Research Article - (2021) Volume 12, Issue 11
Concepts, Current Status, Approaches in Transdermal Drug Delivery System Technologies
Ghazi ME Hussein1, Babiker M Elhaj2 and Heyam Saad Ali3*Abstract
The transdermal patch refers to the medical adhesive area which is kept on a human skin for the transition of a particular drug dosage across human skin to bloodstream. The essence of this activity is to prop up healing to the injured section on the body. The patch allows a controlled release of medication in the bloodstream, both via the porous polymeric membrane film covering the drug reservoir and via body temperature dissolving bony stratum of the medicine rooted in the adhesive or the paste. The film can be either in in form of a solid polymeric material or a residual film. The former form allows a sustained release while the other exhibits a rapid absorption is the leverage which the Transdermal medication technique has over other routes of therapeutic delivery like topical, oral, intramuscular, intravenous and others. Transdermal drug transmission enables steady flow of the drug in question into the body of the patient to make room for a sturdy profile of the blood level. This normally results into a reduced general side effect which means an improved effectiveness over diverse forms of dosages. The major aim of the Transdermal delivery technique is transition of medication into a universal circulation via the outer layer of the body at a programmed rate with negligible intra-patient as well as inter-patient variations.
Keywords
Transdermal patch, TDDS, Stratum corneum
Introduction
Interest has emerged over few years now for the improvement of new drug delivery techniques for the drug molecules in existence. The growth of new delivery techniques for drugs molecules improves drug performance, effectiveness, safety as well as the patient’s compliance with the general therapeutic advantages to considerable extent. Transdermal Drug Delivery System (TDDS) is self-controlled, distinct dosage forms or “patches” which are administered to the human skin to allow drug passage via the outer covering of the body into the bloodstream at a regulated rate. TDDS are designed dosage forms; have different classification used for delivering a therapeutically efficient quantity of drug through to the victim’s skin (Jampilek J and Brychtova K, 2012).
Transdermal drug delivery System (TDDS) is different from topical drug delivery. Topical drug delivery is an application of a formulation to the skin to locally and directly acting on the skin to treat a local disorder.
Transdermal Drug Delivery System (TDDS) is an application of a formulation to the skin to deliver a drug to the systemic circulation, for example, estradiol patches. The efficacy of these (TDD) products is critically dependent on biological factors, such as the integrity of the skin, and the physicochemical properties of the active ingredient and on the formulation designed to release and deliver the active into or across the skin.
The film can be either in in form of a solid polymeric material or a residual film. The former form allows a sustained release while the other exhibits a rapid absorption, (Lawlor KT and Kaur P, 2015).
The main objective of the transdermal administration method is transferring of drugs into the blood circulation system through the mammalian skin via a prearranged speed with reduced intra and inters victim variation. Presently TDDS has proven to be the best techniques for the application of drug. It lessens the load which the oral method usually imposes on the liver and digestive tract. TDDS quickens the patient compliances as well as reduces fatal side effects caused by the drug’s temporary over dose. This is convenience and easier in most Transdermal delivery medications which require once a week application only. And also enhances; bioavailability, higher regular plasma levels, prolonged action time which will result into a reduced dosing frequency, minimal side effects as well as improved therapy. All is achieved because its maintenance of the plasma levels till the last stages of the application interval as compared to the oral dosage method which causes plasma level decline.
Transdermal delivery is known to offers; controlled, and constant application of drugs, access to a constant delivery of medication using tiny organic half-lives, as well as the removal of pulsed entrance into the circulatory system, that normally results into bad after effects. The merits associated with the Transdermal technique of therapeutic delivery cannot be overemphasized as they are numerous. Some of them range from the restriction of hepatic premium metabolism, quickening of therapeutic effectiveness as well as maintenance of stable plasma drug level. The growth of TDDS and its development requires contribution and participation of all the disciplines and it encompasses basic achievability studies beginning from assortment period of medicinal molecules to demonstration period of adequate change of drug in the in vivo as well as the ex vivo design prior to production of the transdermal delivery arrangement which meet up with the overall rigid specific requirements in the following areas (Hatta I, et al., 2010).
• Molecules of drug; physicochemical, and stability factors,
• Patients; cosmetic appeal and comfort,
• The manufacturer; manufacturability and scale up as well as the requirement of the economy, this is the most important.
Transdermal SCOP being the initial Transdermal system was approved by the FDA guidance for preventing nausea as well as vomiting connected with travel in the year 1979. Majority of the Transdermal patches functions to release active ingredient after some days of application to the skin at zero-order rates for some hours. This is vital for chronic prophylactic conditions therapy. The symptoms of percutaneous drug intake are normally found via assessable blood levels and detectable removal of the drugs and the metabolites that is within the urine due to control the administered drug in the effective therapeutic level (Ebert CD, et al., 1992).
Methodology
TDDD method and diagnosis
The skin as the major body covering organ: A mammalian skin which covers approximately 2 square meters is the major part of the mammalian body which receives nearly 1/3 of human plasma circulation via the mammalian body. The skin functions as permeable membrane which obstructs the Transdermal intake of diverse chemicals as well as biological agents. Having thickness of some millimeters, it is always an available body organ with the following activities to perform (Tanwar H and Sachdeva R, 2016).
• It separates the basic blood flow system from the exterior environment
• It works as a wall obstructing physical, microbiological and chemical attacks.
• It functions like a regulatory thermostat which sustains the body temperature.
• It contributes greatly in blood pressure regulation.
• Guards against UV emission from penetrating the human body
• The skin is a major factor which determines everything concerning drug delivery like penetration as well as assimilation of medicine through the dermis. An ultrastructure and anatomy of the outer layer of the body determines its diffusional resistance (Chandrasekaran SK and Shaw JE, 1978).
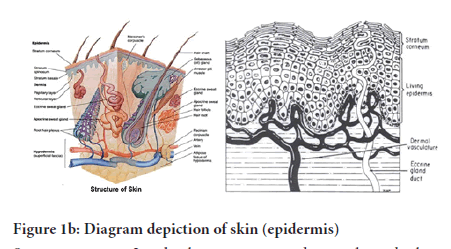
Anatomy of skin: The morphology of the mammalian skin as illustrated in (fig. a) may possibly be classified into 4 major layers, which are:
The practical epidermis
• An overlying dermis
• The epidermis
• A non-practical epidermis (Stratum corneum) (Jensen LB , et al., 2011) (Figure 1a).
Figure 1(a): Diagram depiction of skin (Structure of the Skin) as well as the appendages
The mammalian epidermis: It is an incessantly self-replenishing, squamous epithelium that is stratified and covers the whole exterior layer of the human body as well as basically made up of 2 parts, which are: the viable or living malpighian cells (doable epidermis) with the lifeless horny layer cells or the most famous name-stratum corneum. Doable epidermis is additionally categorized into 4 individual layers: Stratum spinosu, Stratum lucidum, Stratum granulosu and Stratum basale (Figure 1b).
Figure 1(b): Diagram depiction of skin (epidermis)
Stratum corneum: It is the skin outer covering layer and it is also known as the horny layer. It’s the pace restraining obstacle that confines the intimate and noticeable association of chemical compounds. The hurdle natural world of the horny layer relies significantly on its components: 75.00 to 80.00 (%) proteins, 5.00 to 15.00 (%) lipids, as well as 5.00 to 10.00 (%) ondansetron bits and pieces on dehydrated mass root (Gupta PN, et al., 2005).
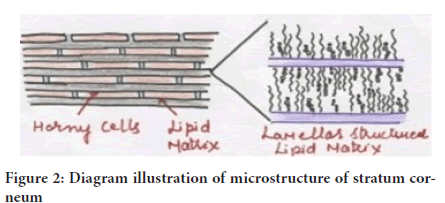
Stratum corneum is about 10mm thick while dry but puffs up more as soon as it is fully drowned. It is lithe but moderately impervious. The structural design of the horny layer (Figure 2) is represented by wall-like figure, having protein blocks as well as lipid mortar. This takes the corneocytes, which is linked through desmosomes or the protein-rich adjuncts of cell crust. The horny skin cell is entrenched within the lipid medium, which plays a considerable act in finding out the element permeability over the skin.
Figure 2: Diagram illustration of microstructure of stratum corneum
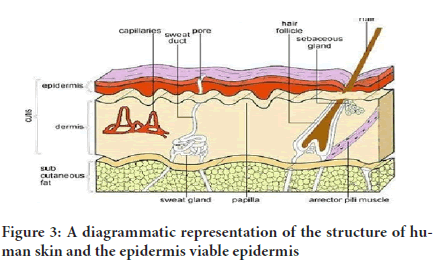
Doable epidermis: They are located under the stratum corneum. They differ in depth with 0.06mm on the eyelids to nearly 0.8mm on the palms. When it comes to the deeper section, this takes in several coverings as stratum granulosum, stratum lucidum, stratum basale, as well as the stratum spinosum. Inside the basale coating, refurbishing of epidermis during mitosis takes place continually and the large number recompenses losses of lifeless corneocytes from a layer of the skin. When cells created via the basale layer budge outside, they change morphologically as well as histochemically, going through keratinization for generating the exterior coat of stratum corneum (Hull W, 2002) (Figure 3).
Figure 3: A diagrammatic representation of the structure of human skin and the epidermis viable epidermis
Dermis: This is the skin layer simply under the epidermis that is 3-5mm thick coat and is made up of a milieu of relative tissues, which has lymph vessels, blood vessels, as well as nerves. The blood supply of the cutaneous possesses essential role in body temperature regulation. In addition, it provides oxygen and nutrients to the skin, as it removes poisons as well as waste products. Capillaries get to 0.2mm within the surface of the skin and give sink states for many molecules piercing the barrier of the skin (Rawlings AV and Harding CR, 2004). The blood supply therefore maintains the dermal absorption of permeate at a very low state, and the ensuing absorption diversity over the epidermis gives the vital dynamic force for Transdermal permeation. When it comes to transdermal delivery of drug, this layer is always seen basically as coagulated water, and thereby offers minimal obstacle to release of many polar drugs, even though the dermal obstacle is slightly important in the delivery of large lipophillic molecules (Rissmann R, et al., 2009).
Hypodermis: This supports the epidermis and dermis. It functions as a fat storeroom module which aids in temperature regulation, mechanical protection and supports nutrition. It transports the major blood vessels with the nerves to the mammalian skin and may possess sensitive pressure organs. When delivering transdermal drugs, drugs must compulsorily pierce across the 3 layers to get to the systemic circulation (Hull W, 2002) (Figure 4).
Figure 4: Basic information of the skin
Results
Percutaneous absorption
For an applied medication to work either systemically or locally, such drug must penetrate via stratum corneum. The definition of percutaneous absorption is said to be the incursion of elements into several skin layers as well as permeation over the skin down to the universal flow. Percutaneous absorption of molecules of drugs is of precise significance during Transdermal medication process. The reason for this is that drugs must be taken in to an appropriate quantity and at the right pace for the attainment and maintenance of systematic and uniform therapeutic levels all through its period of use. Generally, once the drug molecule passes across the stratum corneal obstacle, the transmission into the internal dermal layers as well as general uptake takes place quickly and smoothly (Patel, et al., 2012).
The introduction of curative mediator from the production administered on mammalian outer layer as well as the transportation of the released therapeutic agent into the systematic circulatory system, takes many steps. The procedures are enumerated below as follows (Figure 5)
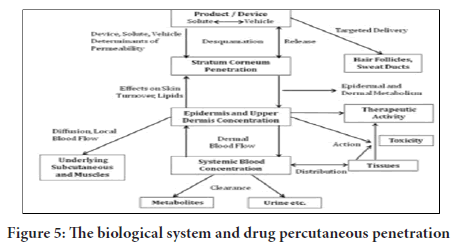
Figure 5: The biological system and drug percutaneous penetration
• Dissolving within and the release stage from the formulated agent
• Segmentation into the outermost skin layer, which is referred to as stratum corneum (SC)
• Movement via SC, which is mostly through the lipidic intercellular path.
• Segmenting from the SC down to the aqueous doable epidermis, movement from the concentrated doable epidermis to the concentrated uppermost dermis, and assimilation is into dermis or the capillary section with microcirculation.
Paths of insemination of medicine via skin
During percutaneous permeation, drug molecules could possibly transfuse via epidermis or move via shunts, especially the ones that are presented using the eccrine glands and the dispersed hair follicles as illustrated (Figure 6a and 6b). In the primary transit diffusion phase, drug molecules might make their way into the skin via hair follicles or the sweat duct to be taken in through via the sebaceous gland and the follicular epithelium (Hatta, et al. 2010). As soon as a stable condition is attained, the circulation and movement via the integral Stratum corneum turns into the fundamental Transdermal permeation pathway.
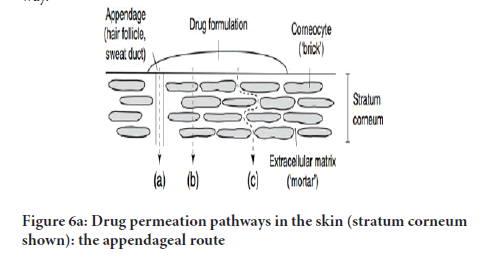
Figure 6(a): Drug permeation pathways in the skin (stratum corneum shown): the appendageal route
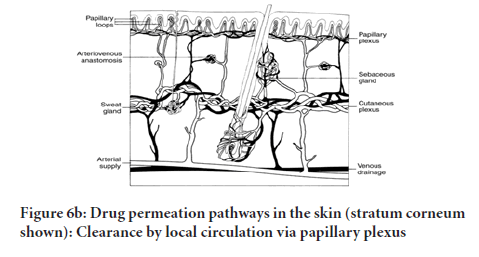
Figure 6(b): Drug permeation pathways in the skin (stratum corneum shown): Clearance by local circulation via papillary plexus
Two major paths are to be defined for skin permeation of molecules applied to the mammalian skin. They are:
1. Transepidermal absorption through two pathways
Intracellular route or pathway: A molecule travelling via the transcellular route partition into and diffuse through the keratinocyte, but in order to move to the next keratinocyte, the molecule must partition into and diffuse through the lipid lamellae between each keratinocyte.
2. Interfollicular pathway or Transfollicular Absorption: The skin appendages (sebaceous and eccrine glands’ pores) are considered as shunts for by passing the stratum corneum. It is important for permeation because the opening of the follicular pore is relatively large and sebum aids in the diffusion of the penetrant (Lawlor KT, et al., 2015).
3.Clearance by Local circulation via papillary plexus Transepidermal absorption: It involves partition of molecules crossing the stratum corneum horny barrier coatings. The two paths of access exist, include: the intercellular route and intracellular as well as (J van Smeden, et al., 2014).
The diffusion mechanism of polar as well as non-polar molecules takes place through the intercellular channel rely on o/w distribution. Movement of Polar molecules takes place via a polar route which consists of bounded water in a hydrated “stratum corneum” (Alexander SPH, et al., 2013).
In non-polar molecules diffusion with dissolution takes place via the “lipid matrix” of “stratum corneum.” Consequently a major pathway of apenetraint was identified using a partition coefficient-log k. All hydrophilic medicines preferentially partition in an intracellular domain, while lipophillic permeate which is octanol or water-log k >2 navigate stratum corneum through an intercellular pathway (Ale I, et al., 2009).
Transfollicular pathway (shunt route): Transfollicular pathway comprises transition through sweat glands as well as hair follicles and connected sebaceous glands. Even though the pathways provide permeability, there importance is minimal due to relatively little area, roughly 0.100% of the whole skin. The path is vital for higher polar molecules and ion, which only just leak into via the Stratum corneum (Anissimov YG and Roberst MS, 2011; Arndats D and Arndts K, 1984).
Barrier role of human skin
Skin is considered as the main access for percutaneous absorption of drug. However, the skin coating of stratum conium is significant in regulating the efficiency of obstruction and represent the obstacle for drug entrance. The dehydrated, keratinized Cells superimpose each other by getting sturdily packed, hindering the entrance of environmental chemical, toxins, radiation, and bacterium from entering to control the human. In addition to the retaining ability of preventing water evaporation from biological fluids. The bottom portion of the human skin comprise of lipid molecules combine to structure a sturdy network, and hence work as a mortar linking the blocks (Anissimov YG and Roberst MS, 2011; Arndats D and Arndts K, 1984). Therefore, to develop an efficient transdermal drug delivery system is to overcome the low permeability of the skin. Numerous chemicals have been reported to have potential enhancing permeation abilities within the skin (Fujii M, et al., 2003; Sabra B, et al. 2008; Nakamura Y, et al., 1996).
Discussion
The fundamentals of transdermal penetration
The skin is top accessible organ and the internal part of the human body. A little part of a millimeter of the tissues demarcates the surface from the capillary network. Furthermore, the Transdermal permeation depends on reactive diffusion (Barbero, AM, 2006).
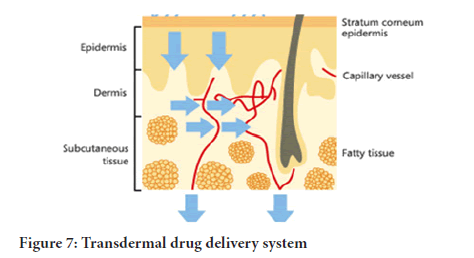
Therefore the stimulation of the therapeutic agents from the formulation administered to the surface of the skin and the transportation to the circulatory system takes a multistep procedure. They steps comprises of the following (Ramteke, et al., 2012) (Table 1) (Figure 7):
| Release from the formation |
|---|
| Diffusion to the stratum corneum |
| Diffusion to the epidermis |
| Diffusion to the dermis |
| Migration to the capillary vessels |
| Migration to the targeted area |
Table 1: Multistep procedure from the formulation administered to the surface of the skin and the transportation to the circulatory system.
Figure 7: Transdermal drug delivery system
1. Movement of the drugs from the formulation
2. Dissolving in and releasing from the product
3. Partition into the stratum corneum as well as diffusion via the lively epidermis
4. Absorption of drugs by the capillary system within dermal papillary plexus covering the surface. This is called absorption by local circulation, where the traversing dissolved enter the circulation by blood flow
5. Absorption by transfollicular route which has a significant impact due to presence of the large pores of the eccrine and sebaceous glands, permit drug molecules through their secretions
6. Segregation into the skin coatings, stratum corneum
7. Movement either via the interlocking channels as in intercellular pathway or intracellular by partition followed by diffusion mechanism based on o/w partition
Characteristic which impacts transdermal delivery
The following are the factors that impact the transdermal delivery-
• Stimulation of a medicament through the vehicle
• Penetration via the mammalian skin barrier (Tanner T and Marks R, 2008)
• Pharmacological response by activation (Liu W, et al., 2017)
The merit from transdermal medication delivery
Transdermal drug delivery offers the best alternative to of IV and oral administration, in terms of reduced side effects, constant drug level, self-administration and unrestricted activity (Stevenson JCL, et al., 1993) (Table 2).
| Merits criteria | IV | Oral | TDDS |
|---|---|---|---|
| reduced side effects | Yes | No | Yes |
| constant drug level | Yes | No | Yes |
| self-administration | No | Yes | Yes |
| unrestricted activity | No | Yes | Yes |
Table 2: Transdermal drug delivery offers the best alternative to of IV and oral administration
Transdermal drug delivery systems have specific advantages such as:
i. Maintain a steady release of drug to the specific site of action, over an extended period of time, minimizing side effect and maximizing therapeutic effects.
ii. Optimize the therapeutic properties of drug: for safer, effective, and reliable.
iii. Improved drug delivery-The drug is distributed locally or in systemic circulation, avoid metabolic or biological barriers, bypassed the GIT, the liver first pass and any and enzymatic type of pitfalls (Singh and Bali A, 2010).
iv. Convenience-Medication outside hospital settings. The therapy could be terminated easily at any stage. It offers suitability for personal administration. It is non-invasive, keeping away from the inconveniences of the parental therapy.
v. Compliance-By reducing drug frequency over the entire course of treatment. The patches requiring administration only once in a week is convenience, and could help in a patient’s adherence to the drug therapy.
vi. Controlled release-TDDS are available which deliver drugs by zero order. Absence of peak in the concentration plasma could lessen the chances of adverse effect. Therefore, the medications, which require complete and steady plasma levels, work perfectly for transdermal medication delivery (Palmer BC and Delouise LA, 2016). Keeping away from medical fluctuation of drug levels, intra and inter patient variation.
vii. The action of the medication which has a stunted half-life gets comprehensive via a reservoir for the medicine in transdermal delivery system plus the regulated release; therefore, it is greatly advantageous in individuals who are unconscious or nauseated.
viii. Transdermal patch is cost effective, due to easy techniques and safe materials which increase productivity.
The demerits associated with the transdermal medicine delivery
What has advantages most definitely have some disadvantages associated with it? The following are the major disadvantages of the transdermal medicinal delivery.
The physicochemical properties drug for penetration through the stratum corneum: Transdermal medical delivery technique cannot be used in the delivery of ionic drugs. Furthermore, there is the need for sufficient aqueous as well as lipid solubility, octanol/ water or a log p between 1-3 for permeate-transverse “stratum corneum” as well as the underlying aqueous cover.
The barrier function of the skin: It changes from one site to another on the same person, from person to person and with age.
Environmental factors: Heat, cold, sweating prevents the patch sticking.
Insecure patches: The patches fall off during bathing, sleeping, walking etc. therefore, the long durational adherences are difficult.
Skin irritation: It may be due to excipient. They may affect allergic reaction (Czarnowicki T, et al., 2014).
• The method cannot deliver medicines in the pulsatile fashion. Transdermal medication delivery cannot actualize the administration of high medical levels in the blood.
• The potent drug is the only suitable candidates when it comes to transdermal patch owing to the natural limitations of the drug entrant imposed because of the impermeability of the skin. Therefore, it cannot work for drugs with high molecular sizes.
The kinetics of the transdermal permeation
This section is vital for effective growth of transdermal arrangement. It involves; sorption using stratum corneum, assimilation of medication via viable epidermis, and absorption in the skin papillary cover (Zidan AS, et al., 2017; Lee AJ, et al., 1998; Khanday MA and Saxena VP, 2010).
Factors that influence transdermal permeation (Schaefer H, et al., 2011)
So many factors impact transdermal permeation, and they are as follows;
• Age
• It has an effect on the drug permeation of through the skin
• Blood flow: (dermal clearance of the molecule transversing the tissue) It tends to decrease with age and could reduce transdermal flux.
• Biological factors: like; skin conditions, age, and regional site of the skin, blood supply, and skin metabolism as well as species differences.
• Thickness of SC.
• Hair follicles: Presence of pores has significant impact in precautious absorption.
• Injury or trauma to the skin
• Hydration of the skin
• Physicochemical factors of the drug; drug concentration, molecular shape and sizes as well as partition coefficient.
• The environmental factors; sunlight, cold season, effect of temperature on the Transdermal patch, and air pollution
• Chemical exposure: for example penetration enhancers in excipients
• Chronic use of certain drugs.
The formation of the transdermal medicinal delivery arrangement
The components of transdermal delivery medication should be carefully chosen to have the following properties-
• Physicochemical features, weight of at most 1000 Daltons, affinity for hydrophilic and lipophilic phase. Great partitioning features and unconducive to drug delivery through the skin. It must be potent , little melting point, non-irritating as well as low half life
• Biological properties; non allergic, effective in mg per day, short half- life biologically, nonirritant, stable in administration, and immune stimulator. The drug must be metabolized within the skin, and must not bound irreversibly
The components of the transdermal drug delivery system include
1. Polymer matrix or matrices
2. The drug
3. The permeation enhancers
4. Other parts (polymeric membrane, backing, lining, rim, adhesive)
Polymer matrix
This is the basis for Transdermal medicine transmission technique? The method of Transdermal administration is formulated since sever al-layered-polymeric laminate in which the medication reservoir/polymer matrix requires dual polymeric coating, the external impervious supporting coating which stops medication loss via assisting layer and the internal polymeric layer which serves as the adhesive (Barba C, et al., 2016). An ideal polymer for Transdermal system must be; nontoxic, stable, affordable, host-friendly available with huge amount of vigorous agents incorporated.
Polymer matrix criteria: It should satisfy the following criteria to release the drug from the device-
• Molecular weight, chemical functionality of the polymer
• It should be stable, non-reactive with the drug.
• Easily manufactured and fabricated into the desired productivity.
• The polymer and its degradation products must be nontoxic.
The mechanical properties of the polymer
i. The mechanical properties of the polymer should not deteriorate excessively when the large amounts of the active agents are incorporated into it (Figure 8).
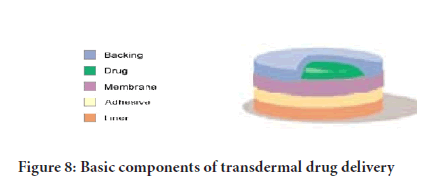
Figure 8: Basic components of transdermal drug delivery
ii. The polymers used in the transdermal drug delivery systems are:
iii. a) Natural polymers: Cellulose derivatives, gelatin, shellac, waxes, gums and their derivatives, starch etc.
iv. b) Synthetic elastomers: Poly butadiene, poly siloxane silicone rubber, nitrile, acrylonitrile, butyl rubber, etc.
v. c) Synthetic polymers: Polyvinyl chloride, polyethylene, poly propylene, polyvinyl pyrrolidone, poly methyl methaacrylate.
For successful development of a transdermal drug delivery, the following are the formulation approaches used in the development of TDDS (Figure 9).
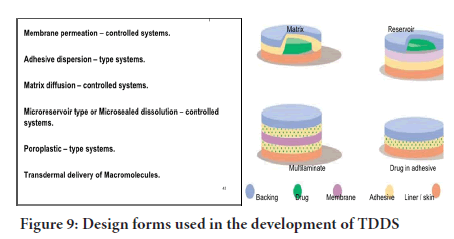
Figure 9: Design forms used in the development of TDDS
Formulation approaches using nanotechnology
Nanotechnology: It is the process of manufacturing, manipulation, and characterization of materials and devices with dimensions at nanometer levels. It is has significant potential in generating nanomaterials (nanocarriers) of , on the scale nano size are potentially useful tools for medicine and biology, as they are of commensurate size to important biological components (e.g. cells, proteins, cell membranes) and thus able to interact in a controlled way at the cellular and molecular levels (Figure 10).
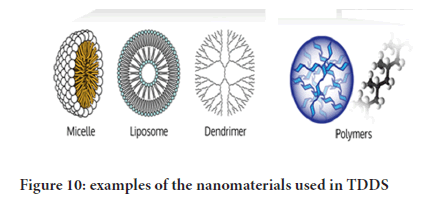
Figure 10: Design forms used in the development of TDDS
Current status and recent technology in transdermal drug delivery system
The micro fabricated kind of microneedles are the hybrids of transdermal patch and the hypodermic needle that is being used for the delivery of the drug effectively with these microscopic needles. The large molecules can even be delivered by their small size across the stratum corneum (Kim KT, et al., 2017; Chen S, et al., 2016; Daddona PE, et al., 2011). The other transdermal drug delivery technology is developed by ALZA Corporation that is known as the Macroflux. This Macroflux is being used for delivering the biopharmaceutical drugs that are controlled in a proper manner that increases the efficacy and bioavailability without any discomfort for the patients (Govil SK, et al., 2019). Currently, great efforts have been collectively exerted to overcome challenges of related to Alza's technology.
The systems based on patch matrix and reservoir forms now are increasingly popular, since the first generation 1981, in the global market in years of FDA approval, as in the figure below (Figure 11).
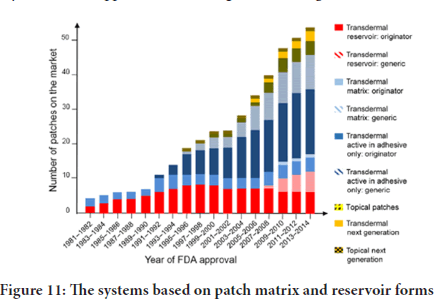
Figure 11: The systems based on patch matrix and reservoir forms
The system uses a titanium based micro projection sort of array that is helpful in creating a superficial pathway around the skin for transportation of the proteins and vaccines for sampling (Gupta PN, et al., 2005).
Another technology is the Metered-Dose Transdermal Spray (MDTS) that was developed in the Victorian College of Pharmacy. It has the ability to expand the overall growth of the TDD system and it is done by broadening the acceptance of patient and also the application of pharmaceutical for the much enhanced form of TDD. Another technology is the Electrically-Based Enhancement techniques that are using the Iontophoresis for facilitating the drug permeation all around the skin by using the electrical application (Oberli MA, et al., 2014).
The future challenges of transdermal drug delivery
Nicotine patch emergence for the past ten years had revolutionized cessation in smoking; treatment of angina was with nitroglycerin, clonidine is for hypertension, and motion sickness was treated with
scopolamine. Estradiol is for treating estrogen deficiency and all the treatment is via patches. Biotech medication was at the development stage then (Himanshu G and Babu RJ, 2013). For the past years now, the quantity of medication developed in patches has not seen any increase; showing little changes in the components of these patch arrangements (Prausnitz MR and Langer R, 2008). Modifications have been mostly hindered by modification of the used materials. This is because restricted quantity of medicines can match with the molecular mass and effective demands of transdermal absorption (Gupta PN, et al., 2005).
Conclusion
The most successful transdermal medical application needs many considerations. Having in mind that the major role of the human skin is containment and protection, it may seem extremely complex to focus on the mammalian skin for delivery of drugs. But, the understanding of the morphology and functioning of the human skin, and the techniques through which these features could be altered, many fresh drug brands are designed for Transdermal administration. The drug properties, the features of the Transdermal equipment, in-vivo model selection and patient’s skin status are very vital for effective and safe drug delivery. The Transdermal drug delivery system may possibly be the best among the most excellent new drug delivery system in future.
References
- Jampilek J, Brychtova K. Azone analogues: Classification, design, and transdermal penetration principles. Med Res Rev. 2012; 32: 907-947.
- Lawlor KT, Kaur P. Dermal contributions to human interfollicular epidermal architecture and self-renewal. Int J Mol Sci. 2015; 16(12): 28098-28107.
- Hatta I, Nakazawa H, Obata Y, Ohta N, Inoue K, Yagu N. Novel method to observe subtle structural modulation of stratum corneum on applying chemical agent. Chem and Phys of Lipids. 2010; 163(4-5): 381-389.
- Ebert CD, Patel D, Heiber W. Method and device for transdermally administering testosterone across nonscrotal skin at therapeutically effective levels. US patent and trademark office. 1992.
- Tanwar H, Sachdeva R. Transdermal Drug Delivery System: A Review. Int J Pharm Sci Res. 2016; 7(6): 2274-90.
- Chandrasekaran SK, Shaw JE. Factors effecting percutaneous absorption of drugs. Curr Probl Dermatol. 1978; 7: 142-155.
- Jensen LB, Petersson K, Nielsen HM. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur J PharmBiopharm. 2011; 79(1): 68-75.
- Gupta PN, Mishra V, Rawat A, Dubey P, Mahor S, Jain S, et al. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: A comparative study. Int J Pharm. 2005; 293: 73-82.
- Hull W. Heat-enhanced transdermal drug delivery: A survey paper. J Appl Res. 2002; 2(1): 1-9.
- Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004; 17(1): 43-48.
- Rissmann R, Oudshoorn MH, Hennink WE, Ponec M, Bouwstra JA. Skin barrier disruption by acetone: observations in a hairless mouse skin model. Arch Dermatol Res. 2009; 301(8): 609-613.
- Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal drug delivery system: A review. The Pharm Innovation. 2012; 1(4): 66-75.
- J van Smeden, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014; 1841: 295-313.
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Mcgrath JC, et al. The concise guide to pharmacology: Overview. Br J Pharmacol. 2013; 170(8): 1449-1458.
- Ale I, Marie J, Maibach H. Skin tolerability associated with transdermal drug delivery systems: An overview. Adv Ther. 2009; 26(10): 920-935.
- Ale I, Lachapelle JM, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: An overview. Adv Ther. 2009; 26(10): 920-935.
- Anissimov YG, Roberst MS. Modelling dermal drug distribution after topical application in human. Pharm Res. 2011; 28(9): 2119-2129.
- Arndats D, Arndts K. Pharmacokinetics and pharmacodynamics of transdermally administered clonidine. Eur J Clin Pharmacol. 1984; 26(1):79-85.
- Fujii M, Takeda Y, Yoshida M, Utoguchi N. Comparison of skin permeation enhancement by 3-l-menthoxypropane-1,2-diol and l-menthol: The permeation of indomethacin and antipyrine. Int J Pharm. 2003; 258(1-2): 217-223.
- Sapra B, Jain S, Tiwary AK. Percutaneous permeation enhancement by terpenes: mechanistic view. AAPS J. 2008; 10(1): 120-132.
- Nakamura Y, Takayama K, Higashiyama K, Suzuki T, Nagai T. Promoting effect of O-ethylmenthol on the percutaneous absorption of ketoprofen. Int J of Pharmaceutics. 1996; 145(1-2): 29-36.
- Barbero AM. Transcellular route of diffusion through stratum corneum: Results from finite element models. J Pharm Sci. 2006; 95(10): 2186-2194.
- Ramteke KH, Dhole SN, Patil SV. Transdermal drug delivery system: A review. J Advanced Sci Res. 2012; 3(1): 22-35.
- Tanner T, Marks R. Delivering drugs by the transdermal route: Review and comment. Skin Res Technol. 2008; 14(3): 249-260.
- Liu W, Teng L, Yu k, Sun X, Fan C, Long C, et al. Design of hydrogels of 5-hydroxymethyl tolterodine and their studies on pharmacokinetics, pharmacodynamics and transdermal mechanism. Eur J Pharm Sci. 2017; 96: 530-541.
- Stevenson JCL, Crook D, Godsland IF, Lees B, Whitehead MI. Oral versus transdermal hormone replacement therapy. Int J Fertil Menopausal Stud. 1993; 38(S1): 30-35
- Singh A, Bali A. Formulation and characterization of transdermal patches for controlled delivery of duloxetine hydrochloride. Anal Sci Technol. 2016; 7(1): 1-13.
- Palmer BC, Delouise LA. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules. 2016; 21(12):1719.
- Czarnowicki T, Krueger JG, Yassky EG. Skin barrier and immune dysregulation in atopic dermatitis: An evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014; 2(4): 371-379.
- Zidan AS, Kamal N, Alayoubi A, Seggel M, Ibrahim S, Rahman Z, et al. Effect of isopropyl myristate on transdermal permeation of testosterone from carbopol gel. J Pharm Sci. 2017; 106(7):1805-1813.
- Lee AJ, King JR, Hibberd S. Mathematical modelling of the release of drug from porous, non-swelling transdermal drug delivery devices. IMA J Math Appl Med Biol 1998; 15: 135-163.
- Khanday MA, Saxena VP. Mathematical study of diffusive fluid transport and distribution in human dermal regions. Anal Theory Appl. 2010; 26(4): 350-358.
- Schaefer H, Redelmeier TE, Lademann J. Relationship between the structure of compounds and their diffusion across membranes. Karger, Switzerland: Basel. 2011.
- Barba C, Alonso C, Marti M, Manich AM, Coderch L. Skin barrier modification with organic solvents. BBA Biomembranes. 2016; 1858(8): 1935-1943.
- Kim KT, Lee J, Kim MH, Park JH, Lee JY, Song JH, et al. Novel reverse electrodialysis-driven iontophoretic system for topical and transdermal delivery of poorly permeable therapeutic agents. Drug Delivery. 2017; 24(1): 1204-1215.
- Chen S, Qin M, Han Y, Zhao L, Fu Y, Shang Y, et al. Assessment of the efficacy of drug transdermal delivery by electro-phonophoresis in treating tuberculous lymphadenitis. Drug Delivery. 2016; 23(5):1588-1593.
- Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1-34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. 2011; 28(1): 159-165.
- Govil SK, Rudnic EM, Sterner DG. Transdermal nitroglycerin patch with penetration enhancers. US Patent 5,262,165, Schering Corporation. Lane ME. Skin penetration enhancers. Int J Pharm. 1993; 447: 12-21.
- Oberli MA, Schoellhammer CM, Langer R, Blankschtein D. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther Deliv. 2014; 5: 843-857.
- Himanshu G, Babu RJ. Transdermal delivery: Product and patent update. Recent Pat Drug Deliv Formul. 2013; 7(3): 184-205.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008; 26: 1261-1268.
Author Info
Ghazi ME Hussein1, Babiker M Elhaj2 and Heyam Saad Ali3*2Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, University of Science and Technology of Fujairah, Fujairah, UAE
3Department of Pharmaceutics, College of Pharmacy, University of Khartoum, Khartoum, Sudan
Received: 03-Jun-2021 Accepted: 17-Jun-2021 Published: 24-Jun-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3