Research Article - (2024) Volume 15, Issue 6
Abstract
Cytoprotective agents are defined as the drugs that are used with certain types of chemotherapy to protect the body from or minimize the side effects of chemotherapy. The leaves of the Camellia sinensis plant are used to make both green and black tea. Most of the Green Tea Polyphenols (GTPs) are flavonols commonly known as catechins. Green tea and black tea flavonoids are known to protect your heart. Due to polyphenols, both green tea and black tea have anti- inflammatory, anti-oxidant, cytoprotective action in cancer and advised for consumption of same. The present study was undertaken to determine and compare the cytoprotective effectiveness of aqueous extracts of green tea and black tea. Black and green tea were procured from Amazon subjected for various in vitro Models. Here to evaluate the cytoprotective effect of black tea and green tea we used brine shrimp model, and Allium cepa model. As a part of study, we evaluated the cytoprotective potential of black and green tea using Brine shrimp model. Presence of test drug but less as compared to that control. So, it can be said that it may give protective action against toxicity of Methotrexate (MTX). In Allium cepa model, width and length of roots was observed to evaluate the cytoprotective effect. Growth in length and width of roots was observed higher in presence of test drug but less as compared to that control. So, it can be said that it may give protective action against toxicity of MTX. From the present study, it can be concluded that the aqueous extracts of black tea and green tea showed cytoprotective activity.
Keywords
Camellia sinensis, Cytoprotective agents, Allium cepa model, Brine shrimp model
Introduction
Cytoprotective drugs are essential in the battle against cancer. While anti-cancer treatments are important for fighting malignancy, they often come with severe side effects that harm patients. These treatments don’t just affect the cancer cells but can also damage healthy ones. That’s where, cytoprotective drugs come in. These medications are specifically designed to work alongside chemotherapy, safeguarding the body from its harsh side effects. There are several cytoprotective drugs available, including amifostine, mesna and glutamine (Hogle WP, 2007). These drugs play a pivotal role in mitigating the adverse effects of chemotherapy, allowing patients to undergo their cancer treatment with greater comfort and fewer complications.
Cancer is a disease characterized by the uncontrolled division and spread of cells, primarily caused by changes in DNA. These genetic alterations can result from various factors, such as errors during cell division, exposure to harmful environmental substances like tobacco smoke and UV rays, or even genetic inheritance (Blackadar CB, 2016). Recognizing the signs and symptoms of cancer, such as breast changes, bladder problems, fatigue, neurological issues, skin changes, hoarseness, or unexplained fevers is important for early detection.
The etiological factors contributing to cancer are varied, including tobacco and alcohol use, an unhealthy diet, physical inactivity and exposure to air pollution. In 2020, cancer posed a significant global burden with 19.3 million new cases and 10 million deaths reported (Sung H, et al., 2021). Medications like doxorubicin are employed in cancer treatment, both in combination with other drugs and on their own, addressing a range of cancer types (Ujah GA, et al., 2021). However, despite advancements in cancer treatment, side effects remain a concern including pain, fatigue, anaemia, mouth issues, nausea, weight changes, dietary problems and hair loss along with bleeding, bone density loss, fertility issues, and nerve problems.
To counteract these side effects and improve the quality of life for cancer patients, cytoprotective drugs have been developed. These agents work in tandem with chemotherapy to shield the body from the harsh repercussions of treatment. Some notable cytoprotective drugs include amifostine, dexrazoxane, mesna and glutamine. Furthermore, adopting a healthy diet can also contribute to reducing the side effects of cancer treatment (Hogle WP, 2007). One example of this is black tea, which has been consumed for thousands of years and is known for its potential health benefits. It contains various components such as alkaloids, theobromine, caffeine, theophylline, polyphenols, amino acids, polysaccharides and volatile oils. Polyphenols, particularly catechins are abundant in both green tea and black tea and have demonstrated their ability to protect the heart, prevent blood vessel plaque formation, and provide anti-inflammatory and antioxidant properties (Li S, et al., 2013). As a result, black tea and its extracts are recommended for their cytoprotective actions in cancer management (Beltz AL, et al., 2006).
In the ongoing battle against cancer, cytoprotective drugs and lifestyle adjustments, including dietary choices, are becoming increasingly important tools for improving the well-being of patients undergoing treatment (Zhang Y, et al., 2012; Huang JM, et al., 2002). These measures help minimize the adverse effects of cancer therapy, offering hope for a brighter future for those affected by this disease.
Therefore, this study was undertaken to determine and compare the cytoprotective effectiveness of aqueous extracts of green tea, black tea by using brine shrimp and Allium cepa in vitro Models.
Materials and Methods
An in vitro study was conducted at the Department of Pharmacy, RK University, Rajkot, India, to check and compare the cytoprotective effectiveness of aqueous extracts of green and black tea by using brine shrimp and Allium cepa models (Mercado SA and Caleño JD, 2020; Ali MM, et al., 2022).
Preparation of aqueous tea extracts
Two different types of tea leaves of the brand, Lipton tea and Tata tea gold care were used for the study including green tea and black tea. 10 grams of each tea leaf sample was weighed and added to 100 ml of boiling distilled water and further boiled for 30 minutes. The extract obtained was reduced to 10 ml over a water bath to obtain maximum (~100%) concentration.
Evaluation of cytoprotective activity of green and black tea extracts using brine shrimp model
Preparation of brine shrimp model: To get started, put the brine shrimp capsule in a tightly sealed container that’s dry and cold (below 50ºF). Then, use a cone-shaped bottle with a light bulb at the top to provide light as shown in the below picture. Next, fill the cone-shaped container halfway with seawater, make sure it has enough air, and turn on the light. Add one brine shrimp capsule and keep doing this for 24 hours. After 24 hours, turn off the light and stop the airflow. Let the baby brine shrimp (called nauplii) settle at the bottom of the bottle. This gives them time to settle down and be ready for the next steps (Figure 1).
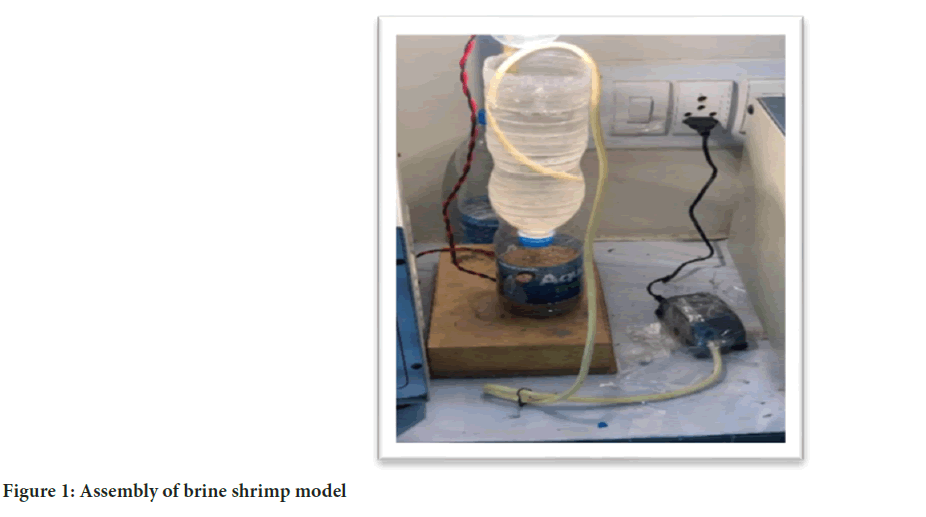
Figure 1: Assembly of brine shrimp model
Cytoprotective test by using brine shrimp in vitro model: Now, 10 living nauplii were taken from the bottle and were placed in petri dishes using pipette. These petri dishes were then divided into different categories, standard, control and test, each receiving different doses. During the dosing process, it’s important to keep the petri dishes continuously supplied with air and light. After waiting for 30 minutes, number of nauplii under alive condition and number of nauplii passed away in each petri dish were carefully counted. Once all the nauplii in each group have perished, an observation table was created to record whether the nauplii in the normal, control, standard and test groups were dead or alive (Figures 2 and 3).
Figure 2: Microscopic view of nauplii

Figure 3: Cytoprotective test using brine shrimp model
Evaluation of cytoprotective activity of black and green tea in Allium cepa: First, a test solution with various concentrations and standard solution using gallic acid were prepared. Next, some onions were taken which were washed with both water and different concentrations of solutions. Then, a beaker was taken and these solutions were poured. The onions were placed on the beaker so that their roots hang down into the solutions and were allowed to sit for about a week, allowing time for the roots to grow properly; this set up was observed carefully during this period. We regularly checked and measured the length and counted the number of roots that developed. This way, we kept tracking the growth of onions in response to different solutions and concentrations (Figure 4).
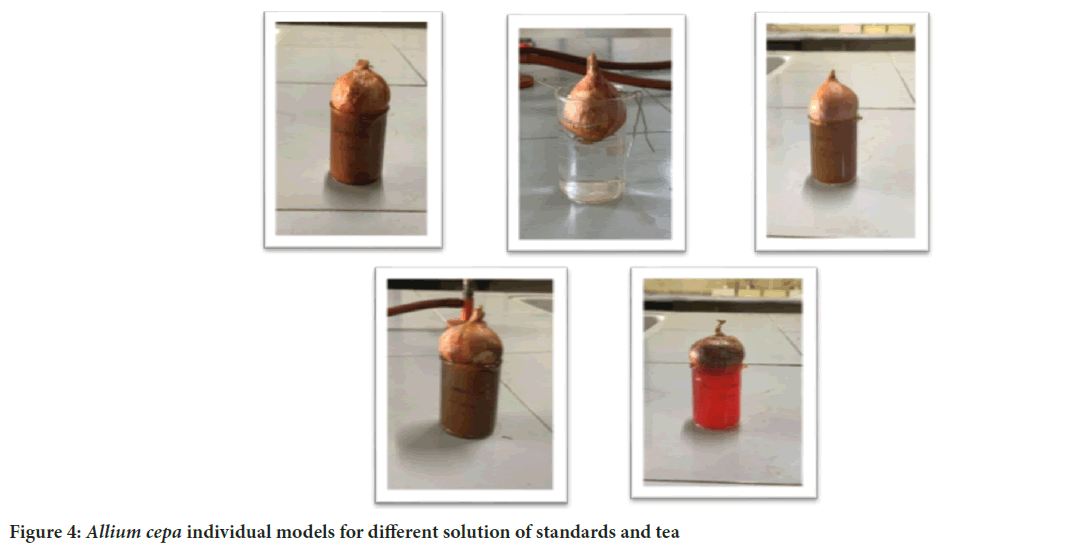
Figure 4: Allium cepa individual models for different solution of standards and tea
Statistical analysis Data was analysed using Analysis of Variance (ANOVA) for determination of variance. Data were considered significantly different from each other if p ≤ 0.05 and if p ≤ 0.001 then the difference between data where consider highly significant.
Results and Discussion
Brine shrimp model
Brine shrimp model was performed as per mentioned protocol. In this model, we used the gallic acid as a standard drug, MTX as disease control and water as control and 2 different doses of black and green tea are used. Dose of test drug was black tea T1 (500 μg/ml) while that of green tea T1 (500 μg/ml) (Tables 1 and 2).
| Group | Time (min) | |||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | |
| Control | 10 | 10 | 9 | 8 | 6 | 5 |
| Standard (MTX) | 10 | 7 | 5 | 2 | 0 | 0 |
| Standard (gallic acid) | 10 | 10 | 10 | 9 | 9 | 9 |
| Black tea (T1) | 10 | 9 | 9 | 8 | 7 | 7 |
| Green tea (T2) | 10 | 9 | 9 | 9 | 8 | 8 |
Table 1: Observation table in brine shrimp model
| Group | Mean survived nauplii |
|---|---|
| Control | 8 |
| Standard (MTX) | 4 |
| Standard (gallic acid) | 9.5 |
| Black tea T1 | 8.33 |
| Green tea T2 | 8.83 |
| p-value | 0.001 |
Table 2: Mean number of survived nauplii after 150 min with 30 min interval with different types of tea extracts, as per ANOVA test
Anticancer agents play a vital role in the cure of patients suffering from malignancy. Though, the chemotherapeutic agents are associated with various adverse effects which produce significant toxic symptoms in the patients.
ytoprotective agents are defined as the drugs that are used with certain types of chemotherapy to protect the body from or minimize the side effects of chemotherapy. As a part of study, we evaluated the cytoprotective potential of black tea and green tea.
The pharmacological models were used to evaluate the cytoprotective potential effect of the black tea and green tea, e.g., brine shrimp model and Allium cepa model. As a part of study, we evaluated the cytoprotective potential of black tea and green tea using brine shrimp model. In that model, it is believed that nauplii is rapidly dividing structure which is identical to that of cancerous cell. That’s why if nauplii can survive after introducing the drug to them, it may be considered as its cytoprotective potential. In this model, control (water) and disease control (MTX) shown significant reduction in nauplii count in respective timeline (Figures 5 and 6). But when test drug is introduced, difference in nauplii count was found statistically significant. This data revealed that tea has cytoprotective action. In detail green tea has more cytoprotective as compared to black tea as nauplii survive well in green tea rather than black tea.
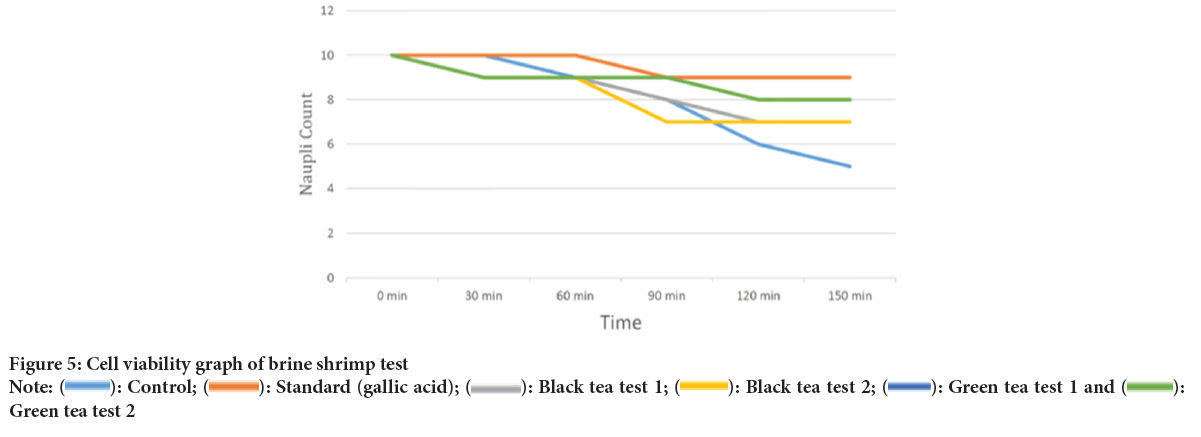
Figure 5: Cell viability graph of brine shrimp test
Note: ( ): Control; (
): Control; ( ): Standard (gallic acid); (
): Standard (gallic acid); ( ): Black tea test 1; (
): Black tea test 1; ( ): Black tea test 2; (
): Black tea test 2; ( ): Green tea test 1 and (
): Green tea test 1 and ( ):Green tea test 2
):Green tea test 2
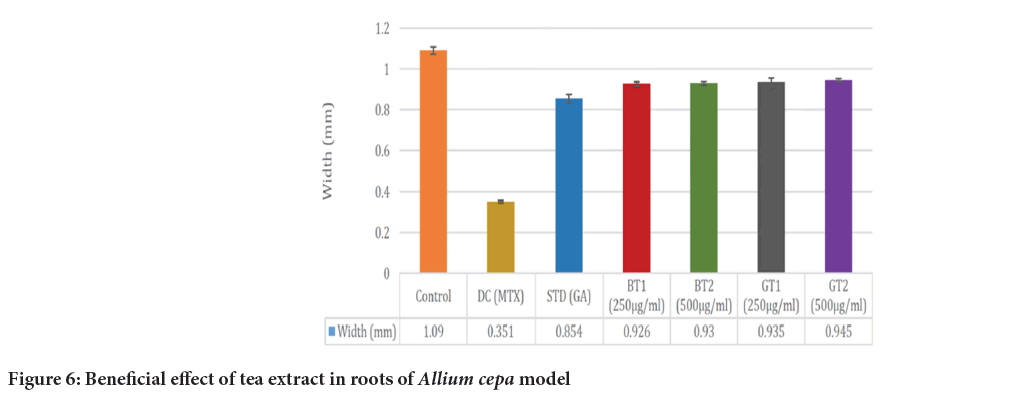
Figure 6: Beneficial effect of tea extract in roots of Allium cepa model
In Allium cepa model, width and length of roots was observed to evaluate the cytoprotective effect (Tables 3 and 4). The cytoprotective effect was observed at the different concentration of test drug (black tea and green tea), Disease Control (DC) (MTX) and standard drug gallic acid and as well as with control. The growth in length of roots as well as width of roots was inhibited in disease control. However, growth in length and width of roots was observed higher in presence of test drug but less as compared to that control group (Table 5). So, it can be said that it may give protective action against toxicity of MTX.
| Group | p-value |
|---|---|
| Black tea vs. gallic acid | 0.057 |
| Green tea vs. gallic acid | 0.109 |
| Black tea vs. green tea | 0.410 |
Table 3: Comparison of survived nauplii after 150 min with 30 min interval with different types of tea, as per Turkey’s post hoc analysis
| Length of Allium cepa | |||||
|---|---|---|---|---|---|
| Reading | Control | Disease Control (DC) MTX | Standard (gallic acid) | Binding Test 1 (BT1) | Generation Type 1 (GT1) |
| 1 | 12.3 | 5.9 | 12 | 12.1 | 12.6 |
| 2 | 12.5 | 5.8 | 12.5 | 12 | 12.4 |
| 3 | 12 | 5.6 | 12.6 | 12.2 | 12.3 |
| 4 | 12.6 | 5.8 | 12.8 | 12 | 12.2 |
| 5 | 12.5 | 6 | 12.6 | 11.9 | 12 |
Table 4: Result of length of roots of Allium cepa
| Width (mm) of Allium cepa | |||||
|---|---|---|---|---|---|
| Reading | Control | Disease Control (DC) MTX | Standard (gallic acid) | BT1 | GT2 |
| 1 | 1.3 | 0.65 | 0.9 | 0.97 | 0.9 |
| 2 | 1.35 | 0.62 | 0.95 | 0.96 | 0.92 |
| 3 | 1.32 | 0.63 | 0.83 | 0.98 | 0.95 |
| 4 | 1.25 | 0.65 | 0.85 | 0.93 | 0.96 |
| 5 | 1.2 | 0.64 | 0.88 | 0.94 | 0.89 |
Table 5: Result of width of roots of Allium cepa
Conclusion
The study showed that both black tea and green tea can protect cells. We found this out by doing different tests, like using brine shrimp, using Allium cepa model. Our results tell us that both black tea and green tea are good at protecting cells, but green tea is even better than black tea.
Limitations
This study had certain limitations that should be acknowledged. One notable limitation was the use of a high concentration of tea extract, which does not accurately reflect typical consumption levels. To enhance the study’s comprehensiveness, it would have been valuable to explore the effectiveness of tea extracts at various lower concentrations.
References
- Hogle WP. Cytoprotective agents used in the treatment of patients with cancer. Semin Oncol Nurs. 2007; 23(3): 213-224.
[Crossref] [Google Scholar] [Pubmed]
- Blackadar CB. Historical review of the causes of cancer. World J Clin Oncol. 2016; 7(1): 54.
[Crossref] [Google Scholar] [Pubmed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3): 209-249.
[Crossref] [Google Scholar] [Pubmed]
- Ujah GA, Nna VU, Suleiman JB, Eleazu C, Nwokocha C, Rebene JA, et al. Tert-butylhydroquinone attenuates doxorubicin-induced dysregulation of testicular cytoprotective and steroidogenic genes, and improves spermatogenesis in rats. Sci Rep. 2021; 11(1): 5522.
[Crossref] [Google Scholar] [Pubmed]
- Li S, Lo CY, Pan MH, Lai CS, Ho CT. Black tea: Chemical analysis and stability. Food Funct. 2013; 4(1): 10-18.
[Crossref] [Google Scholar] [Pubmed]
- Beltz AL, Bayer KD, Moss LA, Simet MI. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006; 6(5): 389-406.
[Crossref] [Google Scholar] [Pubmed]
- Zhang Y, Mu J, Han J, Gu X. An improved brine shrimp larvae lethality microwell test method. Toxicol Mech Methods. 2012; 22(1): 23-30.
[Crossref] [Google Scholar] [Pubmed]
- Huang JM, Nakade K, Kondo M, Yang CS, Fukuyama Y. Brine shrimp lethality test active constituents and new highly oxygenated seco-prezizaane-type sesquiterpenes from Illicium merrillianum. Chem Pharm Bull. 2002;50(1):133-136.
[Crossref] [Google Scholar] [Pubmed]
- Mercado SA, Caleño JD. Cytotoxic evaluation of glyphosate, using Allium cepa L. as bioindicator. Sci Total Environ. 2020; 700: 134452.
[Crossref] [Google Scholar] [Pubmed]
- Ali MM, Fatima A, Nawaz S, Rehman A, Javed M, Nadeem A. Cytotoxic and genotoxic evaluation of bisphenol S on onion root tips by Allium cepa and comet tests. Environ Sci Pollut Res Int. 2022; 29(59): 88803-88811.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Bhargav Kamani, Savankumar Chhatrola* and Tejas GanatraCitation: Kamani B: Comparative Assessment of Extracts of Camellia sinensis in In Vitro Models
Received: 03-Jun-2024 Accepted: 19-Jun-2024 Published: 26-Jun-2024, DOI: 10.31858/0975-8453.15.6.189-193
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






