses caused necrosis and hydropic degeneration of the liver and kidneys and heart inflammatory manifestation with myofibril irregular heartbeat (di Noia J, 2014; Bettega PV, et al.., 2016).
Conclusion
Watercress is a perennial herb that grows in flowing water bodies and is tetraploid in nature with an unknown diploid ancestor. N. officinale has been used by several ethnic groups for various therapeutic and dietary purposes. It is regarded as the food with the highest nutritional value, and the largest concentration of vitamins, minerals and phytonutrients, which makes it a “powerhouse” of food. The present review, investigated the chemical components, pharmacological properties, and therapeutic potential of plant. It is a remarkable medicinal plant with a wide variety of pharmacological activities that could be used in a number of medical applications due to its potent efficacy.
Moreover, watercress is a rich source of calcium, potassium, salt, and magnesium, which are vital for human health. We can infer from this study that watercress indeed is a potent herb which acts as a great pharmacological and food source. Despite its ethnobotanical and ethnoveterinary importance and the ready availability of this plant, no studies demonstrating its efficacy in treating traumatic ulcers, which are very common in dentistry, have been conducted so far.
References
- Angiosperms Division Magnoliophyta General Features Britannica. University of Chicago. 1993.
- Berkes F, Turner NJ. Knowledge, learning and the evolution of conservation practice for social-ecological system resilience. J Hum Ecol. 2006; 34: 479-494.
- Ramawat KG, Mérillon JM. Bioactive Molecules and Medicinal Plants. Berlin: Springer. 2008.
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994; 264(5157): 375-382.
[Crossref] [Google Scholar] [Pubmed]
- Akinmoladun AC, Ibukun EO, Afor E, Obuotor EM, Farombi EO. Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Sci Res Essay. 2007; 2(5): 163-166.
- Goh SH. Malaysian Medicinal Plants for the Treatment of Cardiovascular Diseases. 1995.
- Hussain F, Islam M, Zaman A. Ethnobotanical profile of plants of Shawar valley, district Swat, Pakistan. Int J Biol Biotechnol. 2006; 3(2): 301-307.
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants, National Institute of Science Communication and Information Resources. New Delhi: CSIR. 2006.
- Rasheed S, Khuroo AA, Ganie AH, Mehraj G, Dar GH. Correct taxonomic delimitation of Nasturtium microphyllum Rchb. from Nasturtium officinale R. Br. (Brassicaceae) in Kashmir Himalaya, India. J Asia-Pac Biodivers. 2018; 11(1): 154-157.
- Chaudhary SA, Hisham HA, Mohamed DO. A review on phytochemical and pharmacological potential of watercress plant. Asian J Pharm Clin Res. 2018; 11(12): 102-107.
- Prajapati ND, Purohit SS, Sharma AK, Kumar T. In A Handbook of Medicinal Plants: A Complete Source Book. 2003.
- Nasturtium officinale-R.Br. Plants for a Future. 2024.
- Blüthner WD. Nasturtium officinale R. Br.: Watercress. Medicinal, Aromatic and Stimulant Plants. 2020.
- Deni B. Encyclopaedia of herbs and their uses. The Royal Horticulture Society, London, UK. 2008: 181.
- Zargari A. Medicinal plants, vol 1, Tehran. 1990.
- Zargari F, Ghorbanihaghjo A, Babaei H, Farajnia S, Roodbari NH. The effect of hydroalcoholic extract of Nasturtium officinale R. Br on antioxidant status and DNA damage in liver and kidney rats exposed to arsenic. Med J Tabriz Univ Med Sci. 2014; 36(3): 44-51.
- Ozen T. Investigation of antioxidant properties of Nasturtium officinale (watercress) leaf extracts. Acta Pol Pharm. 2009; 66(2): 187-193.
[Google Scholar] [Pubmed]
- Palaniswamy UR, McAvoy RJ, Bible BB, Stuart JD. Ontogenic variations of ascorbic acid and phenethyl isothiocyanate concentrations in watercress (Nasturtium officinale R. Br.) leaves. J Agric Food Chem. 2003; 51(18): 5504-5509.
[Crossref] [Google Scholar] [Pubmed]
- Chen L, Mohr SN, Yang CS. Decrease of plasma and urinary oxidative metabolites of acetaminophen after consumption of watercress by human volunteers. Clin Pharmacol Ther. 1996; 60(6): 651-660.
[Crossref] [Google Scholar] [Pubmed]
- Zeb A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L). Springerplus. 2015; 4(1):714.
[Crossref] [Google Scholar] [Pubmed]
- Chai TT, Ooh KF, Quah Y, Wong FC. Edible freshwater macrophytes: A source of anticancer and antioxidative natural products-A mini-review. Phytochem Rev. 2015; 14: 443-457.
- Zafar R, Zahoor M, Shah AB, Majid F. Determination of antioxidants and antibacterial activities, total phenolic, polyphenol and pigment contents in Nasturtium officinale. Pharmacologyonline. 2017; 1: 11-18.
- Shahani S, Behzadfar F, Jahani D, Ghasemi M, Shaki F. Antioxidant and anti-inflammatory effects of Nasturtium officinale involved in attenuation of gentamicin-induced nephrotoxicity. Toxicol Mech Methods. 2017; 27(2): 107-114.
- Haro G, Iksen I, Rumanti RM, Marbun N, Sari RP, Gultom RP. Evaluation of antioxidant activity and minerals value from watercress (Nasturtium officinale R. Br.). Rasayan J Chem. 2018; 11(1): 232-237.
- Ginting H, Dalimunthe A, Reveny J. Acute toxicity effect of the ethanolic extract of watercress herb (Nasturtium officinale R. Br.) in mice. Indones J Cancer Chemoprev. 2016; 7(1): 9-16.
- Gonçalves EM, Cruz RM, Abreu MT, Brandão TR, Silva CL. Biochemical and colour changes of watercress (Nasturtium officinale R. Br.) during freezing and frozen storage. J Food Eng. 2009; 93(1): 32-39.
- Pandey Y, Bhatt SS, Debbarma N. Watercress (Nasturtium officinale): A potential source of nutraceuticals. Int J Curr Microbiol App Sci. 2018; 7(2): 2685-2691.
- Pradhan S, Manivannan S, Tamang JP. Proximate, mineral composition and antioxidant properties of some wild leafy vegetables. J Sci Ind Res. 2015; 74: 155-159.
- Al-Snafi AE. Encyclopedia of the constituents and pharmacological effects of Iraqi medicinal plants. Khanna: Rigi Publication. 2015.
- Gil V, MacLeod AJ. Degradation of glucosinolates of Nasturtium officinale seeds. Phytochemistry. 1980; 19(8): 1657-1660.
- Rubin E, Aziz ZA, Surugau N. Glucosinolates content of in vitro grown Nasturtium officinale (watercress). ASM Sci J. 2018; 11: 132-139.
- Lee TH, Khan Z, Subedi L, Kim SY, Lee KR. New bis-thioglycosyl-1, 1′-disulfides from Nasturtium officinale R. Br. and their anti-neuroinflammatory effect. Bioorg Chem. 2019; 86: 501-506.
[Crossref] [Google Scholar] [PubMed]
- Aires A, Carvalho R, Rosa EA, Saavedra MJ. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CyTA-J Food. 2013; 11(4): 343-351.
- Camponogara C, Silva CR, Brusco I, Piana M, Faccin H, de Carvalho LM, et al. Nasturtium officinale R. Br. effectively reduces the skin inflammation induced by croton oil via glucocorticoid receptor-dependent and NF-κB pathways without causing toxicological effects in mice. J Ethnopharmacol. 2019; 229: 190-204.
[Crossref] [Google Scholar] [Pubmed]
- Afsharypuor S, Salehi MS. Volatile constituents of leaves and stems of Nasturtium officinale R. Br. J Essent Oil Res. 2008; 20(6): 517-518.
- Bahramikia S, Yazdanparast R. Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. J Acupunct Meridian Stud. 2010; 3(4): 283-290.
[Crossref] [Google Scholar] [Pubmed]
- Zeb A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). Springerplus. 2015; 4(1): 714.
[Crossref] [Google Scholar] [Pubmed]
- Esteban R, Barrutia O, Artetxe U, Fernández‐Marín B, Hernández A, García‐Plazaola JI. Internal and external factors affecting photosynthetic pigment composition in plants: A meta‐analytical approach. New Phytol. 2015; 206(1): 268-280.
[Crossref] [Google Scholar] [Pubmed]
- Bahramikia S, Yazdanparast R. Effect of hydroalcoholic extracts of Nasturtium officinale leaves on lipid profile in high-fat diet rats. J Ethnopharmacol. 2008; 115(1): 116-121.
[Crossref] [Google Scholar] [Pubmed]
- Yaricsha CA. ACE inhibitory activity, total phenolic and flavonoid content of watercress (Nasturtium officinale R. Br.) extract. Pharmacog J. 2017; 9(2).
- Bayrami A, Ghorbani E, Pouran SR, Habibi-Yangjeh A, Khataee A, Bayrami M. Enriched zinc oxide nanoparticles by Nasturtium officinale leaf extract: Joint ultrasound-microwave-facilitated synthesis, characterization, and implementation for diabetes control and bacterial inhibition. Ultrason Sonochem. 2019; 58: 104613.
[Crossref] [Google Scholar] [Pubmed]
- Gill CI, Haldar S, Boyd LA, Bennett R, Whiteford J, Butler M, et al. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am J Clin Nutr. 2007; 85(2): 504-510.
[Crossref] [Google Scholar] [Pubmed]
- Zafar R, Zahoor M, Shah AB, Majid F. Determination of antioxidants and antibacterial activities, total phenolic, polyphenol and pigment contents in Nasturtium officinale. Pharmacologyonline. 2017; 1: 11-8.
- Ek P, Araujo AC, Oliveira SM, Ramos IN, Brandao TR, Silva CL. Assessment of nutritional quality and color parameters of convective dried watercress (Nasturtium officinale). J Food Process Preserv. 2018; 42(2): e13459.
- Alibas I. Determination of drying parameters, ascorbic acid contents and color characteristics of nettle leaves during microwave‐, air‐and combined microwave-air‐drying. J Food Process Eng. 2010; 33(2): 213-233.
- Kaskey JB, Tindall DR. Physiological aspects of growth and heteroblastic development of Nasturtium officinale under natural conditions. Aquat Bot. 1979; 7: 209-229.
- Sadeghi H, Mostafazadeh M, Sadeghi H, Naderian M, Barmak MJ, Talebianpoor MS, et al. In vivo anti-inflammatory properties of aerial parts of Nasturtium officinale. Pharm Biol. 2014; 52(2): 169-174.
[Crossref] [Google Scholar] [Pubmed]
- Akkol EK, Yalçın FN, Kaya D, Çalış İ, Yesilada E, Ersöz T. In vivo anti-inflammatory and antinociceptive actions of some Lamium species. J Ethnopharmacol. 2008; 118(1): 166-172.
[Crossref] [Google Scholar] [Pubmed]
- Shahani S, Behzadfar F, Jahani D, Ghasemi M, Shaki F. Antioxidant and anti-inflammatory effects of Nasturtium officinale involved in attenuation of gentamicin-induced nephrotoxicity. Toxicol Mech Methods. 2017; 27(2): 107-114.
[Crossref] [Google Scholar] [Pubmed]
- Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplant Proc. 2007; 39(4): 864-865.
[Crossref] [Google Scholar] [Pubmed]
- Karami M, Nosrati A, Naderi M, Makhloogh M, Shahani S. Protective effects of Nasturtium officinale against gamma-irradiation-induced hepatotoxicity in C57 mice. Res J Pharmacog. 2015; 2(2): 19-25.
- Carrasco G, Moggia C, Osses IJ, Álvaro JE, Urrestarazu M. Use of peroxyacetic acid as green chemical on yield and sensorial quality in watercress (Nasturtium officinale R. Br.) under soilless culture. Int J Mol Sci. 2011; 12(12): 9463-9470.
[Crossref] [Google Scholar] [Pubmed]
- Karami M, Mostafazadeh M, Sadeghi H, Sadeghi H, Mehraban F, Kokhdan EP, et al. Nephroprotective effect of Nasturtium officinale (watercress) ethanol extract and vitamin E on vancomycin-induced nephrotoxicity in rats. Jundishapur J Nat Pharm Prod. 2018; 13(1).
- Abdel-Naim AB, Abdel-Wahab MH, Attia FF. Protective effects of vitamin E and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacological Research. 1999; 40(2): 183-187.
[Crossref] [Google Scholar] [Pubmed]
- Clemente M, Miguel MD, Felipe KB, Gribner C, Moura PF, Rigoni AG, et al. Acute and sub-acute oral toxicity studies of standardized extract of Nasturtium officinale in Wistar rats. Regul Toxicol Pharmacol. 2019; 108: 104443.
[Crossref] [Google Scholar] [Pubmed]
- Fuchs TC, Frick K, Emde B, Czasch S, Landenberg FV, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicologic Pathol. 2012; 40(7): 1031-1048.
[Crossref] [Google Scholar] [Pubmed]
- Ginting H, Dalimunthe A, Reveny J. Acute toxicity effect of the ethanolic extract of watercress herb (Nasturtium officinale R. Br.) in mice. Indones J Cancer Chemoprevent. 2016; 7(1): 9-16.
- di Noia J. Defining powerhouse fruits and vegetables: A nutrient density approach. Prev Chronic Dis. 2014; 11.
[Crossref] [Google Scholar] [Pubmed]
- Bettega PV, Johann AC, Alanis LR, Bazei IF, Miguel OG. Experimental confirmation of the utility of Nasturtium officinale used empirically as mouth lesion repairing promotor. Clin Exp Pharmacol. 2016; 5(201): 2161-1459.
Author Info
Nitisha Negi1,2*, Sukirti Upadhyay1 and Mahendra Rana22Department of Pharmaceutical Sciences, Kumaun University, Nainital, Uttarakhand, India
Citation: Negi N: An Overview on Phytopharmacological Perspectives of a Potential plant Species: Nasturtium officinale
Received: 06-Aug-2024 Accepted: 22-Aug-2024 Published: 29-Aug-2024, DOI: 10.31858/0975-8453.15.8.257-262
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






