Review Article - (2021) Volume 12, Issue 11
An Overview on Various Analytical Methods for Estimation of Atenolol and Amiodarone from its Bulk and Pharmaceutical Dosage Forms
Sadaf Arshad Rangrez* and Vinayak GawareAbstract
The main objective of this review is to unify and interpret widely scattered information of reported studies on potential, reliable and efficient analytical methodologies which can estimate Atenolol and Amiodarone separately. The information and suggested outlined below may facilitate and guide further needed studies to optimize the use of analytical techniques like High Performance Liquid Chromatography (HPLC), Bioanalytical Methods, UV Spectroscopy, Stability indicating RP-HPLC methods etc. for determination of Atenolol and Amiodarone in formulation. From the reviewed literature it is obvious that HPLC is a commonly available method of testing in pharmaceutical laboratory so this method should be of choice for complete determination of Atenolol and Amiodarone. Selection of analytical methods is determined by several factors such as speed, convenience, specificity, accuracy, precision, sensitivity, selectivity, cost, availability of instruments, technical expertise and the number of samples to be analyzed.
Keywords
Atenolol, Amiodarone, Analytical estima - tion, HPLC, UV
Introduction
Atenolol is a beta blocker medication primarily used to treat high blood pressure and heart-associated chest pain. Atenolol, however, does not seem to improve mortality in those with high blood pressure (AHFS, 2018; Tomiyama H and Yamashina A, 2014; DiNicolantonio JJ, et al., 2015). Other uses include the prevention of migraines and treatment of certain irregular heartbeats. It is taken by mouth or by injection into a vein. It can also be used with other blood pressure medications (British National Formulary, 2018).
Common side effects include feeling tired, heart failure, dizziness, depression, and shortness of breath. Other serious side effects include bronchospasm. Use is not recommended during pregnancy and alternative drugs are preferred when breastfeeding. It works by blocking β1-adrenergic receptors in the heart, thus decreasing the heart rate and workload.
Atenolol was patented in 1969 and approved for medical use in 1975. It is available as a generic medication. In 2018, it was the 42nd most commonly prescribed medication in the United States, with more than 18 million prescriptions (Beard Jr EL, 2001; Ali MU, et al., 2018; Florey K, 1981; Akiful HM, et al., 2012; Godge RK, et al., 2017) (Figure 1).
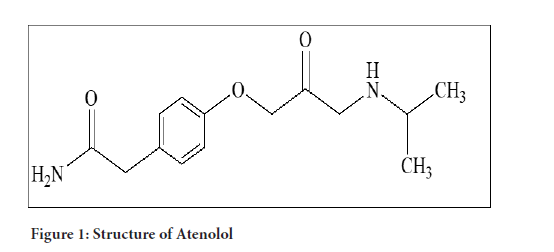
Figure 1:Structure of Atenolol
Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of irregular heartbeats (Beard Jr EL, 2001). This includes Ventricular Tachycardia (VT), Ventricular Fibrillation (VF), and wide complex tachycardia, as well as atrial fibrillation and paroxysmal supraventricular tachycardia (Ali MU, et al., 2018). Evidence in cardiac arrest, however, is poor. It can be given by mouth, intravenously, or intraosseously. When used by mouth, it can take a few weeks for effects to begin. Common side effects include feeling tired, tremor, nausea, and constipation (Florey K, 1981). As amiodarone can have serious side effects, it is mainly recommended only for significant ventricular arrhythmias. Serious side effects include lung toxicity such as interstitial pneumonitis, liver problems, heart arrhythmias, vision problems, thyroid problems, and death If taken during pregnancy or breastfeeding it can cause problems in the fetus. It is a class III antiarrhythmic medication. It works partly by increasing the time before a heart cell can contract again.
Amiodarone was first made in 1961 and came into medical use in 1962 for chest pain believed to be related to the heart. It was pulled from the market in 1967 due to side effects. In 1974 it was found to be useful for arrhythmias and reintroduced. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2017, it was the 196th most commonly prescribed medication in the United States, with more than two million prescriptions (Figure 2).
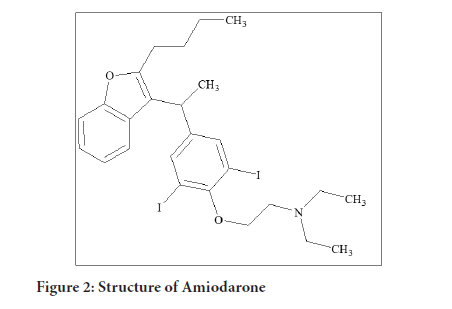
Figure 2: Structure of Amiodarone
Analytical Methods
Analytical methods development and validation play important roles in the discovery, development, and manufacture of pharmaceuticals. Pharmaceutical products formulated with more than one drug, typically referred to as combination products, are intended to meet previously unmet patients need by combining the therapeutic effects of two or more drugs in one product. These combination products can present daunting challenges to the analytical chemist responsible for the development and validation of analytical methods. This review contains the various simultaneous estimation methods (spectrophotometric, High Performance Liquid Chromatography (HPLC) and High-Performance Thin Layer Chromatography (HPTLC) which are employed for the quantitative estimation of drug products containing antihypertensive analytes. The official test methods that result from these processes are used by quality control laboratories to ensure the identity, purity, potency, and performance of drug products.
Validation of RP-HPLC method
The developed method for estimating Atenolol and amiodarone was validated for the following parameters according to ICH guidelines (Nilesh S, et al., 2021; Sabir AM, 2013; ICH I, 2005; Hausherr A, et al., 2020; Pendbhaje NS, et al., 2021; Sule S, et al., 2014).
Filtration analysis: A filtration study of an analytical technique explores the filter's interference with extraneous materials, deposition on the filter bed, and filter compatibility with the sample.
Specificity: Specificity is the capacity to access the analyte unequivocally in the presence of potentially present components.
Linearity and range: The ability of an analytical method to elicit test results that are proportional to the concentration of an analyte in samples within a given range, either directly or through a well-defined mathematical transformation.
Decisiveness The statistical treatment of test results obtained by examination of samples with analyte concentrations around the claimed spectrum determines the analytical method's linearity.
As a function of analyte concentration, the region is graphically plotted. Curve fitting percentages are measured. Acceptance is a state of mind. Criteria include: The plot should be linear, with the origin at the middle. The correlation coefficient (r2) must be greater than 0.999.
Accuracy (%Recovery): The closeness of agreement between the value accepted as a standard true value or an accepted reference value and the value of the value found is expressed by the analytical procedure's accuracy.
Criteria for acceptance: The average recovery rate should be between 98.00 and 102.00 percent. The Relative Standard Deviation (RSD) does not exceed 2.0%.
Precision: When a technique is applied repeatedly to several Samplings of a homogeneous sample, the precision of an analytical method is the degree of agreement among individual test results. A Standard Deviation or Relative Standard Deviation is used to express the accuracy of an empirical system. There are two levels of precision: repeatability and intermediate precision. It is carried out on a sample API. From the same sample matrix, render six separate test solutions of the 100 percent test concentration. Every test solution should be injected twice.
Accuracy in the middle:
Precisely in the middle of the day: It is carried out by making another researcher analyse the data on a different day to ensure that the findings are repeatable. Samples prepared in the same way that the Repeatability parameter samples were (6 Samples prepared).
Criteria for acceptance: For test results, the percent RSD of 6 samples
NMT 2.0% was used.
NMT 2.0% for test results, percent RSD of total 12 samples.
(6 of Repeatability and 6 of Intermediate precision)
Robustness: The robustness of an analytical technique is a measure of its ability to remain unaffected by minor yet deliberate changes in system parameters, and it indicates its efficiency during regular use.
Detection:
Limit Of Detection (LOD): Under the specified experimental conditions, the lowest concentration of the analyte in the sample that the system can detect but not necessarily quantify simply means that the sample is below or above a certain threshold. Limits are defined in percentages or parts per million. The detection limit will be calculated not only by the measurement technique, but also by the type of instrument used.
S/N= 2/1 or 3/1
Where, S=Signal, N=Noise It may be calculated based on the Standard Deviation (SD) of the response and slope of the curve(S).
LOD=3.3 (SD)/S
Where, SD=Standard deviation, S=Slope
Limit Of Quantitation (LOQ): The lowest amount of analyte in a sample that can be calculated with reasonable precision and accuracy under the specified experimental conditions is known as the limit of quantitation (LOQ). It is expressed as the percentage of analyte in the sample (e.g., parts per billion). The S/N ratio should not be less than 10 and the RSD should be less than 3%.
S/N= 10/1
Where S=Signal N=Noise It may be calculated based on the Standard Deviation (SD) of the response and slope of the curve(S).
LOQ=10 (SD)/S Where, SD=Standard deviation, S=Slope
Experimental work Literature survey revealed that was determined by UV-visible spectroscopy and HPLC. In the current work, the authors have proposed a simple, specific, valid and robust RP-HPLC method for the estimation of Atenolol and amiodarone in pharmaceutical active substance form (Tables 1 and 2).
| Sr. No. | Name of author | Name of journal | Title of article | Analytical conditions |
|---|---|---|---|---|
| UV Spectrophotometric | ||||
| 1 | Madhurai P, et al. (Madhurai P, et al., 2015) | International Journal of Pharmaceutical Chemical and Biological Science | Quantitative estimation of atenolol in pharmaceutical Dosage forms by using visible spectroscopy. | Solvent-Distilled water |
| λ max-549 nm | ||||
| Beer-Lambert’s limits (µg/mL)- | ||||
| 2-10 | ||||
| linear regression equation- | ||||
| Y=0.0572 C+0 .0033 | ||||
| correlation coefficient-0.9990 | ||||
| % RSD-0.319 | ||||
| % Recovery-99.5%% | ||||
| LOD-5.88 µg/mL | ||||
| LOQ-17.83 µg/mL | ||||
| Turbidimetric analysis | ||||
| 1 | Al-Awadie NS and Khudhair AF (Al-Awadie NS and Khudhair AF, 2014) | Iraqi Journal of Science | Determination of Atenolol in pharmaceutical formulations by continuous flow injection analysis via turbidimetric (T180o) and scattered light effect at two opposite position (2N90o) using Ayah 4SW-3D-T180-2N90-Solar-CFI Analyzer | Turbidimetric: T180º |
| Scattered light effect at two opposite position (2N90º). | ||||
| Incident light in namely +90º and -90º | ||||
| Linearity of Atenolol is ranged from (0.1-11) mmol. L-1 | ||||
| Correlation coefficient-0.938 | ||||
| LOD-0.05 mmol. L-1 | ||||
| Kinetic method | ||||
| 1 | Fadnis AG and Agarwal R (Fadnis AG and Agarwal R, 2015) | J Chem Pharm Res | Kinetic method for estimation of Atenolol | Fixed Time Method: |
| 103 [Unknown-1] moldm-3= 3.75(calculated): 3.75(actual) | ||||
| 103 [Unknown-2] moldm-3=6.26 ± 0.01(calculated): 6.25(actual) | ||||
| Bioanalytical methods | ||||
| 1 | Yilmaz B, et al. (Yilmaz B, et al., 2012) | Journal of Chromatographic Science | HPLC Method for Determination of Atenolol in Human Plasma and Application to a Pharmacokinetic Study in Turkey. | Column-Ace C18 reverse-phase column |
| M.P- methanol=water (50:50, v/v) | ||||
| λ max-549 nm | ||||
| Beer-Lambert’s limits- | ||||
| 5-150 ng/mL | ||||
| correlation coefficient-0.9990 | ||||
| % RSD-0.319 | ||||
| % Recovery-98.4% | ||||
| LOD-1.5 ng/mL | ||||
| LOQ-5 ng/mL | ||||
| Stability-Indicating HPLC method | ||||
| 1 | Belal F, et al. (Belal F, et al., 2008) | Journal of Chromatography Separation Technique | Stability-indicating HPLC Method for the Determination of Atenolol in Pharmaceutical Preparations. | Column-C8 Column (250 mm × 4.6 mm i.d., 5 μm) |
| M.P-acetonitrile: methanol:0.02 M phosphate buffer, pH 5 (20:20:60) | ||||
| Flow rate-1 ml/min | ||||
| λ max-226 nm | ||||
| Beer-Lambert’s limits- | ||||
| 0.05-10 μg/ml | ||||
| Correlation coefficient-1 | ||||
| % Recovery-100.4% | ||||
| LOD-0.01 μg/mL | ||||
| LOQ-0.03 μg/mL | ||||
| stability-indicating capability-acid and base media | ||||
| UV-HPLC | ||||
| 1 | Goebel K and Rolim CM (Goebel K and Rolim CM, 2007) | Latin American Journal of Pharmacy | Validation of UV Spectrophotometric and HPLC Methods for Quantitative Determination of Atenolol in Pharmaceutical Preparations. | Column-Purospher RP-18 (250 mm × 4.6 mm, 5 μm) |
| Solvent-10 mM ammonium acetate buffer (pH 7.0) and acetonitrile (80:20 v/v) | ||||
| λ max-275 nm | ||||
| Beer-Lambert’s limits (µg/mL)- | ||||
| 2-10 | ||||
| Correlation coefficient-0.9990 | ||||
| % Recovery-98.5% | ||||
| Reverse Phase High Performance Liquid Chromatography | ||||
| 1 | Kumar N, et al. (Kumar N, et al., 2010) | E-Journal of Chemistry | Estimation of Atenolol by Reverse Phase High Performance Liquid Chromatography | Column-ODS and dimensions of column was 25 mm × 4.6 mm |
| M.P-phosphate buffer and acetonitrile (53:47 v/v) | ||||
| λ max-230 nm | ||||
| Flow rate-2.1. mL/min | ||||
| Beer-Lambert’s limits- | ||||
| 5-150 ng/mL | ||||
| correlation coefficient-0.9990 | ||||
| % RSD-0.6 | ||||
| % Recovery-99.6% | ||||
| LOD-510 ng/mL | ||||
| LOQ-120 ng/mL | ||||
| 2 | Kori S, et al. (Kori S, et al., 2013) | International Journal of Science and Research | Method Development and Validation of Atenolol Drug by Spectrophotometric and HPLC Technique in Forensic Application | Column-RPC18 column |
| λ max-226 nm | ||||
| Flow rate-2.1. mL/min | ||||
| Beer-Lambert’s limits- | ||||
| 25-50 µg/mL | ||||
| correlation coefficient-0.9990 | ||||
| % RSD-0.5 | ||||
| % Recovery-99.5% | ||||
| LOD-2.00 µg/mL | ||||
| LOQ-6.3 µg/mL | ||||
| 3 | Baskara BL (Baskara BL, 2011) | Asian journal of applied science | Facile and Rapid RP-HPLC for method determination of Atenolol in pharmaceutical formulation. | Column-Atlantis dC18 |
| λmax-225 nm | ||||
| Flow rate-1.00 ml/min | ||||
| Beer-Lambert’s limits- | ||||
| 1-100 µg/mL | ||||
| correlation coefficient-0.999 | ||||
| % Recovery-98.03-102.5% | ||||
| LOD-0.4 µg/mL | ||||
| LOQ-1.0 µg/mL | ||||
Table 1: Analytical methods used for the estimation of Atenolol from bulk and formulations
| Sr. No. | Name of author |
Name of journal | Title of article | Analytical conditions |
| UV -HPLC | ||||
| 1 | Al-Rimawi F (Al-Rimawi F, 2010) | Pharmaceutica Analytica Acta | Validation of an HPLC-UV Method for the Determination of Amiodarone Impurities in Tablet Formulations | Column- C18 column |
| λ max-240 nm | ||||
| Mob. Phase-buffer solution pH 5.0, methanol, and acetonitrile (30:30:40, v/v/v) | ||||
| Beer-Lambert’s limits (µg/mL)- | ||||
| 0.005-0.015 | ||||
| Correlation coefficient-0.9990 | ||||
| % Recovery-99.7% | ||||
| LOD-0.0005 µg/mL | ||||
| LOQ-0.0002 µg/mL | ||||
| Bioanalytical methods | ||||
| 1 | Rodrigues M, et al, (Rodrigues M, et al., 2013) | Journal of Chromatographic Science | A Rapid HPLC Method for the Simultaneous Determination of Amiodarone and its Major Metabolite in Rat Plasma and Tissues: A Useful Tool for Pharmacokinetic Studies | Column-LiChroCART Purospher Star C18 column (55 3 4 mm, 3 mm) |
| λ max-254 nm | ||||
| Mob. Phase- phosphate buffer (50 mM) with 0.1% formic acid (pH 3.1)-methanol-acetonitrile (45:5:50, v/v/v) | ||||
| Flow rate-1.3 mL/min | ||||
| Beer-Lambert’s limits (µg/mL)- | ||||
| 0.1-15 | ||||
| correlation coefficient-0.995 | ||||
| % Recovery-97.7 % | ||||
| 2 | Jun AS and Brocks DR (Jun AS and Brocks DR,2001) | Journal of pharmaceutical science | High performance liquid chromatographic assay of amiodarone in rate plasma. | Column-C8 analytical column |
| λ max-242 nm | ||||
| Mob. Phase- buffer solution pH 5.0, methanol, and acetonitrile (30:30:40, v/v/v) | ||||
| Correlation coefficient-0.998 | ||||
| % Recovery-75-82% | ||||
| LOD-0.035 µg/mL | ||||
| LOQ-0.035 µg/mL | ||||
| RP-HPLC | ||||
| 1 | Babji P, et al. (Babji P, et al., 2013) | Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry | Development and validation of RP-HPLC method for amiodarone tablets in pharmaceutical dosage forms | Column-Hypersil BDS column |
| λ max-240 nm | ||||
| Mob. Phase-Acetonitrile: 0.5%Triethylamine Buffer pH to 6.5 with orthophosphoric acid (75:25) | ||||
| Flow rate-2.0 mL/min | ||||
| Correlation coefficient-0.9990 | ||||
| % Recovery-99.7-100.1% | ||||
| 2 | Thyagarajapuram N and Alexander KS (Thyagarajapuram N and Alexander KS,2003) | Journal of Liquid Chromatography and Related Technologies | A Simplified Method for the Estimation of Amiodarone Hydrochloride by Reverse‐Phase High Performance Liquid Chromatography | Column-Novapak C8 column 3.9 150 mm-particle size of 4 mm |
| λ max-240 nm | ||||
| Flow rate-1.5 mL/min | ||||
| Mob. Phase-methanol, water, and acetic acid in a 95:4:1 | ||||
| Linear equation-y ¼ 27683x þ 42192 | ||||
| Correlation coefficient-0.94 | ||||
| % Recovery-99.7% | ||||
| LOD-3.12 µg/mL | ||||
| LOQ-0.936 µg/mL | ||||
| 3 | Amit G, et al. (Amit G, et al., 2018) | World Journal of Pharmaceutical Research | Development and validation of new RP-HPLC method for analysis of amiodarone hydrochloride and its related compounds | Column-Column-Novapak C8 column 3.9* 150 mm-particle size of 4 mm |
| λ max-240 nm | ||||
| Flow rate-1.0 mL/min | ||||
| Mob. Phase- Acetonitrile: Water (80:20) | ||||
| Degradation Study-acid hydrolysis (1 M HCl at 60°C for 3 hrs), | ||||
| basic hydrolysis (1 M NaOH at 60°C for 3 hrs) | ||||
| oxidation (6% H2O2 at 60°C for 3 hrs) | ||||
| Stability indicating RP-HPLC method | ||||
| 1 | Mallu UR, et al. (Mallu UR, et al., 2010) | Drug Invention Today | Method Development of stability indicating HPLC method for the determination of Amiodarone Hydrochloride in pharmaceutical dosage form | Column-Column-Novapak C8 column 3.9* 150 mm-particle size of 4 mm |
| λ max-240 nm | ||||
| Flow rate-1.0 mL/min | ||||
| Mob. Phase-acetate buffer-Acetonitrile (15:85 v/v) | ||||
| Linearity range-12.5 to 75 µg/mL | ||||
| Correlation coefficient-0.999 | ||||
| % Recovery-99.24 % | ||||
| 2 | Ana Silva Coelho et.al (Coelho AS, et al., 2020) | Brazilian Journal of Pharmaceutical Sciences | Stability-indicating HPLC method for determination of amiodarone hydrochloride and its impurities in tablets: a detailed forced degradation study | Column-Agilent Zorbax Eclipse XDB-C18 column (100 × 3.0 mm, 3.5 µm). |
| λ max-242 nm | ||||
| Flow rate-1.0 mL/min | ||||
| Mob. Phase- 50 mM acetate buffer pH 5.5 (A) and a mixture of methanol-acetonitrile (3:4, v/v) (B) in gradient elution | ||||
| Linear equation- y=151.12x-877.23 | ||||
| Correlation coefficient-0.999 | ||||
| %RSD-1.29 | ||||
| % Recovery-99.7% | ||||
| LOD-8.0 µg/mL | ||||
| LOQ-9.0 µg/mL | ||||
Table 2: Analytical methods used for the estimation of Amiodarone from bulk and formulations
Conclusion
Presented work is focused on the use of different analytical methods like High Performance Liquid Chromatography (HPLC), Bioanalytical Methods, UV Spectroscopy, Stability indicating RP-HPLC methods etc. for determination of Atenolol and Amiodarone in formulation as well as in API. From the reviewed literature it is obvious that HPLC is a commonly available method of testing in pharmaceutical laboratory so this method should be of choice for complete determination of Atenolol and Amiodarone. No one analytical methods are available in market for the simultaneous estimation of the Atenolol and amiodarone in Pharmaceutical dosage form and bulk drugs.
References
- AHFS. Atenolol Monograph for Professionals". Drugs. 2018.
- Tomiyama H, Yamashina A. Beta-blockers in the management of hypertension and/or chronic kidney disease. Int J Hypertens. 2014; 14.
- DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D'Ascenzo F, Cerrato E, et al. ß-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart. 2015; 2(1).
- British National Formulary: BNF 76. Pharmaceutical Press. 2018; 151-153.
- Beard Jr EL. The american society of health system pharmacists. JONA'S Healthcare Law, Ethics Regul. 2001; 3(3): 78-79.
- Ali MU, Fitzpatrick-Lewis D, Kenny M, Raina P, Atkins DL, Soar J, et al. Effectiveness of antiarrhythmic drugs for shockable cardiac arrest: a systematic review. Resuscitation. 2018; 132: 63-72.
- Florey K. Analytical profiles of drug substances and excipients. Academic press. 1981.
- Akiful HM, Nivedita G, Prashanth KK, Pradeep KT, Hasan AS, Prakash VD. Simultaneous estimation of atenolol and chlorthalidone as bulk and in tablet dosage form using UV-spectrophotometry. J Pharm Biol Sci. 2012; 1(4): 20-23.
- Godge RK, Shinde GS, Dighe NS. Quantitative estimation and validation of atenolol and amlodipine besylate by absorbance ratio (Q) method. J Ejpmr. 2017; 4(7): 412-415.
- Nilesh S, Pendhbaje R, Nirmal V, Ashwini A, Jamdhade S, Pathan M. Method Development and Validation by HPLC: A Brief Review. Research and Reviews: A Journal of Pharmaceutical Science. 2021; 12(1): 27-39.
- Sabir AM. HPLC method development and validation -A review. Int Res J Pharm. 2013; 4(4): 39-46.
- ICH I. Q2 (R1): Validation of analytical procedures: text and methodology. International Conference on Harmonization. 2005.
- Hausherr A, Roessle C, Pinet E, Vasseur V, Abarou T, Benakouche S, et al. Development and validation of a new HPLC method for the analysis of a novel oral suspension formulation of 50 mg/ml ursodeoxycholic acid for newborns. Pharm Technol Hosp Pharm. 2020; 5(1).
- Pendbhaje NS, Jamdhade AA, Pathan SM, Nirmal RV. A Review on Quantification of Brexpiprazole in Its Bulk and Pharmaceutical Dosage Form by Various Analytical Methods. International Journal of Pharmaceutical Research and Applications. 2021; 6(1): 1118-1132.
- Sule S, Ambadekar S, Nikam D, Sule A, Bhure S. A practical approach to RP HPLC analytical method development. World J Pharm Sci. 2014; 3(9).
- Madhurai P, Babu GR, Rao PS, Kiran BS, Kumari MV. Quantitative estimation of atenolol in pharmaceutical dosage forms by using visible spectroscopy. Int J Pharm Chem Biol Sci. 2015; 5(3).
- Al-Awadie NS, Khudhair AF. Determination of Atenolol in pharmaceutical formulations by continuous flow injection analysis via turbidimetric (T180o) and scattered light effect at two opposite position (2N90o) using Ayah 4SW-3D-T180-2N90-Solar-CFI Analyser. Iraqi J Sci. 2014; 55(1): 12-26.
- Fadnis AG, Agarwal R. Kinetic method for estimation of Atenolol. J Chem. 2011; 3(6): 899-904.
- Yilmaz B, Arslan S, Asci A. HPLC method for determination of atenolol in human plasma and application to a pharmacokinetic study in Turkey. J Chromatogr Sci. 2012; 50(10): 914-919.
- Belal F, Elbrashy A, Eid M, Nasr JJ. Stability-indicating HPLC method for the determination of quetiapine: application to tablets and human plasma. J Liq Chrom Relat Tech. 2008; 31(9): 1283-1298.
- Goebel K, Rolim CM. Validation of UV spectrophotometric and HPLC methods for Quantitative determination of atenolol in pharmaceutical preparations. Lat Am J Pharm. 2007; 26(5): 765-760.
- Kumar N, Verma N, Songh O, Joshi N, Singh KG. Estimation of atenolol by reverse phase high performance liquid chromatography. E J Chem. 2010; 7(3): 962-966.
- Kori S, Goyal J, Sharma S, Pahade NK, Tandekar M. Method Development and Validation of Atenolol Drug by Spectrophotometric and HPLC Technique in Forensic Application. Int J Sci Res. 2013; 4: 438.
- Baskara BL. Facile and Rapid RP-HPLC for method determination of Atenolol in pharmaceutical formulation. Asian J Pharm Sci. 2011; 4(3): 306-313.
- Al-Rimawi F. Validation of an HPLC-UV method for the Determination of Amiodarone Impurities in Tablet Formulations. Pharm Anal Acta. 2010; 1(1): 1000105.
- Rodrigues M, Alves G, Ferreira A, Queiroz J, Falcão A. A rapid HPLC method for the simultaneous determination of amiodarone and its major metabolite in rat plasma and tissues: a useful tool for pharmacokinetic studies. J Chromatogr Sci. 2013; 51(4): 361-370.
- Jun AS, Brocks DR. High-performance liquid chromatographic assay of amiodarone in rat plasma. J Pharm Pharmaceut Sci. 2001; 4(3): 263-268.
- Babji P, Prasadarao M, Narasimharao D, Shankar S, Beravalli R. Development and validation of RP-HPLC method for Amiodarone tablets in pharmaceutical dosage forms. Asian journal of pharmaceutical analysis and medicinal chemistry. 2013; 1(3): 155-161.
- Thyagarajapuram N, Alexander KS. A simplified method for the estimation of amiodarone hydrochloride by reverse-phase high performance liquid chromatography. J Liq Chromatogr Relat. 2003; 26(8): 1315-1326.
- Amit G, Deepesh P, Advait A. Development and validation of new RP-HPLC method for analysis of amiodarone hydrochloride and its related compounds. World J Pharm Res. 2018; 7(18).
- Mallu UR, Reddy KH, Bobbarala V, Penumajji S. Method Development of stability indicating HPLC method for the determination of Amiodarone Hydrochloride in pharmaceutical dosage form. Drug Invent. 2010; 2(2).
- Coelho AS, Ribeiro IF, Lages EB. Stability-indicating HPLC method for determination of amiodarone hydrochloride and its impurities in tablets: a detailed forced degradation study. Braz J Pharm Sci. 2020; 56.
Author Info
Sadaf Arshad Rangrez* and Vinayak GawareReceived: 31-May-2021 Accepted: 14-Jun-2021 Published: 21-Jun-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






