Review Article - (2024) Volume 15, Issue 8
Abstract
Pharmaceutical waste can result from many activities and locations in a healthcare facility. A compounding pharmacy on site generates drug waste. Anywhere if medicines are employed the site can have spills, half- used bottles, Intravenous (IV) equipment with residual medicine on it. Waste drugs or pharmaceuticals require special treatment and their management seems to be a challenge. Small quantities at households can often be thrown away in the municipal waste stream (perhaps with some method of denaturing or making the drugs undesirable to interlopers). Large quantities kept at pharmacies, distribution centers, hospitals, etc., must be managed to minimize the risk of release or to exposure to workers and the public. This category of waste includes expired, unused and contaminated pharmaceutical products including vaccines and biological products used for therapy. Prescription and over-the-counter drugs end up as pharmaceutical waste as belongings used in pharmacies like gloves, masks, bottles, etc. In the past, medical waste was routinely disposed by flushing it down the drain. At that time, society was unaware of the potential harm these drugs could cause to the environment. Now, biologists have discovered traces of pharmaceuticals remaining in fish and aquatic ecosystems, highlighting the negative consequences of improper drug disposal. As responsible citizens and waste managers, it is crucial to prioritize the principle of prevention. While pharmaceutical wastes can be hazardous under the Resource Conservation and Recovery Act (RCRA), in many instances, managing pharmaceutical waste is a vital aspect of healthcare and environmental preservation. In a world where medical breakthroughs have resulted in a multitude of pharmaceuticals, appropriate handling and disposal of these substances have become increasingly important.
Keywords
Pharmaceutical waste, Pollution, Biomedical waste, Regulatory bodies
Introduction
Biomedical waste typically comprises of solid or liquid waste generated during the diagnosis, treatment, immunization of human beings or animals, research related to these activities or in the production and testing of biological materials. According to the World Health Organization (WHO), roughly 85% of hospital waste is non-toxic, with about 10% being infectious and the remaining 5% being non-infectious as it contains hazardous chemicals like methyl chloride and formaldehyde. The primary concern of hospital waste lies within the transmission of infectious diseases such as hepatitis B or C viruses, through syringes and needles posing highest health risk. Unfortunately, hospital waste has often been inadequately managed and simply disposed off. When hospital waste is improperly disposed, it can become hazardous, especially when mixed with municipal solid waste and left in uncontrolled or illegal landfills near residential areas and slums. This improper disposal can endanger human health by contributing to the spread of diseases like Acquired Immunodeficiency Syndrome (AIDS), hepatitis, plague, cholera, etc. Waste generated in healthcare facilities pose high potential risk of infection and injure more than any other type of waste.
RCRA was enacted in 1976 and it governs the management of solid and hazardous waste generated within the United States. In the previous several years, the Environmental Protection Agency (EPA) and state environmental protection inspectors have determined that healthcare facilities have not been managing hazardous waste in compliance with RCRA. A number of pharmaceuticals and formulations meet the definition of hazardous waste under RCRA. EPA and some state environmental agencies are now necessitating healthcare facilities to identify, segregate, appropriately label, store, transport and dispose these hazardous wastes in compliance with RCRA regulations. As a result of this focus on the part of regulators, surveyors for the Joint Commission (JC) are also including pharmaceutical waste management in their survey questions.
These guidelines discuss categorizing pharmaceutical waste, maintaining and updating an inventory of pharmaceutical waste streams, managing waste storage sites throughout the Military Treatment Facility (MTF), and disposing the waste material (Pratyusha K, et al.., 2012). The determination of these guidelines explains to provide policy and guidelines for MTFs generating pharmaceutical waste and ensure the Implementation of Reference, 40 CFR 260-279, EPA Hazardous Waste Management Regulations. Additionally, it also explains to provide Best Management Practice (BMP) guidelines for the management of other non-RCRA pharmaceutical waste (Bridges JS, 1990).
Literature Review
Importance of pharmaceutical waste disposal
Almost everyone has been goes to a hospital or a clinic at some point during their lives. These hospitals or clinics, even the smallest of them, use hundreds or sometimes even thousands of pharmaceuticals every day. It is a wonder where all of this pharmaceutical waste goes. Pharmaceutical waste is quite hazardous. It is totally different from regular waste and therefore special measures are required to dispose of it properly. It is a combination of different types of wastes (Halling-Sørensen BN, et al.., 1998).
Types of pharmaceutical waste
Communal wastes and biomedical wastes are known as general health care wastes, hazardous health care wastes, health care risk wastes or special wastes (Majara M and Leduka RC, 2009). Biomedical wastes are further classified as listed-
Infectious waste: It contains pathogens like bacteria, viruses, parasites or fungi which can lead to diseases in susceptible hosts when found in detectable concentrations (Figure 1). Infectious wastes, which are also known as biomedical or hazardous waste, refer to materials that have the potential to carry infectious agents, which can pose health risks to humans and the environment. These materials are typically generated in healthcare settings, research laboratories and other facilities where biological or medical procedures are conducted.
Figure 1: Biomedical or infectious waste
These further include microbial cultures, infectious agent stocks from pathological labs, and waste generated during procedures on infected patients (Benotti MJ and Brownawell BJ, 2009). For instance, disposable towels, gowns, aprons, gloves, tissues and materials or instruments that have been use during surgeries and autopsies on patients suffering from infectious diseases.
Chemical waste: This approach is primarily employed for the treatment of liquid waste, such as blood, stools, urine, potent antioxidants, aldehydes and phenol compounds; it effectively eliminates or deactivates microorganisms. Chemical disinfection is also applied to microbiological cultures, mutilated sharps and shredded solid materials. The disinfectant’s effectiveness is influenced by the duration of interaction with the waste, the concentration of the chemical, and the type of chemical used. However, chemical disinfection is toxic, prohibiting discharge into surface water and restricting large quantities in sewages. Users must follow required precautions during the disinfection process due to potential hazardous effects.
Chemical waste encompasses surplus, unused or undesired chemicals, particularly those posing harm to human health or the environment (Figure 2). It can be categorized as hazardous waste, non-hazardous waste, universal waste and household hazardous waste.
Figure 2: Chemical waste, causing harm to human health
Pathological waste: Pathological wastes encompass tissues, human carcasses, blood, body fluids, body parts and human fetuses. These anatomical wastes are typically categorized as a subset of infectious wastes. Sharp objects such as knives, broken glass, hypodermic needles, and scalpels, etc., are highly hazardous due to their potential to cause cuts or wounds, whether infected or not (Figure 3).
Figure 3: Hazardous pathological waste
These objects seem to be like a special category of garbage from hospitals and labs. Handling and throwing away these items need extra care because they could be harmful. There are strict rules to make sure it’s done safely, protecting both the environment and people’s health. It should be a thought to keep everyone safe from the potentially risky stuff used in healthcare.
Waste containing heavy metals: Biomedical wastes, particularly those containing heavy metals, originate mainly from sources like garden pesticides, pharmaceuticals, personal healthcare products and mercury wastes from broken clinical equipment (Figure 4). These wastes, which are rich in heavy metals are often highly toxic and can leach into the soil, causing contamination with metals such as lead, copper and zinc (Joshi SW, et al.., 2017). This poses environmental concerns and emphasizes the need for proper disposal and management practices to prevent soil pollution.
Figure 4: Waste with high content of heavy metals
High levels of heavy metals in biomedical waste, such as lead, mercury and cadmium can harm ecosystems and human health (Larsson DJ, et al.., 2007). Implementing strict regulations, effective waste management practices and promoting clean industrial processes are essential for minimizing the impact of heavy metal pollution (Tiwari AV and Kadu PA, 2013). Persistent exposure to heavy metals from waste can lead to various health issues, including neurological disorders, organ damage and developmental problems, especially in children. It highlights the importance of adopting sustainable practices to reduce heavy metal emissions and safeguard both the environment and human well-being (Mohan S and Gandhimathi R, 2009).
Pharmaceutical waste: Proper disposal is necessary for expired drugs, unused, spilt, or contaminated pharmaceutical items, including vaccines and sera (no longer in use). Pharmaceutical waste encompasses packaging materials like glass bottles and aluminum packs that have been in contact with drug products (Figure 5). Ensuring responsible disposal is vital to prevent environmental contamination and safeguard public safety.
Figure 5: Pharmaceutical waste
Inappropriate disposal of pharmaceutical waste can lead to environmental pollution and potential health risks (Patneedi CB and Prasadu KD, 2015) (Figure 6). Therefore, healthcare facilities, pharmacies and individuals must adhere to regulations and best practices to minimize the impact on ecosystems and public health. Effective pharmaceutical waste management involves raising awareness, educating healthcare professionals and implementing efficient collection and disposal systems (Kadam A, et al.., 2016). Additionally, promoting research and development of eco-friendly packaging materials and drug formulations can contribute to reducing the environmental footprint of the pharmaceutical industry.
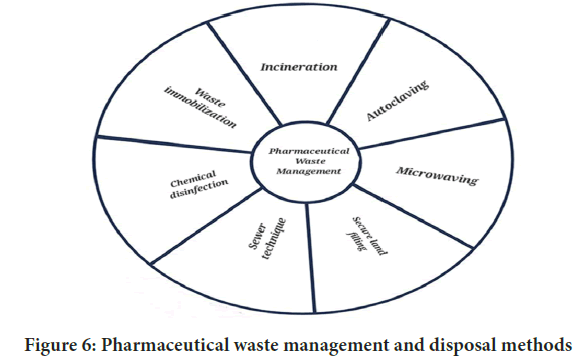
Figure 6: Pharmaceutical waste management and disposal methods
Ultimately, a comprehensive approach to pharmaceutical waste management is crucial for maintaining environmental sustainability and protecting communities from the potential hazards associated with improper disposal practices. Pharmaceutical waste consists of unused or expired medications. Improper disposal, such as throwing them in regular trash or flushing them, can have adverse environmental and public health effects. To address this, local guidelines should be adhered for safe disposal, often involving collection programs or designated drop-off points. Responsible disposal helps safeguard the environment and community well-being.
Discussion
Sources of pharmaceutical waste
Researchers have recently quantified pharmaceutical wastes in the environment, despite their presence for decades which are listed below-
• Wastes disposal from pharmacies.
• Defective landfills causing leaching of drugs.
• Direct and improper disposal of unused/expired medications by patients into the waste and also through excretion of urine or feces.
• Drugs released from sources like aquaculture medicated feed, molecular farming, pest control drugs, etc.
• Physician samples which are given by companies to medical representatives for sales promotion purpose, even in many developing countries like India.
• Animal healthcare facilities produce pharmaceutical waste from expired or unused medications.
• Pharmaceutical manufacturing processes may generate waste, including unused or rejected products and by-products.
• Pharmaceutical waste can enter the environment through improper disposal methods, such as flushing medications down the toilet, leading to water contamination (Bruce GM, et al.., 2010).
Pharmaceutical waste management and disposal methods
The specified technologies for pharmaceutical waste treatment and disposal in India’s pharmaceutical waste rules include the following-
Incineration: It serves as a disposal technique wherein solid wastes undergo combustion, transforming them into gaseous byproducts and residue. This method is also referred to as thermal treatment which is valuable for managing solid waste and includes the waste that is found in wastewater as well. This process typically reduces the volume by 20%-30%. Incinerators convert waste materials into heat, gas, steam and ash, and this method is employed on both small and large scales by the industries (Figure 7). While recognized for handling hazardous waste, incineration is controversial due to the emission of gaseous pollutants. It is not suitable for certain materials like pressurized gas containers, significant chemical wastes, halogenated chemicals, plastics with halogens, mercury and cadmium-containing waste, and radiographic waste, incineration requires careful disposal, resulting in production of ash in secure landfills. Skilled operators are important for implementing this technique.
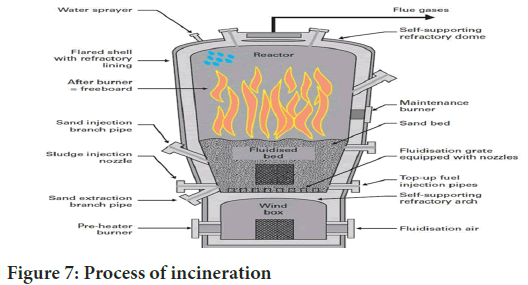
Figure 7: Process of incineration
Autoclaving method: In this method, biomedical waste comes into direct contact with saturated steam in a pressure vessel at specified time and temperature to eliminate pathogens. To ensure effective disinfection, guidelines are set for minimum temperature, pressure, and autoclave residence time in biomedical waste management. Autoclaving results in waste suitable for landfill disposal alongside municipal waste. The process also generates a wastewater stream, necessitating proper disposal with regulated controls (Kolpin DW, et al.., 2002). Operating the autoclave requires a qualified technician and involves moderate investment and operating costs. Autoclaving stands as a pivotal sterilization technique, employing saturated steam in a pressurized chamber. This method ensures the thorough elimination of pathogens from biomedical waste through carefully regulated time and temperature parameters. Adhering to biomedical waste management guidelines, autoclaves enforce specific requirements for minimum temperature, pressure, and residence time.
Microwaving method: Applying an electromagnetic field for the conduction-based destruction of infectious components in Biomedical Waste (BMW) is effective when exposed to Ultraviolent (UV) radiation. However, this method is not suitable for human anatomical, animal, chemical, pharmaceutical waste and large metal parts (Figure 8).
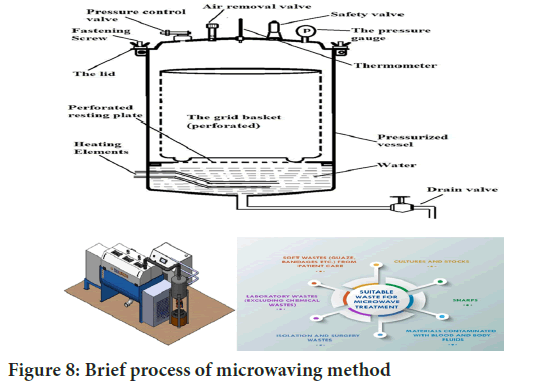
Figure 8: Brief process of microwaving method
The microwaving method demands minimal electrical energy and allows for landfill disposal of the generated waste. Economically advantageous, it requires moderate investment and incurs low operating costs. Nonetheless, drawbacks include the need for qualified technicians and the potential for frequent breakdowns in shredders. On the other hand, microwaving method emerges as a viable alternative, demanding minimal electrical energy and allowing for the landfill disposal of the generated waste. One of its primary advantages lies within its economic feasibility, requiring a moderate investment and boasting low operating costs. Despite these merits, it’s crucial to acknowledge the drawbacks associated with this technology.
Secure land filling: Waste deposition typically involves compaction for increased density and stability, coupled with covering to deter vermin. Biomedical waste guidelines mandate the secure landfill disposal of discarded medicines, cytotoxic drugs, solid chemical waste, and incineration ash. Secure land filling, the method for solid waste and hazardous substance disposal, is widely used globally, often in remote, unused are as away from cities (Figure 9). Properly designed and managed landfills prove effective, hygienic, and economical. However, inadequate management poses environmental hazards, with gas byproducts like methane or carbon dioxide causing odor and contributing to greenhouse gases. Modern landfill techniques include leachate management and gas extraction for electricity generation.
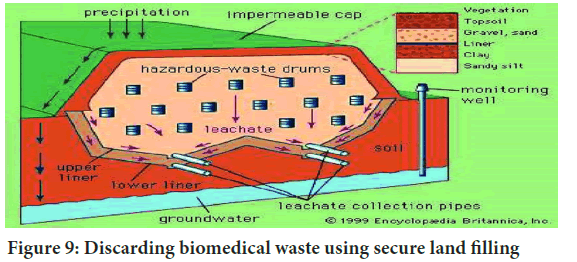
Figure 9: Discarding biomedical waste using secure land filling
Landfill management is crucial for handling waste effectively. Secure land filling, a common method, involves compacting waste to enhance density and stability while preventing vermin attraction. Biomedical waste, including discarded medicines and cytotoxic drugs, follows strict rules for disposal in secure landfills, safeguarding against environmental hazards.
Sewage method: Certain pharmaceuticals, such as liquids like syrups and IV fluids, are diluted with water and released into sewages in minimal amounts over time, generally without significant impact on public health or the environment (Figure 10). Diluted liquid pharmaceuticals or antiseptics can be safely disposed of in fast-flowing watercourses. In cases of damaged or deteriorating sewages, the expertise of a hydrogeologist or sanitary engineer may be necessary. In specific situations where sewages are in disrepair or have been damaged due to various factors, consulting a hydrogeologist or sanitary engineer becomes crucial. Their expertise can aid in assessing potential risks and proposing solutions to mitigate any adverse effects on the environment or public health. It’s noteworthy that the disposal of pharmaceuticals, even in diluted form, requires careful consideration to prevent any unintended consequences. The fast-flowing watercourses are often chosen as a disposal method to facilitate further dilution and dispersion.
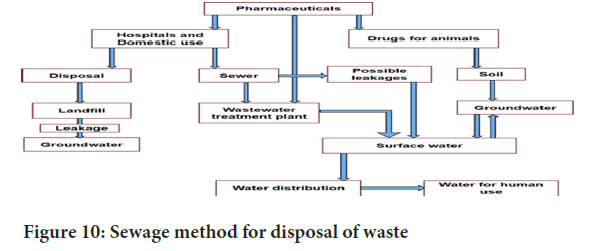
Figure 10: Sewage method for disposal of waste
Biomedical and it’s classification of waste
Biomedical waste, originating from the diagnosis, treatment, or immunization of humans or animals, as well as the production or testing of biological materials, comprises solid or liquid waste. According to the WHO, approximately 85% of hospital waste is non-hazardous, with 10% being infectious and the remaining 5% non-infectious but containing some chemical components like methyl chloride and formaldehyde. The primary concern with infectious waste lies in the potential transmission of Human Immunodeficiency Virus (HIV) and hepatitis B or C viruses, particularly through needles and syringes. Health care facilities, including wards, delivery rooms, emergency and outpatient services, operation theaters, laboratories, and pharmaceutical stores, are significant sources of biomedical waste. Those at risk of exposure include healthcare personnel, patients and visitors, as well as waste management facility employees and scavengers.
Biomedical waste in healthcare facilities primarily emanates from wards, delivery rooms, emergency and outpatient services, operation theaters, laboratories, and pharmaceutical and chemical stores. This poses exposure risks for healthcare personnel such as doctors, nurses, healthcare assistants, maintenance staff, and support personnel involved in waste handling, transportation, and laundry. Additionally, patients and their visitors, as well as waste management facility employees and scavengers, are also susceptible to potential exposure due to biomedical wastes.
Biomedical waste encompasses materials generated during healthcare activities, and its sources within healthcare facilities extend to various departments. Wards, delivery rooms, emergency and outpatient services, operation theaters, laboratories, and pharmaceutical and chemical stores are key contributors to the production of biomedical waste. This waste poses potential exposure risks to a range of individuals. Classification of biomedical waste can be categorized into hazardous and non-hazardous waste (Table 1).
| Hazardous waste | Non-hazardous waste |
|---|---|
| It is harmful to human health or environment, if not disposed properly | It does not directly cause harm to human health or environment, but it cannot dump in sewer line due to risk |
| It includes explosives, flammable liquids/solids, poisous, toxic, ecotoxic, infectious substances | It includes paper, plastic, metals, glass, etc. |
| It is regulated under the Resource Conservation and Recovery Act (RCRA) | It is regulated under state and local governments through the federal government |
| It might be produced from companies and households as well as worksites | It can be produced from general waste like food, bathroom garbage, industrial or agricultural sources. |
| It is categorized as listed and characteristic waste | Disposable method varies as per the regulations governing them |
| Examples: Pesticides, herbicides, industrial solvents, fluorescent light bulbs and mercury containing batteries. | Examples: Agricultural waste, batteries, construction debris, industrial waste, medical waste, municipal solid waste, scrap tires, etc. |
Table 1: Classification of biomedical waste
Potential solution for pharmaceutical waste
To appropriately manage hazardous pharmaceutical waste, healthcare institutions may need to establish new waste categories. All facilities should reassess existing policies to align with state and federal regulations for pharmaceutical waste and environmental compliance. Utilizing computerization, automation, and bar-code scanning technology can enhance the creation of secure and efficient pharmaceutical waste management processes.
Waste management team
Establishing an interdepartmental, multidisciplinary team is recommended for ensuring compliance with RCRA and state regulations. This team would assess current practices, identifying gaps in pharmaceutical waste management and promptly addressing them to enhance compliance. Additionally, the team could act as the facility’s point of contact with the regional EPA office and potentially liaise with state environmental or sanitary offices and external consultants.
Inventory management
To reduce hazardous pharmaceutical waste, healthcare facilities should maintain minimum inventory levels, prioritize the use of older stock, and employ strategies such as rotating inventory. Additionally, minimizing unwanted or expired medications, utilizing multidose vials, preparing patient-specific oral syringes, centralizing disposal of physician’s samples, and avoiding unnecessary prescriptions, especially antibiotics, are recommended practices (Wu M, et al.., 2009). Items not requiring special handling, like unit dose packaging for non-P-listed items, empty medication vials with non-P-listed contents, and partially used nonhazardous items, can be disposed of in the municipal trash or sewage system. Empty containers of nonhazardous items can also be discarded in the trash.
Reverse distribution
Pharmacies can reduce pharmaceutical waste through reverse distribution, returning unused but viable pharmaceuticals to manufacturers for credit. EPA exempts health care facilities from treating returned pharmaceuticals as “discarded materials,” shifting disposal responsibility to the reverse distributor, subject to compliance with Return Industry Association (RIA) standards. Pharmaceutical waste handled through reverse distribution doesn’t contribute to a facility’s hazardous waste generator status.
State and county activity
Healthcare facilities should be aware that certain states and counties have regulations more stringent than federal RCRA standards. To understand applicable requirements, it is advisable for facilities to communicate with their state EPA or relevant regulatory authority.
Pharmaceutical waste management in India
India’s economic growth results in a significant increase in waste production, posing environmental hazards. The biomedical waste management and handling rule of 1998, enacted in July of that year, aimed to control and address the issue. In 2016, the central government notified the biomedical waste management rules, placing responsibility on state pollution control boards to enforce these regulations. These rules apply to individuals involved in the generation, collection, reception, storage, transportation, treatment, disposal, or handling of biomedical waste in any capacity.
The economic development in India has led to a substantial surge in waste generation, contributing to adverse effects on the environment. To address this concern, the biomedical waste management and handling rule of 1998 was implemented in July 1998. Subsequently, the central government introduced the biomedical waste management rules in 2016. State pollution control boards are entrusted with the responsibility of overseeing and enforcing these regulations. The rules encompass all individuals engaged in the various stages of biomedical waste management, including generation, collection, reception, storage, transportation, treatment, disposal or handling in any form. This regulatory framework seeks to mitigate the environmental impact of biomedical waste and ensure a systematic and responsible approach to its management across the nation. Pharmaceutical waste management in India faces challenges related to improper disposal, lack of awareness, and inadequate regulatory frameworks. Efforts are being made to establish guidelines for safe disposal and encourage practices within the pharmaceutical industry (Table 2). Increasing awareness among healthcare professionals and the public is crucial for effective implementation of waste management strategies.
| Schedules | Guidelines |
|---|---|
| 1 | Treatment and disposal of biomedical waste |
| 2 | Generated waste is segregated into different containers or bags |
| 3 | Containers are labelled |
Table 2: Different schedules and guidelines
Minimizing pharmaceutical waste
While developing your pharmaceutical waste management program, it’s important to recognize constraints in substituting less hazardous drugs due to the therapeutic effects of the chemicals. Nevertheless, focusing on waste reduction can help mitigate compliance challenges, reduce costs, and minimize risks. The following section explore various opportunities of waste minimization.
i. Incorporating lifecycle impacts into the purchasing process
ii. Optimizing the utilization of opened chemotherapy vials
iii. Enforcing a samples policy
iv. Properly labeling drugs for home use
v. Prudently priming and flushing IV lines with saline solution
vi. Assessing container size in relation to usage
vii. Substituting prepackaged unit dose liquids with patient-specific oral syringes
viii. Managing controlled substances
ix. Ensuring safe delivery of chemotherapy drugs
x. Regularly monitoring expiry dates on emergency syringes
xi. Fine-tuning inventory controls to minimize outdated products
xii. Exploring management options
xiii. Preparing for implementation
• Identifying satellite accumulation areas
• Assessing storage accumulation areas
• Initiating a pilot program
Conclusion
Pharmaceutical and biomedical waste poses hazards to human and animal health, as well as the environment. Managing this waste is a challenge for medical personnel, recycling industries, government administrations, policy planners, and quality assurance teams. Both governmental and non-governmental organizations are actively addressing this issue, creating provisions for proper waste disposal. Continuous development of new classifications and effective techniques for medical waste removal is crucial to reduce management costs. Healthcare professionals, including physicians, pharmacists, and nurses, play a role in waste disposal, and collaboration between government, NGOs, and the public is essential to alleviate the environmental impact of unused and expired drugs. Overall, proper waste management is vital for ensuring the safety of both health and the environment.
References
- Pratyusha K, Gaikwad NM, Phatak AA, Chaudhari PD. Review on: Waste material management in pharmaceutical industry. Int J Pharm Sci Rev Res. 2012; 16(2): 121-129.
- Bridges JS. Experience with the EPA manual for waste minimization opportunity assessments. International Atomic Energy Agency (IAEA). 1990.
- Halling-Sørensen BN, Nielsen SN, Lanzky PF, Ingerslev F, Lützhøft HH, Jørgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere. 1998; 36(2): 357-393.
[Crossref] [Google Scholar] [Pubmed]
- Majara M, Leduka RC. Health-care waste practices in selected health-care facilities in Maseru. 2009.
- Benotti MJ, Brownawell BJ. Microbial degradation of pharmaceuticals in estuarine and coastal seawater. Environ Pollut. 2009; 157(3): 994-1002.
[Crossref] [Google Scholar] [Pubmed]
- Joshi SW, Remya Devi PS, Lali AM, Gantayet LM, Verma R. Kinetic separation of cobalt from zirconium by cation exchange process. Sep Sci Technol. 2017; 52(4): 712-721.
- Larsson DJ, de Pedro C, Paxeus N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J Hazard Mater. 2007; 148(3): 751-755.
[Crossref] [Google Scholar] [Pubmed]
- Tiwari AV, Kadu PA. Biomedical waste management practices in India-A review. Int J Curr Eng Technol. 2013; 3(5): 2030-2033.
[Crossref] [Google Scholar] [Pubmed]
- Mohan S, Gandhimathi R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J Hazard Mater. 2009; 169(1-3): 351-359.
[Crossref] [Google Scholar] [Pubmed]
- Patneedi CB, Prasadu KD. Impact of pharmaceutical wastes on human life and environment. Rasayan J Chem. 2015; 8(1): 67-70.
- Kadam A, Patil S, Patil S, Tumkur A. Pharmaceutical waste management an overview. Indian J Pharm Sci. 2016; 9(1).
[Crossref] [Google Scholar] [Pubmed]
- Bruce GM, Pleus RC, Snyder SA. Toxicological relevance of pharmaceuticals in drinking water. Environ Sci Technol. 2010; 44(14): 5619-5626.
[Crossref] [Google Scholar] [Pubmed]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, et al. Pharmaceuticals, hormones and other organic wastewater contaminants in US streams, 1999-2000: A national reconnaissance. Environ Sci Technol. 2002; 36(6): 1202-1211.
[Crossref] [Google Scholar] [Pubmed]
- Wu M, Atchley D, Greer L, Janssen S, Rosenberg D, Sass J. Dosed without prescription: Preventing pharmaceutical. Natural Resources Defense Council (NRDC). 2009.
Author Info
Pratik Pandit, Rishikesh Misal, Gaurav Pawar and Somnath Davkar*Citation: Pandit P: Approaches of Pharmaceutical Waste Management and Handling Vol
Received: 06-Aug-2024 Accepted: 22-Aug-2024 Published: 29-Aug-2024, DOI: 10.31858/0975-8453.15.8.251-256
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3