Research Article - (2022) Volume 13, Issue 9
Abstract
Introduction: Calotropis procera is a shrub had been used traditionally for the treatment of many diseases including analgesic, anti-inflammatory, hepatic and other diseases. The flower of the plant has not been investigated for its analgesic activity. The present study aims High Pressure Thin Layer Chromatography (HPTLC) and Gas Chromatography-Mass Spectrometry (GC-MS) analysis of Ethanolic Extract of Calotropis procera to evaluate the analgesic effects of Calotropis procera as well as assessment of analgesic activity in animal models.
Methods: The ethanolic extracts were prepared using a rotary evaporator and the maceration procedure. The phytocompounds characterization was done by GC-MS and HPTLC. The hot plate method, acetic acid- induced writhing test, and tail-flick test were used to determine the analgesic activity of various doses (100, 200, 300 mg/kg body weight) of the Ethanolic Extract of Calotropis procera flower (EECP).
Conclusion: The extract has shown showed significant analgesic activity in the all experiments at a dose of 300 mg/kg. We found that even at low doses, analgesic effectiveness could be achieved via central and peripheral mediated action. The ethanol extract’s HPTLC and GC-MS analysis revealed number of compounds with potent analgesic and antioxidant activity.
Keywords
Calotropis procera, Gas Chromatography-Mass Spectrometry (GC-MS), High Pressure Thin Layer Chromatography (HPTLC), Analgesic, Anti-inflammatory
Introduction
Medicinal plants are the most valuable asset to human health, and they provide a rich source of novel potential chemicals with a variety of therapeutic actions (Sajeesh T and Parimelazhagan T, 2014). Non-Steroidal Anti-Inflammatory medications (NSAIDs) are used to treat muscle pain, dysmenorrhea, arthritic diseases, pyrexia, gout, and migraines, as well as being utilized as opi- oid-sparing medicines in some cases of severe trauma (Ghlich- loo I and Gerriets V, 2021). Gastrointestinal, liver, and kidney dysfunction are all adverse effects of NSAIDs (Hörl WH, 2010). In the development of new alternative to NSAIDS, plant extract and their phytoconstituents may play crucial role for safe use anal- gesic. Many medicinal plants have been used traditionally having anti-inflammatory and analgesic effects.
Calotropis procerais known by several names around the world, including apple of calotrope, huge milkweed, sodom, Indian milk- weed, rubber tree, wild cotton, and usher (Dhileepan K, 2014). Calotropis procerais found in Africa, Western Asia, the Arabian Peninsula, and India (GRIN-Global, 2021). The plant have hepa- toprotective, anti-inflammatory, antidiarrheal properties and antiepileptic activities (Silva MC, et al., 2010; Quazi S, et al., 2013; Jalalpure SS, et al., 2009; Obese E, et al., 2021).
Phytocompounds found in C. procerainclude flavonoids, tannins, terpinoids, saponins, steroids, linoleic acid, amino acid, palmitic acid, and fatty ethyl esters (Kumar A, et al., 2022). Many of these compounds are having anti-inflammatory, analgesic and anti- oxidant properties. The present study is focused on phytochem- ical characterization using HPTLC and GC-MS, as well as the an- alysis of analgesic effect of Ethanolic Extract of Calotropis proceraflower on mice.
Materials and Methods
Animals
Swiss Albino mice (25-35 g) were obtained from the animal house at Institute of Medical Sciences (IMS), Banaras Hindu University, Varanasi. Animals were acclimatized in a temperature-controlled environment and given free access to tap water and food. Accord- ing to Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) criteria, the study was approved by the Central Animal Ethical Committee of Banaras Hindu University (Reg. No. 542/GO/Rebi/S/02/CPCSEA dated 26.5.2017).
Drugs, chemicals and reagents
Diclofenac (Sigma-Aldrich), morphine (Cipla Healthcare Ltd., India) and organic solvents (ethanol, petroleum ether and ethyl acetate) were purchased from Sisco Research Laboratories Pvt. Ltd.
Preparation of extracts
The flowers of Calotropis procera were collected in Varanasi area of Uttar Pradesh, India. The plant was authenticated by Assistant Professor, A. K. Kushwaha, and a voucher specimen no. (2019- 02) was deposited in the herbarium of the Department of Dra- vyaguna, Institute of Medical Sciences, Banaras Hindu University. Flowers of Calotropis procerawere dried and milled in powder form at room temperature. The shade dried power of the flower was macerated with 95% ethanol (1:7 w/v). The extract was con- centrated using rotary evaporator (Buchi R-210 Advanced, Switz- erland). Percentage yield was obtained 13.8% w/w. The extract was kept at room temperature until they were evaluated (Costa AR, et al., 2020).
High Pressure Thin Layer Chromatography (HPTLC)
To obtain an appropriate resolution chromatogram, various mobile phases were examined, with the final mobile phase consisting of toluene, Chloro- form, and Ethyl Alcohol in the ratio 4:4:1. Sample-applicated plates were put in the twin trough chamber (CAMAG) until the solvent front reached the maximum distance (10 10 cm plate). A dryer was used to dry the plates. The plate was scanned at 254 nm after this plate was derivatized (post derivatization) in Anisaldehyde-Sulphuric acid reagent and heated at 110°C for 5 minutes before being observed at 366 nm. All the analysis retention factor and area percent of the individual bands on the Thin Layer Chromatography (TLC) plate were performed by CAMAG TLC scanner and win CATS planar chromatography manager software.
GC-MS analysis
GC-MS analysis was performed on the primary extract EECP. For Gas Chromatography, GC Program GCMS-QP2010 Ultra were used and in- strument conditions such as ion source temp 220°C, interface temperature 270°C, solvent cut time 2.50 min, relative detector gain mode and thresh- old 1000 were used. MS conditions were as start time 3.0 min, end time: 39.98 min, start m/z: 40.0, and end m/z: 650, scan speed 0.33 and Acquisi- tion (ACQ) mode used for analysis.
Antinociceptive activity
Tail flick method: Analgesic activity was measured using the tail-flick method. Swiss albino mice of either sex (25-30 gm) were divided into five groups of five mice each. Group 1 was given vehicle, while Group 2 was given morphine (10 mg/kg i.p.). EECP flower were given to groups 3, 4, and 5 at dosages of 100, 200, and 300 mg/kg p.o., respectively. The tail-flick latency was measured using an analgesiometer (Techno, India). The cur- rent flowing through the nichrome wire was kept constant at 6 amp. Radi- ant heat (55°C ± 2°C) was applied to the tail and maintained at a distance of 2.5 cm from the root. To avoid tissue damage, the reaction time threshold was set at 10 seconds. The control, standard, and test groups’ average scores were recorded. Five minutes before the test, morphine was administered, and one hour before the test, the extract dosage was given orally.
Hot plate test: Swiss albino mice (25-30 gm) were placed into five groups, each with five mice. The first group received vehicle, whereas the second received morphine (10 mg/kg i.p.). Groups 3, 4, and 5 received EECP flower at doses of 100, 200, and 300 mg/kg p.o., respectively. Animals were paced on a hot plate (analgesiometer) at a constant temperature of 55°C ± 1°C. At 15, 30, 60, 90, and 120 minutes following the first thermal stimulus, the time taken to jump and withdraw the paws was measured (Iauk L, et al., 1993).
Acetic acid induced writhing assay: The writhing response in the mice was monitored for 20 minutes after 5 minutes of intraperitoneal (i.p.) in- jection of acetic acid (0.3 ml, 3 percent). 1 hour before the test, 100, 200, and 300 mg/kg body weight. of EECP were given orally (Fadda AA and Elattar KM, 2015). These groups’ writhing was measured and compared to the control group. The standard drug was used as diclofenac (10 mg/kg) (Iauk L, et al., 1993).
Statistical analysis
The results of tail flick test, and hot plate test were analyzed using two-way ANOVA followed by Dunnett’s multiple comparisons test while results of acetic acid writhing test was analyzed using one-way ANOVA followed by Dunnett’s multiple comparisons test. The data was presented in the form of a mean ± Standard Error of the Mean (SEM). The statistical analysis was carried out using the GraphPad Prism 8.0.2 software. In the comparison of outcomes, p value of less than 0.05 was considered significant.
Results
High Performance Thin Layer Chromatography (HPTLC) Analysis
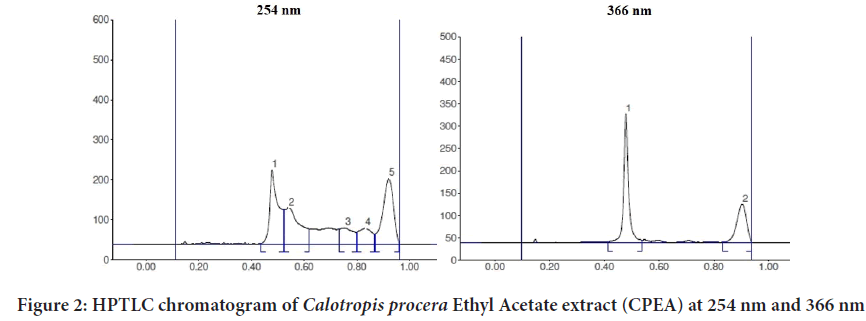
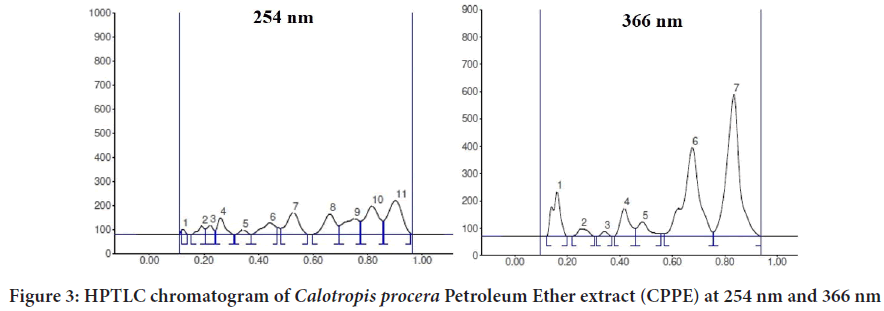
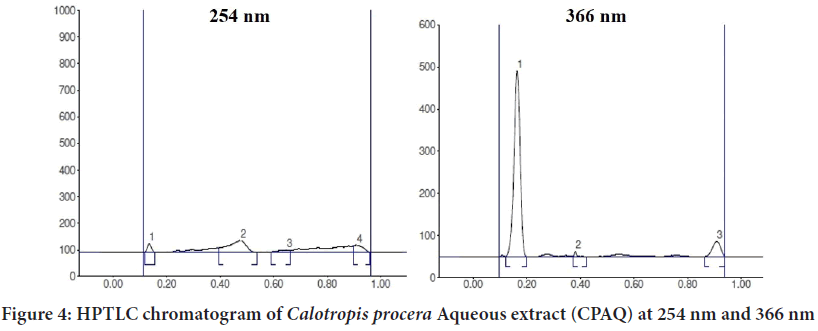
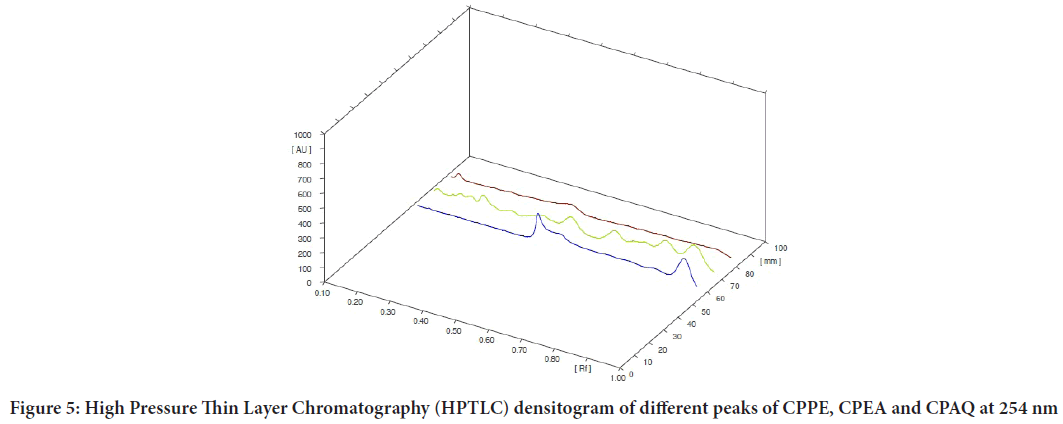
The EECP extract (10 g) was separated using a separating funnel and polarity-based solvents, yielding three fractions (Calotropis procera Ethyl Acetate (CPEA), Calotropis proceraPetroleum Ether (CPPE), and Calot- ropis proceraAqueous (CPAQ)), which were then dried at room temper- ature. The yields were found to be 1.8 percent, 2.6 percent, and 1.4 percent, respectively. The three fractions were used for injection on an HPTLC ap- paratus after partition chromatography. Figures 1-5 exhibits various peaks, their Retention factor (Rf) values, and the area percentage of each peak. CPPE has the most peaks in comparison to CPAQ and CPEA. When seen at a wavelength of 366 nm, some peaks were more visible after deriva- tization. The peak’s intensity was clearly reflected by the area percentage (Tables 1-6).
| Peak | Start Retention factor (Rf) | Start height | Max Rf | Max% | End Rf | End height | Area | Area% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.44 | 1.7 | 0.48 | 35.66 | 0.52 | 85.9 | 4357.3 | 27.72 |
| 2 | 0.53 | 86 | 0.54 | 17.35 | 0.62 | 37.8 | 3739.3 | 23.69 |
| 3 | 0.74 | 38 | 0.76 | 7.86 | 0.8 | 29.5 | 1408.3 | 1480.3 |
| 4 | 0.8 | 29.7 | 0.83 | 7.86 | 0.87 | 25.1 | 1454 | 1454 |
| 5 | 0.87 | 25.5 | 0.92 | 31.46 | 0.97 | 2.6 | 4734.9 | 4734.9 |
Table 1: High performance thin layer chromatography chromatogram of Calotropis procera Ethyl Acetate extract (CPEA) at 254 nm
| Peak | Start Rf | Start height | Max Rf | Max% | End Rf | End height | Area | Area% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.41 | 2.1 | 0.48 | 287.7 | 0.54 | 5.4 | 3728.6 | 63.18 |
| 2 | 0.84 | 0.5 | 0.91 | 22.95 | 0.94 | 2.1 | 2172.6 | 36.82 |
Table 2: High performance thin layer chromatography chromatogram of CPEA at 366 nm
| Peak | Start Rf | Start height | Max Rf | Max% | End Rf | End height | Area | Area% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.12 | 19.9 | 0.12 | 3.04 | 0.14 | 0.4 | 194.7 | 0.85 |
| 2 | 0.15 | 0.1 | 0.19 | 4.76 | 0.2 | 23 | 582.5 | 2.54 |
| 3 | 0.21 | 23.2 | 0.22 | 5.35 | 0.24 | 15.7 | 661.1 | 2.89 |
| 4 | 0.24 | 16.8 | 0.26 | 9.4 | 0.31 | 0 | 1322.4 | 5.78 |
| 5 | 0.31 | 0.2 | 0.34 | 2.67 | 0.37 | 1.2 | 373.5 | 1.63 |
| 6 | 0.37 | 1.3 | 0.44 | 6.61 | 0.47 | 30.3 | 1695.3 | 7.41 |
| 7 | 0.48 | 26.3 | 0.53 | 12.45 | 0.58 | 1.7 | 2985.4 | 13.04 |
| 8 | 0.6 | 0 | 0.66 | 11.62 | 0.7 | 37.2 | 2632.2 | 11.5 |
| 9 | 0.7 | 37.5 | 0.75 | 9 | 0.77 | 56.1 | 2715.5 | 11.86 |
| 10 | 0.78 | 56.2 | 0.82 | 16.01 | 0.86 | 57.2 | 4413.5 | 19.28 |
| 11 | 0.86 | 57.4 | 0.9 | 19.09 | 0.96 | 5.7 | 5373.3 | 23.21 |
Table 3: High performance thin layer chromatography chromatogram of Calotropis procera Petroleum Ether extract (CPPE) at 254 nm
| Peak | Start Rf | Start height | Max Rf | Max% | End Rf | End height | Area | Area% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.12 | 0.6 | 0.16 | 13.5 | 0.2 | 0 | 3649.4 | 8.89 |
| 2 | 0.22 | 0.6 | 0.25 | 2.34 | 0.3 | 0 | 795.1 | 1.94 |
| 3 | 0.31 | 0.1 | 0.34 | 1.55 | 0.37 | 4 | 312.2 | 0.76 |
| 4 | 0.38 | 0.1 | 0.42 | 8.55 | 0.46 | 29.3 | 2579 | 6.28 |
| 5 | 0.46 | 29.6 | 0.49 | 4.43 | 0.56 | 10.4 | 1679.9 | 4.09 |
| 6 | 0.57 | 10.3 | 0.68 | 26.87 | 0.76 | 15.4 | 13925.3 | 33.91 |
| 7 | 0.76 | 15.6 | 0.84 | 42.77 | 0.94 | 0.6 | 18127.8 | 44.14 |
Table 4: High performance thin layer chromatography chromatogram of CPPE at 366 nm
| Peak | Start Rf | Start height | Max Rf | Max% | End Rf | End height | Area | Area% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.12 | 0.9 | 0.14 | 28.17 | 0.16 | 0.1 | 365.5 | 10 |
| 2 | 0.4 | 16.9 | 0.48 | 39.35 | 0.54 | 0.6 | 2229.5 | 60.98 |
| 3 | 0.59 | 2.5 | 0.65 | 8.7 | 0.66 | 10.1 | 314.2 | 8.59 |
| 4 | 0.9 | 25.3 | 0.91 | 23.78 | 0.96 | 0.5 | 746.7 | 20.42 |
Table 5: High performance thin layer chromatography chromatogram of Calotropis procera Aqueous extract (CPAQ) at 254 nm
| Peak | Start Rf | Start height | Max Rf | Max% | End Rf | End height | Area | Area% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.12 | 0.4 | 0.16 | 90.07 | 0.2 | 0 | 7284 | 89.38 |
| 2 | 0.37 | 1.1 | 0.38 | 2.51 | 0.42 | 0 | 73.4 | 0.9 |
| 3 | 0.86 | 0.1 | 0.91 | 7.42 | 0.94 | 1.6 | 791.6 | 9.71 |
Table 6: High performance thin layer chromatography chromatogram of CPAQ at 366 nm
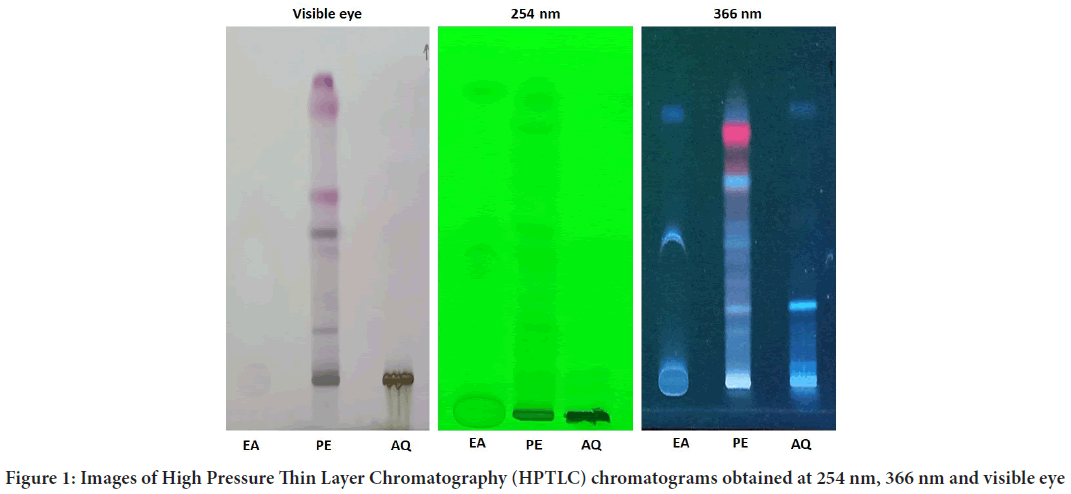
Figure 1: Images of High Pressure Thin Layer Chromatography (HPTLC) chromatograms obtained at 254 nm, 366 nm and visible eye
Figure 2: HPTLC chromatogram of Calotropis procera Ethyl Acetate extract (CPEA) at 254 nm and 366 nm
Figure 3: HPTLC chromatogram of Calotropis procera Petroleum Ether extract (CPPE) at 254 nm and 366 nm
Figure 4: HPTLC chromatogram of Calotropis procera Aqueous extract (CPAQ) at 254 nm and 366 nm
Figure 5: High Pressure Thin Layer Chromatography (HPTLC) densitogram of different peaks of CPPE, CPEA and CPAQ at 254 nm
Gas Chromatography-Mass Spectrometry (GC-MS)
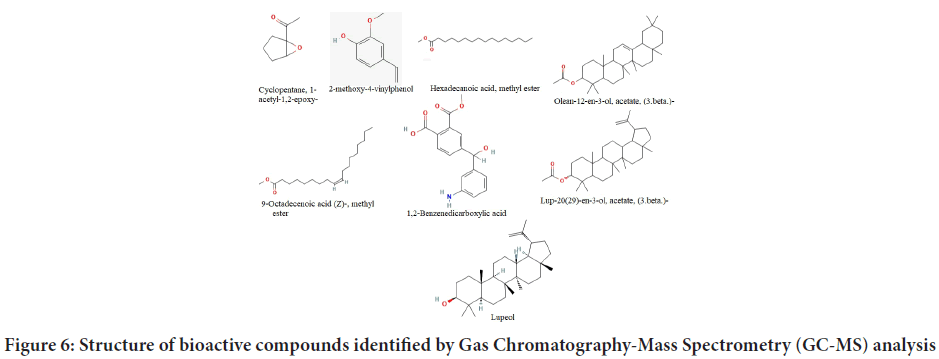
GC-MS spectra analysis of the EECP indicated the existence of various phytochemical components (Figure 6). By comparing their mass spectra to those in the NIST libraries, (Table 7) the phyto-compounds were iden- tified and characterized. The biological active compounds cyclopentane, 1-acetyl-1,2-epoxy-, 2-methoxy-4-vinylphenol, hexadecanoic acid, methyl ester n-hexadecanoic acid, 9-Octadecenoic acid (Z)-methyl ester, 1,2-Ben- zenedicarboxylic acid, Olean-12-en-3-ol, acetate, (3.beta.)-Lup-20(29)-en- 3-ol, acetate, (3 beta) and lupeol were identified and characterized.
| R time | Area | Area% | Compounds |
|---|---|---|---|
| 3.722 | 3542922 | 1.29 | Cyclopentane, 1-acetyl-1,2-epoxy |
| 6.632 | 7436247 | 2.7 | 2-methoxy-4-vinylphenol |
| 13.669 | 5903910 | 2.15 | Hexadecanoic acid, methyl ester |
| 14.14 | 17559366 | 6.38 | n-Hexadecanoic acid |
| 15.364 | 2963999 | 1.08 | 9-Octadecenoic acid (Z)-methyl ester |
| 19.059 | 2823082 | 1.03 | 1,2-Benzenedicarboxylic acid |
| 29.08 | 22689685 | 8.25 | Olean-12-en-3-ol, acetate, (3 beta) |
| 30.086 | 33430866 | 12.15 | Lup-20(29)-en-3-ol, acetate, (3 beta) |
| 30.398 | 7143539 | 2.6 | Lupeol |
Table 7: Major bioactive compounds identified by Gas Chromatography-Mass Spectrometry (GC-MS) analysis
Figure 6: Structure of bioactive compounds identified by Gas Chromatography-Mass Spectrometry (GC-MS) analysis
Antinociceptive activity
Tail flick test: At the 5, 15, 30, 60, and 90 minute time points of the study, the tail flick test revealed that all groups’ reaction time to radiant heat was significantly increased (p<0.001) compared to the control group (Figure 7).
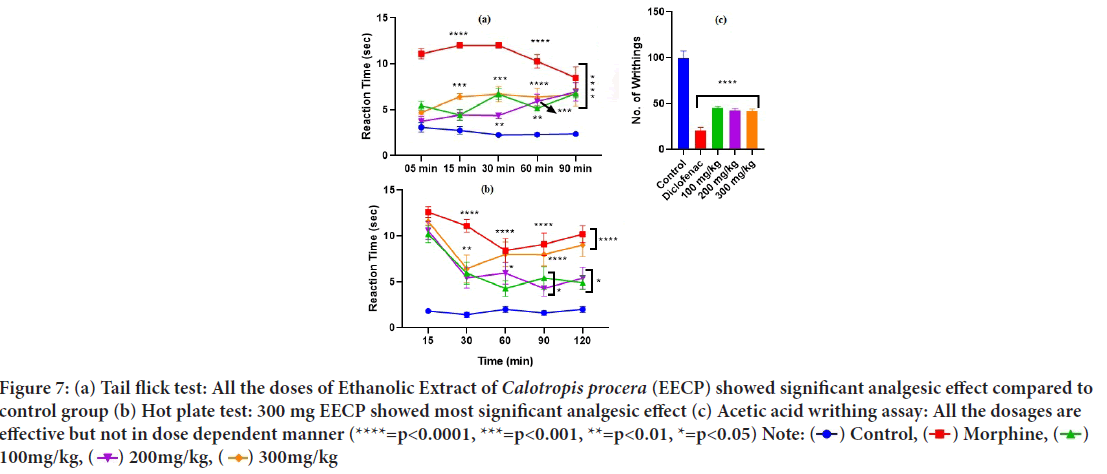
Figure 7: (a) Tail flick test: All the doses of Ethanolic Extract of Calotropis procera (EECP) showed significant analgesic effect compared to
control group (b) Hot plate test: 300 mg EECP showed most significant analgesic effect (c) Acetic acid writhing assay: All the dosages are
effective but not in dose dependent manner (****=p<0.0001, ***=p<0.001, **=p<0.01, *=p<0.05) Note:  Control,
Control,  Morphine,
Morphine,  100mg/kg,
100mg/kg,  200mg/kg,
200mg/kg,  300mg/kg
300mg/kg
Hot plate test: When mice were placed on a hot plate, the time it took for them to jump and remove their paws has been observed. All the treatment groups’ reaction times increased significantly (p<0.001) as compared to the control group at 15, 30, 60, 90, and 120 minutes (Figure 7).
Acetic acid writhing assay: EECP produced a significant decrease in the acetic acid induced writhing’s at all dosage. The number of writhing’s ob- served at 100 mg/kg, 200 mg/kg and 300 mg/kg doses were 45.6 ± 1.7, 42.4 ± 2.2 and 41.4 ± 2.7, respectively, as against the control group (99.2 ± 8.0) (p<0.001) (Figure 7).
Discussion
In the present study the EECP extract was subjected to HPTLC and GC- MS analysis, further extract was analyzed for analgesic activity. The re- sults of HPTLC showed many peaks in the partitioned fraction. Further, GC-MS spectra analysis of the EECP indicated the existence of various phytochemical components. By comparing their mass spectra to those in the National Institute of Standards and Technology (NIST) libraries, the phyto-compounds were identified and characterized. These are the biologically active compounds such as lupeol, in an study, has shown po- tent analgesic and anti-inflammatory activity (Rathinavel T, et al., 2021), whereas n-hexadecanoic acid has shown an anti-inflammatory property that inhibits phospholipase A2. The analgesic assays were used to look into both central and peripherally mediated actions. Three animal models were used to investigate the analgesic effects of EECP. In tail flick latency, EECP flower exhibits strong analgesic effect. However, some research suggests that the clinical outcomes of the tail flick test are disputed (Deuis JR, et al., 2017). At a dose of 300 mg/kg, the analgesic effect of EECP flower was most noticeable in the hot plate test. Supraspinal reactions are shown on the hot plate, while spinal responses are shown on the tail flick (Rezaee-Asl M, et al., 2014). While the tail flick test is simple to perform, it’s important to note that spinally transected rats exhibit a similar behavioral response, suggesting that the tail withdrawal response is a spinal reflex rather than a measure of pain behaviour requiring higher brain centers. This implies that alterations in motor processing could affect the tail flick response (Deuis JR, et al., 2017). The use of acetic acid to cause abdominal constriction is a sensitive method for identifying analgesics with peripheral effect (Yimer T, et al., 2020). Injections of acetic acid into the peritoneal cavity cause inflam- matory mediators (histamine and bradykinin) to be released, stimulating nociceptive nerve fibres. Data is sent to the spinal cord and higher brain regions viathese fibres, where it is integrated and modified (Ahmed S,et al., 2015). In an acetic acid-induced writhing test, EECP flower significantly reduced the average number of writhings when compared to the control group at all three doses. Antioxidants helps to mitigates oxidative stress (Singh P, et al., 2022). Presence of antioxidants and other anti-inflamma- tory compounds in the extract may be responsible for analgesic activity of the extract.
Conclusion
The HPTLC and GCMS results revealed the presence of bioactive com- pound with analgesic property. Ethanolic Extract of Calotropis proceraflower has pain ameliorative effect in acetic acid writhing test, hot plate and tail flick tests. These findings indicated that antinociceptive effect was mediated by both cerebral and peripheral pathways.
Acknowledgements
The authors are thankful to Dr. Vibhav Gautam, Assistant professor, Cen- tre of Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, for providing his lab facility.
References
- Sajeesh T, Parimelazhagan T. Analgesic, anti-inflammatory, and GC-MS studies on Castanospermum australe A. Cunn. and C. Fraser ex Hook. Scientific World Journal. 2014.
[Crossref] [Google Scholar] [Pubmed]
- Ghlichloo I, Gerriets V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). StatPearls. 2021.
- Hörl WH. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals. 2010; 3(7): 2291-2321.
[Crossref] [Google Scholar] [Pubmed]
- Dhileepan K. Prospects for the classical biological control of Calotropis procera (Apocynaceae) using coevolved insects. Biocontrol Sci Technol. 2014; 24(9): 977-998.
- GRIN-Global. Gene bank of Calotropis procera (Aiton) W. T. Aiton. GR Database of the National Genebank of Tunisia (GRIN-Global). 2021.
- Silva MC, da Silva AB, Teixeira FM, de Sousa PC, Rondon RM, Júnior JE, et al. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pac J Trop Med. 2010; 3(4): 332-336.
- Quazi S, Mathur K, Arora S. Calotropis procera: An overview of its phytochemistry and pharmacology. Indian J Drugs. 2013; 1(2): 63-69.
- Jalalpure SS, Salahuddin M, Imtiyaz Shaikh M, Manvi FV. Anticonvulsant effects of Calotropis procera root in rats. Pharm Biol. 2009; 47(2): 162-167.
- Obese E, Biney RP, Henneh IT, Adakudugu EA, Anokwah D, Agyemang LS, et al. The anticonvulsant effect of hydroethanolic leaf extract of Calotropis procera (Ait) R. Br.(Apocynaceae). Neural Plast. 2021.
[Crossref] [Google Scholar] [Pubmed]
- Kumar A, Kumar B, Kumar R, Kumar A, Singh M, Tiwari V, et al. Acute and subacute toxicity study of ethanolic extract of Calotropis procera (Aiton) Dryand flower in Swiss albino mice. Phytomedicine Plus. 2022; 2(2): 100224.
- Costa AR, de Lima Silva JR, de Oliveira TJ, da Silva TG, Pereira PS, de Oliveira BEF, et al. Phytochemical profile of Anacardium occidentale L.(cashew tree) and the cytotoxic and toxicological evaluation of its bark and leaf extracts. S Afr J Bot. 2020; 135: 355-364.
- Iauk L, Galati EM, Kirjavainen S, Forestieri AM, Trovato A. Analgesic and antipyretic effects of Mucuna pruriens. Int J Pharmacogn. 1993; 31(3): 213-216.
- Fadda AA, Elattar KM. Design and synthesis of some enaminonitrile derivatives of antipyrine as potential novel anti-inflammatory and analgesic agents. J Biosci Med. 2015; 3(11): 114.
- Rathinavel T, Ammashi S, Shanmugam G. Analgesic and anti-inflammatory potential of Lupeol isolated from Indian traditional medicinal plant Crateva adansonii screened through in vivo and in silico approaches. J Genet Eng Biotechnol. 2021; 19(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017; 10: 284.
[Crossref] [Google Scholar] [Pubmed]
- Rezaee-Asl M, Sabour M, Nikoui V, Ostadhadi S, Bakhtiarian A. The study of analgesic effects of Leonurus cardiaca L. in mice by formalin, tail flick and hot plate tests. Int Sch Res Notices. 2014.
[Crossref] [Google Scholar] [Pubmed]
- Yimer T, Birru EM, Adugna M, Geta M, Emiru YK. Evaluation of analgesic and anti-inflammatory activities of 80% methanol root extract of Echinops kebericho M. (Asteraceae). J Inflamm Res. 2020; 13: 647.
[Crossref] [Google Scholar] [Pubmed]
- Ahmed S, Naved A, Khan RA, Siddiqui S. Analgesic activities of methanol extract of Terminalia chebula fruit. Pharmacology and Pharmacy. 2015; 6: 547-553.
- Singh P, Verma K, Dixit J, Rai V, Narayan G, Tiwari KN, et al. Panchvalkal (polyherbal formulation) mitigates STZ induced type 2 DM by modulating the expression of Hexokinase (HX), Lactate Dehydrogenase (LDH), Triphosphate Isomerase (TPI). Phytomedicine Plus. 2022; 2(1): 100193.
Author Info
Ashutosh Kumar1, Brijesh Kumar1*, Rajesh Kumar1, Ajay Kumar1, Manish Singh1, Pratistha Singh2, Vinod Tiwari3 and Anshuman Trigunayat12Department of Opthalmology, University of California, Los Angeles, USA
3Department of Pharmaceutical Engineering and Technology, Indian Institute of Technology (BHU), Varanasi, India
Citation: Kumar A: Assessment of Pain-Ameliorative Potential of Ethanolic Extract of Calotropis procera and its Active Components characterization by HPTLC and GC-MS Analysis
Received: 29-Aug-2022 Accepted: 23-Sep-2022 Published: 30-Sep-2022, DOI: 10.31858/0975-8453.13.9.581-586
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3