Research Article - (2023) Volume 14, Issue 6
Abstract
The goal of this study was to see whether the aqueous and ethanolic extracts of Bauhinia variegata Linn. root had any antinociceptive effects in streptozotocin (STZ)-induced diabetic rats. A diabetic animal was created by injecting STZ (60 mg/kg, intraperitoneally) once. Hot-plate test, tail-flick test, and formalin were used to determine nociceptive thresholds after five weeks of diabetes induction. Grip strength was used to determine muscle strength during diabetic neuropathic pain. We looked at total protein and blood glucose biochemical indicators to identify an oxidative stress marker, antioxidant levels in the sciatic nerve (LPO, SOD, CAT, and GSH).
In comparison to control rats, diabetic rats had significantly higher levels of nociception and hyperalgesia. Repeated treatment of Bauhinia variegata extract decreased mechanical allodynia and restored the physiological feeling of heat pain in diabetic rats, induced with streptozotocin (STZ). The extracts significantly (p<0.001) improved the diabetic rats’ lipid peroxidation status and antioxidant enzyme (superoxide dismutase, catalase) levels.
Keywords
Diabetic neuropathy, Neurodegeneration, Nociception, Bauhinia variegata Linn, Polyphenols
Introduction
Diabetes, often known as Diabetes Mellitus (DM), is a group of metabolic illnesses characterized by chronically increased blood sugar levels (hyperglycaemia). Common symptoms include frequent urination, increased thirst, and increased appetite (WHO, 2022). Diabetes, if left untreated, may have a variety of negative health implications. Acute consequences include hyperosmolar hyperglycemia, diabetic ketoacidosis, and even death (WHO, 2020). Serious long-term complications include cardiovascular illness, stroke, chronic renal disease, foot ulcers, eye damage, nerve damage, and cognitive impairment (Kitabchi AE, et al., 2009; 39. Weerateerangkull P, et al., 2008). Diabetes is caused either due to insufficient pancreatic insulin synthesis or insufficient insulin utilization by the body’s cells. Insulin is the hormone responsible for promoting the entrance of glucose from meals into cells for utilization as energy. Diabetes mellitus is classified into three types-Type 1 diabetes which is caused by the pancreas failure to produce adequate insulin due to loss of beta cell (Kitabchi AE, et al., 2009). This kind was formerly described as “insulin-dependent diabetes mellitus” or “juvenile diabetes” (WHO, 2022). The loss of beta cells is caused by an immunological response (Risérus U, et al., 2009) which is unknown to find out what triggered this immune response (WHO, 2022). Although Type 1 diabetes is most often diagnosed in childhood or adolescence, it may also attack adults.
Insulin resistance is a condition in which cells do not respond properly to insulin, is a precursor to type 2 diabetes (WHO, 2022). If the disease develops, an insulin shortage may ensue. This kind was formerly known as “adult-onset diabetes” or “non-insulin-dependent diabetes mellitus” (WHO, 2022). Type 2 diabetes is more common in the elderly, but younger individuals are increasingly developing it at a faster rate owing to an increase in childhood obesity. The most common cause is a combination of high body weight and inadequate exercise.
Gestational diabetes, is the third primary type of diabetes which affects pregnant women who have never had the condition. Blood sugar levels in women with gestational diabetes frequently returns to normal after delivery. Women who had gestational diabetes during their pregnancy, on the other hand, are more likely to develop type 2 diabetes later in life. Insulin injections are the sole approach to treat type 1 diabetes. Type 2 diabetes may be avoided and controlled by eating a well-balanced diet, exercising often, maintaining a healthy body weight and avoiding tobacco use. Type 2 diabetes may be treated with oral anti-diabetic medications, either with or without insulin (WHO, 2020). Managing blood pressure and maintaining sufficient foot and eye care are critical for persons suffering from this condition. Oral medications and insulin both may produce low blood sugar (hypoglycaemia) (Irwin RS and Rippe JM, 2010). For those with type 2 diabetes, weight reduction surgery may be a possibility (CDC, 2019). Often, gestational diabetes resolves completely once the baby is delivered. Diabetes affected 463 million people worldwide in 2019 (8.8% of the adult population), with type 2 diabetes accounting for over 90% of occurrences. Male and female rates are similar (Vos T, et al., 2012). Trends indicate that the rates will continue to rise. Diabetes increases the likelihood of dying young by at least double. Diabetes claimed the lives of nearly 4.2 million people in 2019. It is the world’s seventh leading cause of death. Diabetes-related medical expenses were estimated to cost the global economy $727 billion in 2017. Diabetes cost the United States around $327 billion in 2017 (WHO, 2020). Diabetes patients spend 2.3 times more on medical treatment on average.
Signs and symptoms
Despite the fact that they are not diabetes-specific, a variety of additional signs and symptoms might suggest the onset of the condition. In addition to the usual symptoms, these include blurred vision, headache, fatigue, poor wound healing, and itchy skin. Long-term high blood sugar levels may cause glucose absorption in the lens of the eye, affecting the curvature of the lens and affecting vision. Moreover, diabetic retinopathy may cause long-term vision loss. Unintentional weight loss, polyuria, polydipsia, and polyphagia (increased hunger), are typical signs of uncontrolled diabetes, as shown in Figure 1. Type 1 diabetes symptoms may arise rapidly (within weeks or months), but type 2 diabetes symptoms may occur gradually or possibly not at all.
Figure 1: Summary of the key signs and symptoms of diabetes
Causes
Diabetes mellitus is classified into six types-type 1 diabetes, type 2 diabetes, hybrid forms of the disease, hyperglycemia first seen during pregnancy, “unclassified diabetes,” and “other particular kinds.” (American Diabetes Association, 2018) “Hybrid kinds of diabetes” include type 2 diabetes that is prone to ketosis and immune-mediated adult diabetes. Gestational diabetes mellitus is defined as “hyperglycemia discovered during pregnancy” (type 1 or type 2 diabetes first diagnosed during pregnancy). The “other special categories” are made up of over a dozen separate criteria. Diabetes is a more complex condition than previously thought, with people experiencing a wide range of symptoms.
Type 1 diabetes
Type 1 diabetes is characterized by the loss of insulin-producing beta cells in the pancreatic islet, resulting in insulin insufficiency. This kind is further subdivided into immune-mediated and idiopathic subgroups. The bulk of type 1 diabetes is immune-driven, with an autoimmune attack mediated by T cells resulting in the loss of beta cells and, ultimately, insulin (Rother KI, 2007). It is responsible for around 10% of diabetes mellitus cases in North America and Europe. When symptoms first occur, the majority of patients are in good health and at a healthy weight. Insulin sensitivity and response is often typical, particularly early on. While it was often known as “juvenile diabetes” because it usually affected children and the majority of patients with type 1 diabetes today are adults. The term “brittle” diabetes, also known as unstable diabetes or labile diabetes, was formerly used to describe the significant and frequent variations in blood sugar levels that occur in insulin-dependent diabetes for no obvious reason. This term, however, should not be utilized since it lacks a biological base (Merck manual professional, 2010). Nevertheless, symptoms of type 1 diabetes include fluctuating and unexpected high and dangerously low blood sugar levels and the risk of diabetic ketoacidosis. Other complications include infection, gastroparesis (irregular dietary carbohydrate absorption), and endocrinopathies (such as Addison’s disease). Another issue is an impaired counter-regulatory response to low blood sugar (Merck manual professional, 2010). It is estimated that this condition occurs in 1%-2% of patients with type 1 diabetes. A variety of genes, including particular HLA genotypes, are known to impact the risk of type 1 diabetes. Diabetes may be caused by one or more environmental causes, such as a viral infection or poor diet, in people who have a genetic risk. While additional viruses have been proposed, there is currently insufficient clear evidence to support this notion in humans (Petzold A, et al., 2015; Butalia S, et al., 2016). While the mechanism is not fully understood, research suggests that gliadin (a protein present in gluten) is one dietary component that may contribute to the formation of type 1 diabetes. Type 1 diabetes may strike at any age, and the majority of cases are found in adulthood. When type 1 diabetes emerges in adults, it is referred to as Latent Autoimmune Diabetes of Adults (LADA), and it progresses more slowly than in children. As a result of this discrepancy, some individuals refer to this condition as “type 1 diabetes.” Adults with LADA are sometimes misdiagnosed as having type 2 diabetes because of their age rather than a reason.
Type 2 diabetes
Type 2 diabetes is characterized by insulin resistance and perhaps reduced insulin production (Shoback DM and Gardner DG, 2018). It is considered that the insulin receptor has a role in the decreased sensitivity of body tissues to insulin. Nevertheless, there are specific problems which are still unknown. Diabetes mellitus cases with a recognized defect are classified differently. Type 2 diabetes accounts for 95% of all cases and is also the most common (WHO, 2020). Many people with type 2 diabetes exhibit indications of prediabetes (impaired fasting glucose and/or impaired glucose tolerance) before they meet the criteria for the condition (American Diabetes Association, 2016). By adopting lifestyle changes or taking medicines that raise insulin sensitivity or lower the liver’s production of glucose, prediabetes may be delayed or avoided from progressing to overt type 2 diabetes. Type 2 diabetes is mostly caused by genetics and lifestyle decisions (Risérus U, et al., 2009). Obesity (defined as a body mass index more than 30), inactivity, poor diet, stress, and urbanization are all recognized to have a substantial impact in the development of type 2 diabetes (Melmed S, et al., 2012). Excess body fat is connected to 30% of cases in individuals of Chinese and Japanese descent, 60%-80% of cases in people of European and African heritage, and 100% of cases in Pima Indians and Pacific Islanders (Shoback DM and Gardner DG, 2018). People of any weight might have a high waist-hip ratio.
Bauhinia variegata Linn. and its chemical constituents
Mountain Ebony (Bauhinia variegata Linn.), is a medium-sized deciduous tree native to India that may grow to 1,300 meters in the Himalayas. The plant is widely used by tribal people in India and is a component of many traditional medicinal systems, including Ayurveda, Unani, and Homeopathy. Scientists have worked hard to demonstrate the plant’s effectiveness through pharmacological research, taking into account the various traditional claims about its ability to cure a variety of ailments. Grahi, Krimighna, Kushtaghna, Gandamalanashaka, Vranaropaka, Mehaghna, and Raktapittashamak are among the medications shown. Researchers have worked hard to highlight the plant’s organic and concoction potential, pharmacological properties of Bauhinia variegata Linn. including antioxidant activity, nephroprotective activity against growth, hepatoprotective activity against oxidants, and immunomodulatory activity against germs. Kanchanara Guggulu, Kanchan gutika, Gandamala kundan rasa, Gulkand Kanchanara, and Kanchanaburi Kwatha are all real ingredients in Ayurvedic medicine. Genuine components include Ushirasava, Chandanasava, Vidangarishta, Kanchanalak Drava, and Kanchanara Varuna Kwatha (Azevedo CR, et al., 2006). Kanchanara refers to and is used for several types of Bauhinia in Indian medicine. The illustrated Bauhinia variegata Linn. is related to Bauhinia racemosa Linn. and Rakta Kanchnar. Peeta Kanchnar is also known as Shveta Kanchnar in Bhavaprakash, in addition to Bauhinia variegata Linn., Bauhinia purpurea Linn., and Bauhinia tomentosa. Bauhinia Linn. is a tropical plant family that includes shrubs, trees, and sometimes climbers (Caesalpiniaceae). This family is available in 15 distinct types in India (Bairagi SM, et al., 2012). Bauhiniasare mostly produced from seeds, and vegetative proliferation rather than inarching has not been very successful. Several important machinery products, like tannins, fiber, gum, and oil, are derived from Bauhinia species. The bulk of the plant species have been developed for decorative purposes, and they produce aromatic and beautiful blossoms. Moreover, Bauhinias are being grown for reforestation and the manufacturing of wood fleece boards. Among them are B. tomentosa Linn., B. racemosa Lam., B. retusa Roxb., B. purpurea Linn., B. variegata Linn., and B. malabarica Roxb. are found and have widespread usage in conventional drug frameworks (Bodakhe SH and Ram A, 2007) (Table 1).
| Root | Chemical constituents |
|---|---|
| Bauhinia variegata Linn. | Flavanone |
| Dihydro dibenzo xepin | |
| Flavonol glycoside-5, 7, 3, 4-tetrahydroxy-3-methoxy-7-0-alpha-L-rhamnopyranosyl (1-3)-0-beta galactopyranoside | |
| (2S)-5, 7-dimethoxy-3,4-methylenedioxy flavanone | |
| Dihydro dibenzo xepin | |
| 5,6-dihydro-1,7 dihydroxy-3,4-dimethoxy-2-methyldibenz (b,f) oxepin |
Table 1: Chemical constituents of Bauhinia variegata Linn
Pharmacological activities
Hepatoprotective effect: In rats, an ethanolic concentrate of B. variegatastem exhibited chemoprevention against a test liver tumor produced by N-nitrosodiethylamine. Decrease in N-nitrosodiethylamine-induced higher levels of blood glutamate pyruvate transaminase, glutamate oxaloacetate transaminase, basic phosphatase, add up to bilirubin, gamma glutamate Trans peptidase, lipid peroxidase, glutathione peroxidase, and glutathione-S-transferees showed (Konrad RJ, et al., 2001). The stem bark of B. variegatawas concentrated in ethanol, and oral dosages of 100 and 200 mg/kg demonstrated hepatotoxicity in rats exposed to carbon tetrachloride, as well as decreased levels of AST, ALT, ALP, and GGTP. The injury-recovery effects of Bauhinia variegata’s foundation ethanolic and fluid concentrates were studied in pale skinned wistar rats employing extraction and entry point twisted models. The doses utilized were 200 and 400 mg/kg of body weight (b.w). In the extraction wound model and cut injury models, both fluid and ethanol concentrations of the Bauhinia variegata foundation caused significant damage recovery, which was equivalent to the standard (framycetin) in the cut injury model (Azevedo CR, et al., 2006).
Cancer-fighting properties: An in vitro study found that Bauhinia variegata extract has an anticancer effect by reducing the growth of certain cell lines. Another study found that cyclophosphamide-induced mutagenesis in mouse bone marrow cells revealed that methanolic concentrate of Bauhinia variegata leaves at concentrations of 300, 600, and 900 mg/kg demonstrated anti-mutagenic activity by preserving the arrangement of micronucleus and chromosomal aberrations (Bairagi SM, et al., 2012). Streptozotocin (STZ) and alloxan-induced diabetic rats were given oral administration of ethanolic, fluid, and hydro-alcoholic concentrates of Bauhinia variegata’s leaves and stem bark at different dosages, i.e. 200 and 400 mg/kg, which reduced elevated blood glucose levels by enhancing glucose metabolism (Azevedo CR, et al., 2006).
Materials and Methods
Plant material
The root of the Bauhinia variegata Linn. obtained and certified in Bangalore, Karnataka, was given by the Regional Research Institute (Ay). (India). A voucher specimen with the RRCBI MCW 79/4 voucher specimen number has been placed.
Preparation of the root extract
The confirmed root was coarsely crushed and shade dried. Conventional extraction procedures were applied, along with analytical grade solvents. Pet-ether extraction was used to defeat the powdered medicine (60°C-800°C). To make a coarse powder, the root was soxhlet extracted with 90% ethanol (1 kg). The aqueous extract was created by the maceration method. The extracted mixture was concentrated at low pressure to yield ethanol (4.2%) and aqueous extracts (2.4%).
Animals
Healthy Wistar albino rats of either sexes weighing between 150-200 g were utilized in the study. The animals were all bought from the NIMS University’s Central Animal Home. The animals were converted by spending a week in the animal home facilities at the NIMS Institute of Pharmacy in Jaipur. They were housed in polypropylene cages of 32 × 24 × 16 cm with husk bedding in a controlled environment with 12 hours of light and 12 hours of darkness and a constant temperature of 25°C. The animals were fed a standard pellet diet and had unfettered access to water. Permission was acquired from the Institutional Animals Ethics Committee (IAEC) of the NIMS Institute of Pharmacy, Jaipur, to conduct diabetic neuropathy and cardioprotective activities. The registration number is IAEC/NIMS PH/JPR/12/2011.
Diabetic neuropathy model
Study design: The animals, which weighed between 180 and 200 g, were divided into five groups of six rats each. The control group was Group I, the streptozotocin-induced diabetes control group was Group II, and the standard (gabapentin) group was Group III. Aqueous Extracts of Bauhinia variegata groups IV and V (BVAE) and Ethanolic Extracts of Bauhinia variegata (BVEE), respectively (Table 2).
| Groups | Treatment | Before treatment | After treatment | Weight change | Percentage weight change |
|---|---|---|---|---|---|
| Group I | Control | 180.75 ± 6.34 | 208.56 ± 3.46 | 27.81 ± 3.21 | 15.38 ± 2.52 |
| Group II | Diabetic | 182.32 ± 4.54 | 148.34 ± 4.65 | -33.98 ± 2.32 | -18.63 ± 4.11 |
| Group III | CPAE | 183.62 ± 7.43 | 197.54 ± 5.31*** | 13.92 ± 5.31*** | 7.58 ± 3.54 |
| Group IV | BVAE | 175.76 ± 3.45 | 191.23 ± 4.75*** | 15.47 ± 3.45*** | 8.8 ± 2.34 |
| Group V | BVEE | 177.21 ± 6.32 | 196.32 ± 2.34*** | 19.11 ± 4.42*** | 10.78 ± 4.51 |
Note: Results are mean standard deviation, n=6; ***p<0.001 when compared to the diabetes control group, and (p<0.001) when compared to the control group
Table 2: Weights of rats before and after treatment in STZ induced diabetic neuropathy model in rats
Procedure: Healthy albino Wistar strain rats weighing between 150 and 200 g were utilized in both sexes. The animals were given unlimited access to water after a 16-hour feeding fast. The quantity of blood sugar in the rats’ tails was then assessed using a digital display glucometer. The mice were then given an intraperitoneal injection of 60 mg/kg streptozotocin diluted in 0.1 M sodium citrate and citric acid. After that, the animals were detained for 35 days while being fed and watered. Each rat’s blood glucose levels were examined once a week after the medicine or extracts were administered orally. Body weight, grip strength, and pain perception were measured after five weak streptozotocin doses. Once fasting blood glucose levels exceeded 250 mg/dl, animals were divided into groups of six and labeled diabetic (Risérus U, et al., 2009; Melmed S, et al., 2012; Azevedo CR, et al., 2006).
Physical parameters
• Body weight was recorded on the first and last days of the experiment since diabetes in animals progressively affects body weight.
• (Grip-strength) The rota rod device was used in the test to assess muscular strength or neuromuscular function by putting the rats on a horizontal rod moving at a speed of 25 rpm. Rats that could remain on top for at least 25 seconds on three consecutive times were selected for the research. The length of the rod in use was calculated.
Measurement of nociceptive threshold
Tail flick: Animals exhibited abnormal behavior when subjected to aversive stimuli such as tail flicks. Animals’ tails are permitted to dangle freely after they are placed in separate cages. Before testing, the animals are given 30 minutes to become acclimated to their new surroundings. The bottom 5 cm of the rat’s tail has been tagged, and it was immersed in hot water held at 55°C. There were signs of struggle or a delay in the basal tail flick (tail withdrawal reaction). The time restriction was set at ten seconds.
Eddy’s hot plate method: Thermal sensitivity was measured using an eddy’s hot plate while the temperature was kept at 55°C-56°C and observations were made. The cutoff time was set at ten seconds. After oral administration of the test substances and drugs, the average baseline reaction time was measured.
Formalin test: After growing habituated to the box, each animal was given a subcutaneous injection in the hind paw using a 25-gauge syringe needle. It may be used to mimic chemical sensitivity or chemical stimulation. A nociceptive score was determined for each rat by calculating the amount of time spent in each of the four behavioral categories after 15 minutes in a 5 minute clock. The position and posture of the injected hind paw may be recognized from the opposing paw. The injected paw is lifted without having contact with any surface and licked, bit, or shook. The amount of time spent in each category was then multiplied, added, and divided by the total length for each 5 or (300 sec) minute block of time to provide a weighted nociceptive score ranging from 0 to 3. The observations were maintained for 60 minutes after the animals were placed in the box (Bianchi R, et al., 2004; Calcutt NA, et al., 1996; Khalili M, 2009).
Biochemical parameters
Blood glucose level measurement: Blood was drawn from the tails of rats in order to measure blood glucose levels. The plasma was obtained after centrifugation (3000 rpm for 10 minutes at 4°C). Blood glucose was estimated using the GOD-POD kit approach.
Total protein: Since it is considered that the total proteins of sick animals are abnormal, the amount of total protein was assessed at 546 nm using a typical approach and commercially available diagnostic kits.
Enzymatic estimation
Preparation of sciatic nerve for estimation of oxidative stress marker: After the completion of the experiment, the sciatic nerves of three animals from each group were removed, weighed, and homogenized in a teflon glass homogenizer using ice cold phosphate buffer saline 10 times (w/v) (50 mM pH 7.8). The homogenate was centrifuged at 1000 rpm for 3 minutes at 4°C, and the supernatant was separated into two parts. One fraction of the supernatant was used to assess Lipid Peroxidation (LPO), while the other was used to assess Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione (GSH).
Results
Physical parameters
Effect of plant extracts on body weight: After 35 days of STZ induction, diabetic neuropathy was diagnosed using muscular grip strength measurements. The muscular grip strength was dramatically diminished in STZ-treated groups that demonstrated diabetic neuropathy induction. The muscular grip strength in the normal control group was normal (30.33 ± 1.21 min), therefore there was no statistically significant difference discovered in the control group (p<0.001), but there was a significant difference detected in the diabetes induced group (4.17 ± 1.47 min, p<0.001). Gabapentin substantially enhanced grip strength in both test group animals (p<0.001) as compared to the diabetic control group (Table 3). The proportion of weight increase in diabetic rats not getting therapy was much lower when compared to the normal control and treated groups (p<0.001).
| Groups | Treatment | Total protein (g/l) | Grip strength (sec) |
|---|---|---|---|
| Group I | Control | 87.53 ± 3.27 | 30.33 ± 1.21 |
| Group II | Diabetic | 54.36 ± 4.52 | 4.17 ± 1.47 |
| Group III | CPAE | 70.48 ± 2.47** | 18.33 ± 2.32 *** |
| Group IV | BVAE | 71.89 ± 1.46** | 17.67± 1.03 *** |
| Group V | BVEE | 73.38 ± 2.74** | 19.33 ± 2.16*** |
Note: Results are mean standard deviation (SD) and n=6 **p<0.01, ***(p<0.001) when compared to the diabetes control group, and (p<0.001) when compared to the control group
Table 3: Effect of plant extracts on grip strength by using rota rod in STZ induced diabetic neuropathy model in rats
Effect of plant extracts on grip strength: Diabetic neuropathy was detected after 35 days of STZ induction utilizing muscular grip strength assessments. Muscular grip strength was significantly reduced in individuals treated with STZ who developed diabetic neuropathy. There was no statistically significant difference identified in the control group (p=0.001) because the muscular grip strength in the normal control group was normal (30.3 ± 31.21 min), but a significant difference was observed in the diabetes-induced group (4.17 ± 1.47 min, p=0.001). Gabapentin and both test group animals showed significantly stronger grips when compared to the diabetes control group (p<0.001) (Table 3).
Measurement of nociceptive threshold: When the STZ diabetic control group was delivered using the tail flick technique, their response time was significantly (p<0.001) shorter than the normal control group. After 5 weeks of STZ injection, the withdrawal latency in diabetes control rats was found to be shorter (3.23 ± 0.23 s) than in non-diabetic control rats (9.76 ± 0.54 s). This decreased mean tail withdrawal latency is considerably (p<0.05) reduced by BVAE (6.72 ± 0.63 s) and BEE (7.42 ± 0.76 s). Hyperalgesia was significantly reduced (p<0.05) in extract-treated mice (Table 4). In the hot plate technique, STZ-induced diabetes rats had a significantly (p<0.001) lower paw withdrawal reaction (2.27 ± 0.87 s) than control rats (8.14 ± 0.54 s). The paw withdrawal response Standard (6.42 ± 0.25 s), BVAE (5.96 ± 0.58 s), and BVAE (6.53 ± 0.27 s) were significantly (p<0.001) increased when compared to diabetic control rats after treatment with aqueous extract of plants (Table 4). The formalin test findings investigated whether diabetic control group rats had an enhanced nociceptive response and hyperalgesia behavior in response to chemical stimuli. As compared to the diabetic control group (3.21 ± 0.12), the test score of standard (2.15 ± 0.14), BVAE (2.46 ± 0.32), and BEE (2.27 ± 0.07) treated animals was substantially (p<0.01) lower.
| Groups | Treatment | Tail flick (Reaction time in sec) | Hot plate (Reaction time in sec) | Formalin test (Test score) |
|---|---|---|---|---|
| Group I | Control | 9.76 ± 0.54 | 8.14 ± 0.54 | 1.65 ± 0.06 |
| Group II | Diabetic | 3.23 ± 0.23 | 2.27 ± 0.87 | 3.21 ± 0.12 |
| Group III | CPAE | 8.86 ± 0.76** | 7.48 ± 0.25*** | 1.65 ± 0.14** |
| Group IV | BVAE | 6.72 ± 0.63* | 5.96 ± 0.58* | 2.46 ± 0.32*** |
| Group V | BVEE | 7.42 ± 0.76** | 6.53 ± 0.27** | 2.27 ± 0.07*** |
Note: The data were presented as mean S.D. (n=6) and were analyzed using one way ANOVA followed by the dunnett's comparison test where *p<0.05, **p<0.01, ***p<0.001 when compared to the diabetic control group, p<0.001 when compared to the normal control group, ns=when compared to the diabetic control group
Table 4: Effect of various plant extract concentrations on response time in the tail flick, hot plate, tail immersion, and formalin tests in rats with STZ-induced diabetic neuropathy
Biochemical estimation
Effect of plant extracts on plasma glucose level: There was no significant difference in plasma glucose levels between normal non-diabetic rats (120.27 ± 6.21 mg/dl) and diabetic (STZ) control rats (123.87 ± 4.57 mg/dl) before the production of diabetic neuropathy by intraperitoneal injection of STZ. Five weeks following intraperitoneal injection of STZ, diabetic (STZ) control rats had a substantial (p<0.001) rise in plasma glucose level (370.25 ± 6.53 mg/dl) as compared to normal non-diabetic rats. As compared to the diabetic control group, chronic therapy with Gabapentin, BVAE, and BEE for 5 weeks substantially (p<0.001) lowered plasma glucose level (180.37 ± 3.68 mg/dl, 215.82 ± 7.35 mg/dl, 205.62 ± 6.05 mg/dl, respectively) (Table 5).
| Groups | Change in blood glucose levels on different days (mg/dl) | ||||
|---|---|---|---|---|---|
| Day-1 | Day-7 | Day-21 | Day-28 | Day-35 | |
| Group I | 120.27 ± 6.21 | 118.32 ± 3.24 | 121.34 ± 5.43 | 120.26 ± 4.65 | 120.75 ± 4.22 |
| Group II | 123.87 ± 4.57 | 358.75 ± 4.32 | 363.28 ± 6.54 | 368.82 ± 6.43 | 370.25 ± 6.53 |
| Group III | 120.45 ± 4.34 | 327.36 ± 5.32* | 188.32 ± 3.21*** | 208.83 ± 8.42*** | 180.37 ± 3.68*** |
| Group IV | 124.88 ± 8.34 | 323.53 ± 4.57** | 278.21±7.37*** | 218.65 ± 3.55*** | 215.82 ± 7.35*** |
| Group V | 123.76 ± 8.21 | 355.32 ± 5.21ns | 267.54 ± 8.92*** | 199.31 ± 6.87*** | 205.62 ± 6.05*** |
Note: The data were presented as mean S.D. (n=6) and were analysed using one way ANOVA followed by the dunnett's comparison test where *p<0.05, **p<0.01, ***p<0.001 when compared to the diabetic control group, p<0.001 when compared to the normal control group, ns=when compared to the diabetic control group
Table 5: Effect of plant extracts on plasma glucose levels in rats before and after five weeks of STZ therapy
Total protein: In control rats, serum total protein was determined to be (87.53 ± 3.27 g/L) (Table 3). Serum total protein (54.36 ± 4.52 g/L) was considerably (p<0.001) lower in the diabetic control group. Plant extracts of Bauhinia variegata, namely aqueous and ethanolic extracts, substantially (p<0.01) enhanced total protein (75.48 ± 2.47, 71.89 ± 1.46, 73.38 ± 2.74 g/L) in the treatment groups.
Enzymatic estimation: The diabetic control group’s SOD levels were considerably (p<0.001) lower than the normal control group (29.66 ± 0.59). (12.37 ± 0.98). Treatment with aqueous and ethanolic extracts considerably (p<0.001) increased SOD levels as compared to the diabetic group. The diabetes control group’s lipid peroxidation levels (4.51 ± 0.06) were considerably (p<0.001) higher than the control group (1.35 ± 0.03). The lipid peroxidation level in aqueous and ethanolic i.e. BVAE (2.27 ± 0.03) BVEE (2.43 ± 0.05) was considerably (p<0.001) lower in the diabetes group.
Similarly, the diabetic control group had a substantially (p<0.001) lower GSH level (3.82 ± 0.19) than the normal control group (10.26 ± 0.43). As compared to diabetic control groups, extracts treated groups showed substantial (p<0.001) increases in GSH levels. The diabetes control group (2.160.21) had a significant (p<0.001) lower CAT level than the normal control group (7.87 ± 0.23). As compared to diabetic control groups, CAT levels considerably (p<0.001) enhanced therapy in extracted treated groups (Table 6).
| Groups | Treatment | SOD (unit/mg protein) | LPO (nm/mg protein) | GSH (μg/mg protein) | CAT (K/min) |
|---|---|---|---|---|---|
| Group I | Control | 29.66 ± 0.59 | 1.35 ± 0.03 | 10.26 ± 0.43 | 7.87 ± 0.23 |
| Group II | Diabetic | 12.37 ± 0.98 | 4.51 ± 0.06 | 3.82 ± 0.19 | 2.16 ± 0.21 |
| Group III | CPAE | 24.52 ± 0.34*** | 1.59 ± 0.02*** | 8.62 ± 0.42*** | 6.86 ± 0.19*** |
| Group IV | BVAE | 19.32 ± 0.37*** | 2.27 ± 0.03*** | 6.34 ± 0.56*** | 4.57 ± 0.05*** |
| Group V | BVEE | 20.65 ± 0.56*** | 2.43 ± 0.05*** | 7.62 ± 0.45*** | 5.74 ± 0.31*** |
Note: The data were represented as mean S.D. (n=6) and analysed using one-way ANOVA followed by the dunnett's comparison test where *p<0.05, **p<0.01, ***p<0.001 when compared to the diabetic control group, p<0.001 when compared to the normal control group, ns=when compared to the diabetic control group
Table 6: Effect of various Autologous Plasmin Enzyme (APE) dosages on Glutathione (GSH), Lipid Peroxidation (LPO), Superoxide Dismutase (SOD), and Catalase (CAT) levels in the rat sciatic nerve in a STZ-induced diabetic neuropathy model
Morphological changes in sciatic nerve
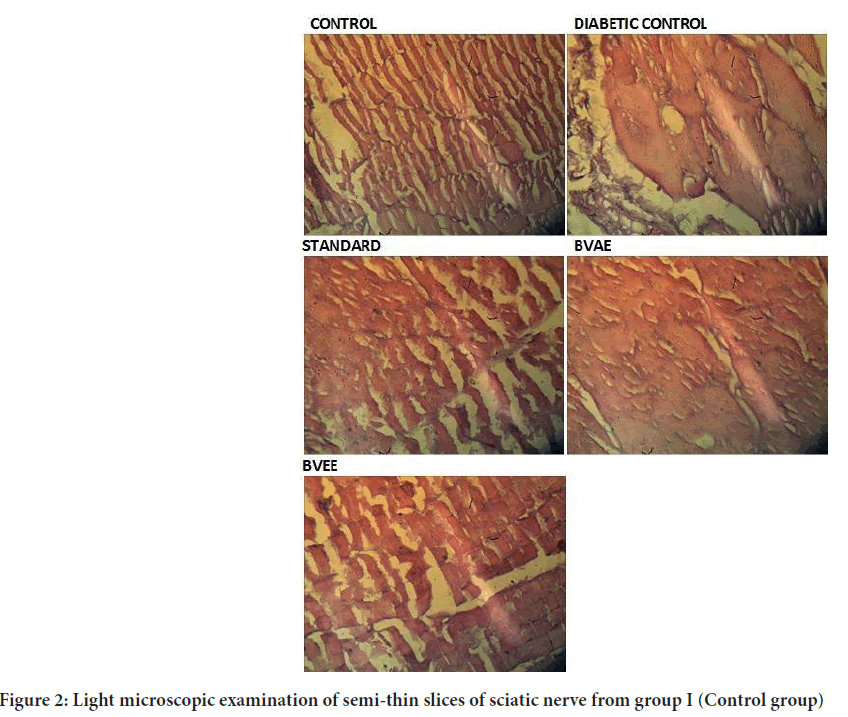
A light microscopic examination of semi-thin slices of sciatic nerve from group I (Control group) revealed numerous myelinated axons and few unmyelinated axons. Blood vessels were seen in the endoneurium between the axons. Collagen fibrils were discovered in the space between nerve axons. The myelin sheaths were made up of many black lamellae that looked close together (Figure 2). A light microscopic examination of semi-thin slices of the diabetes group II (Diabetic group) sciatic nerve revealed many unmyelinated axons of varying sizes. Electron microscopy of the same group revealed splitting in the myelin lamellae. Ranvier’s nodes have uneven neurilemmal terminations. Using light microscopy, the diabetic group’s semi-thin sciatic nerve slices with plant extracts showed numerous myelinated axons. Few axons in those treated with extracts had abnormal myelination. In the connected axon, myelin lamellae seemed to be densely packed. According to quantitative measurement of the myelination state of the nerve fibers using light microscope photomicrographs, hyperglycemia substantially impaired the myelination of nerve fibers in the extraction treatment groups compared to group II. Nevertheless, when compared to group controls, this impact was reduced, indicating that the extracted treated animals had a modest protective effect.
Figure 2: Light microscopic examination of semi-thin slices of sciatic nerve from group I (Control group)
Discussion
Diabetic neuropathy, a devastating side effect of diabetes mellitus, makes patients’ quality of life even worse. Diabetic neuropathy clinical indicators include allodynia, hyperalgesia caused by an enhanced nociceptive response, reduced motor nerve conduction velocity, neuronal hypoxia, lowered threshold to painful stimuli, and so on. Animals exposed to STZ exhibit comparable effects. Clinical pathogenic features, such as biochemical, oxidative, and metabolic changes, have been seen in STZ-injected rats and in humans (Negi G, et al., 2010). The STZ-induced diabetic rat model has been widely explored for the development and effects of the condition (Rees DA and Alcolado JC, 2005). Nevertheless, the STZ dosage and method of administration differ amongst experiments. Despite the fact that a high dose or intravenous route is expected to result in a higher induction rate than a low dose or intraperitoneal route, it may still result in severe diabetes, which can be harmful to the rats’ general health. The rats’ general health, including severe dehydration, electrolyte imbalance, and considerable muscle weakness, may have an effect on their nociceptive threshold (Morrow TJ, 2004; Gong YH, et al., 2011). The findings of this study indicated that a dose of 60 mg/kg STZ delivered intraperitoneally was an appropriate option for creating diabetic animal models without jeopardizing their general health. When streptozotocin is given to test animals, it damages pancreatic beta cells and induces diabetes. It has been claimed that the adverse effects of STZ are caused by oxygen free radicals, namely hydroxyl radicals. Very reactive carbonium radicals generated by the breakdown of STZ molecules may boost the generation of oxygen free radicals. These highly reactive radicals induce nuclear DNA fragmentation in beta cells as well as direct or indirect injury to the islet endothelium. Endogenous CuZn SOD was inactivated in a concentration-dependent manner when intact erythrocytes were exposed to hydrogen peroxide. The precise nature of the decrease in SOD activity may be explained by a direct response to increased formation of active oxygen species such as superoxide and hydroxyl radicals, irreversible inactivation of SOD by its product, hydrogen peroxide, and an increase in non-enzymatic glycation of SOD because zinc is a component of the CuZn catalytic site, and diabetes is associated with low zinc status. It is possible to conclude that the mechanisms of STZ-induced hyperglycemia are attributed to DNA strand breakage in pancreatic islets, stimulation of nuclear poly (ADP-ribose) synthetase, and depletion of intracellular NAD+ and NADP+ levels, which inhibit proinsulin synthesis and induce diabetes, and activation of oxygen species, such as superoxide O2•-, hydrogen peroxide (H2O2 ), hydroxyl radical (•OH as compared to control rats, animals given STZ injections exhibited lower body weights, weaker grips, lower levels of protein synthesis, and lower nociceptive thresholds to painful stimuli (Lenzen S, 2008). Diabetic rats were given plant extracts in a study, and the findings showed an increase in tail flick latencies, tail withdrawal response, paw withdrawal response, reaction time in the grip strength test, and formalin test (Table 7). This might be due to a variety of aetiologies for nociception in diabetic neuropathy rats. The aqueous and ethanolic extracts of Bauhinia variegata significantly (p<0.001) increase body weight in contrast to the diabetic control group, which may be due to its capacity to prevent muscle wasting. These plant extracts’ anabolic and steroidal characteristics imply that the increased body weight in rats treated with the extracts may be due to enhanced metabolic activity (Mu J, et al., 2006; Rahman H and Eswaraiah MC, 2012). In both type 1 and type 2 diabetes, muscle weakness is related with the degree of neuropathy and is hypothesized to be caused by neurogenic atrophy caused by motor fiber axonal degeneration (Akbarzadeh A, et al., 2007). Diabetic rats treated with an aqueous and an ethanolic extract of Bauhinia variegata demonstrated a significant (p<0.001) improvement in motor behavior, particularly grip strength, in the present investigation. In comparison to the diabetic control group, therapy with these indigenous crude extracts resulted in a significant (p<0.001) improvement in grip strength. Due to its anabolic effect, it has the ability to boost physical strength. Allodynia, hyperalgesia from a heightened nociceptive response, reduced motor nerve conduction velocity, neuronal hypoxia, and a diminished tolerance for painful stimuli are clinical symptoms of diabetic neuropathy. Anti-inflammatory drugs counteract this impact by increasing the pain threshold and decreasing the inflammatory process (Ramachandran A, et al., 2009). STZ-induced diabetic mice display similar symptoms, although they take three to four weeks to manifest. The pain threshold was shown to change gradually and rise up to the fifth week of the trial. It’s conceivable that the anti-inflammatory characteristics of all of these plants are what help to alleviate the pain associated with diabetic neuropathy. Diabetic control rats exhibited higher blood sugar levels than normal control rats in the present investigation. Bauhinia variegata has hypoglycemic properties and may help reduce glucose levels by reducing oxidative stress and free radical generation. After three weeks of continuous medication, the group treated with crude extracts had the highest drop in blood glucose levels when compared to diabetic rats induced with STZ. The outcomes observed were decreased free radical generation or increased free radical scavenging. The anti-hyperglycemic impact of aqueous and ethanolic extract in may be caused by a stimulatory effect on the remaining cells to produce more insulin, or by improved peripheral tissue glucose consumption, or by its antioxidant properties. Streptozotocin significantly (p<0.001) reduces the quantity of total protein. Hypoglycemic effects have been discovered in alkaloids, tannins, amino acids, and polyphenols. Total protein synthesis may have decreased as a result of a variety of factors, including an increase in the rate at which amino acids are converted to glucose, a decrease in amino acid uptake, an increase in the rate at which glucogenic amino acids are converted to CO2 and H2O, the loss of the transitional factor, and a decrease in ribosomal protein synthesis. Antioxidant defense systems may increase total protein (Rawi SM, et al., 2011; Rimbau V, et al., 1996). Several studies have linked STZ-induced oxidative stress to diabetic neuropathy in rats, and various plant extracts have shown antioxidative characteristics that decrease oxidative stress. The major components that actively scavenge free radicals are assumed to be flavonoids and polyphenols. Many antioxidants, including tocotrienol, alpha-lipoic acid, and Emblica officinalis, have been shown to increase antioxidant enzyme levels and diminish oxidative stress caused by STZ (Feldman EL, 2003; Vincent AM, et al., 2004). Since extracts include alkaloids, flavonoids, and polyphenols, all of which are known to have antioxidant characteristics, these chemicals are expected to help decrease the oxidative stress caused by ROS. A broad variety of disorders have a major influence on peripheral nerve function. Diabetic neuropathy is one of them. Functional limitations may occur from nerve fiber loss, myelin abnormalities, connective tissue alterations, and vascularization changes (Smith AG and Singleton JR, 2008). Researchers utilized a light microscope in this study to show how rats with short-term hyperglycemia may drastically affect the way their nerve fibers are myelinated. These findings are consistent with previous study, which found that diabetic rats had lower myelinated fiber area, density, diameter, and axon/myelin ratio. Changes in Ranvier nodes and segmental demyelination have also been noted (Jamali R and Mohseni S, 2006; Algaidi S, 2011). Similar structural changes have also been seen in diabetic neuropathy patients. These include nerve fiber loss as well as microvascular abnormalities such as basement membrane thickening and endothelial cell hyperplasia. It’s worth noting that there was a clear correlation between the severity of the neuropathy and the degree of these changes. Many studies have shown a link between these structural changes and the severity of neuropathy symptoms and symptoms (Algaidi S, 2011).
| Groups | Treatment |
|---|---|
| Group I | Normal control |
| Group II | Diabetic control (Streptozotocin 60 mg/kg body weight (b.w)) |
| Group III | Streptozotocin 60 mg/kg b.w+Standard (Gabapentin) |
| Group IV | Streptozotocin 60 mg/kg b.w+Aqueous extracts |
| Bauhinia variegata (400 mg/kg per os (p.o.)) | |
| Group V | Streptozotocin 60 mg/kg b.w+ethanolic extracts |
| Bauhinia variegata (400 mg/kg p.o.) |
Table 7: Effect of plant extracts on grip strength by using Rota rod in STZ induced diabetic neuropathy model in rats
Conclusion
It was intriguing to discover that drinking plant extracts significantly reduced blood glucose levels. While the underlying mechanisms are unknown, extracts may improve endothelial function by decreasing the oxidative stress associated with diabetes and, as a result, insulin resistance. This result, however, raises the possibility that the antioxidant effect is not as beneficial as the antihyperglycemic effect. Yet, similar outcomes were found in a separate study. This study found a link between a decrease in oxidative stress markers and the positive effects of flavonoids, polyphenolic components, and alpha-lipoic acid on structural abnormalities in diabetic rats’ neurons..
References
- Diabetes. World Health Organization (WHO) 2022.
- The top 10 causes of death. World Health Organization (WHO). 2020.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009; 32(7): 1335-1343.
[Crossref] [Google Scholar] [Pubmed]
- Weerateerangkull P, Praputpittaya C, Banjerdpongchai R. Effects of ascorbic acid on streptozotocin-induced oxidative stress and memory impairment in rats. Thai J Pharm Sci. 2008; 20(2): 54-61.
- Irwin RS, Rippe JM. Manual of intensive care medicine. Lippincott Williams and Wilkins. 2010.
- Diabetes report card. CDC. 2019.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012; 380(9859): 2163-2196.
[Crossref] [Google Scholar] [Pubmed]
- Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009; 48(1): 44-51.
[Crossref] [Google Scholar] [Pubmed]
- American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018; 41(5): 917-928.
[Crossref] [Google Scholar] [Pubmed]
- Rother KI. Diabetes treatment-bridging the divide. N Engl J Med. 2007; 356(15): 1499.
[Crossref] [Google Scholar] [Pubmed]
- Diabetes mellitus and disorders of carbohydrate metabolism. Merck manual professional. 2010.
- Petzold A, Solimena M, Knoch KP. Mechanisms of beta cell dysfunction associated with viral infection. Current diabetes reports. 2015; 15: 1-0. [Crossref]
[Google Scholar] [Pubmed]
- Butalia S, Kaplan GG, Khokhar B, Rabi DM. Environmental risk factors and type 1 diabetes: Past, present, and future. Can J Diabetes. 2016; 40(6): 586-593.
[Crossref] [Google Scholar] [Pubmed]
- Shoback DM, Gardner DG. Greenspan’s basic and clinical endocrinology. McGraw-Hill Education. 2018.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 40 (Suppl 1): S11-S24. 2016.
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams textbook of endocrinology. Elsevier Health Sciences. 2012.
- Azevedo CR, Maciel FM, Silva LB, Ferreira AT, Da Cunha M, Machado OL, et al. Isolation and intracellular localization of insulin-like proteins from leaves of Bauhinia variegata. Braz J Med Biol Res. 2006; 39: 1435-1444.
[Crossref] [Google Scholar] [Pubmed]
- Bairagi SM, Aher AA, Nimase PK. In vitro anthelmintic activity of Bauhinia variegata bark (Leguminosae). Int J Pharm Pharm Sci. 2012; 4(3): 672-674.
- Bodakhe SH, Ram A. Hepatoprotective properties of Bauhinia variegata bark extract. Yakugaku Zasshi. 2007; 127(9): 1503-1507.
[Crossref] [Google Scholar] [Pubmed]
- Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE. The potential mechanism of the diabetogenic action of streptozotocin: Inhibition of pancreatic β-cell O-GlcNAc-selective N-acetyl-β-D-glucosaminidase. Biochem J. 2001; 356(1): 31-41.
[Crossref] [Google Scholar] [Pubmed]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004; 101(3): 823-828.
[Crossref] [Google Scholar] [Pubmed]
- Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: Effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996; 68(2-3): 293-299.
[Crossref] [Google Scholar] [Pubmed]
- Khalili M. The effect of oral administration of Withania somnifera root on formalin-induced pain in diabetic rats. Basic Clin Neurosci. 2009; 1(1): 29.
- Negi G, Kumar A, Kaundal RK, Gulati A, Sharma SS. Functional and biochemical evidence indicating beneficial effect of Melatonin and Nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology. 2010; 58(3): 585-592.
[Crossref] [Google Scholar] [Pubmed]
- Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005; 22(4): 359-370.
[Crossref] [Google Scholar] [Pubmed]
- Morrow TJ. Animal models of painful diabetic neuropathy: The STZ rat model. Curr Protoc Neurosci. 2004; 29(1): 9-18.
[Crossref] [Google Scholar] [Pubmed]
- Gong YH, Yu XR, Liu HL, Yang N, Zuo PP, Huang YG. Antinociceptive effects of combination of tramadol and acetaminophen on painful diabetic neuropathy in streptozotocin-induced diabetic rats. Acta Anaesthesiol Taiwan. 2011; 49(1): 16-20.
[Crossref] [Google Scholar] [Pubmed]
- Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008; 51(2): 216-226.
[Crossref] [Google Scholar] [Pubmed]
- Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006; 55(6): 1695-1704.
[Crossref] [Google Scholar] [Pubmed]
- Rahman H, Eswaraiah MC. Simple spectroscopic methods for estimating brain neurotransmitters, antioxidant enzymes of laboratory animals like mice: A review. Pharmatutor. 2012.
- Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi SH, Farhangi A, Verdi AA, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007; 22: 60-64.
[Crossref] [Google Scholar] [Pubmed]
- Ramachandran A, Snehalatha C, Mary S, Selvam S, Kumar CK, Seeli AC, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: Results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia. 2009; 52: 1019-1026.
[Crossref] [Google Scholar] [Pubmed]
- Rawi SM, Mourad IM, Sayed DA. Biochemical changes in experimental diabetes before and after treatment with Mangifera indica and Psidium guava extracts. Int J Pharm Bio Sci. 2011; 2(2): 29-41.
- Rimbau V, Risco E, Canigueral S, Iglesias J. Antiinflammatory activity of some extracts from plants used in the traditional medicine of North‐African countries. Phytother Res. 1996; 10(5): 421-423.
[Crossref] [Google Scholar] [Pubmed]
- Feldman EL. Oxidative stress and diabetic neuropathy: A new understanding of an old problem. J Clin Invest. 2003; 111(4): 431-433.
[Crossref] [Google Scholar] [Pubmed]
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004; 25(4): 612-628.
[Crossref] [Google Scholar] [Pubmed]
- Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008; 14(1): 23-29.
[Crossref] [Google Scholar] [Pubmed]
- Jamali R, Mohseni S. Differential neuropathies in hyperglycemic and hypoglycemic diabetic rats. J Neuropathol Exp Neurol. 2006; 65(12): 1118-1125.
[Crossref] [Google Scholar] [Pubmed]
- Algaidi S. The effect of antioxidants on experimentally induced diabetic peripheral neuropathy in adult male albino rats. J Am sci. 2011; 7(12): 671-677.
Author Info
Rajesh Kumar Sharma1, Junaid Tantray2, Sourabh Kosey1, Akhilesh Patel2, Priya Rani1 and Shobit Raj2*2Department of Pharmacy Practice, NIMS Institute of Pharmacy, Jaipur, India
Citation: Sharma RK: Beneficial Effects of Bauhinia variegata Linn. Root against Streptozotocin Induced Diabetic Neuropathy in Rats
Received: 12-May-2023 Accepted: 26-May-2023 Published: 02-Jun-2023, DOI: 10.31858/0975-8453.14.366-374
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3