Research Article - (2022) Volume 13, Issue 7
Abstract
Tramadol is codeine analogue with 4-phenyl-piperidine group; it has less analgesic power than morphine but with better abuse potential. However, chronic use associated with different adverse effects like anxiety, euphoria, nervousness, insomnia, depression, agitation. In this study, we assessed the outcome behind restriction of dispensing Tramadol in private health sectors in Iraq.
Methods: To assess Tramadol information gathered through searching in VigiBase data and their related Individual Case Safety Report (ICSR) that is officially supported by WHO global database. All reports were analyzed by Vigilyze mining of data and by computing IC25 to evaluate the strength of association between Tramadol and its related disorders, and comparing it with between with worldwide records.
Results: 184 cases were collected across 7 years of Iraqi Pharmacovigilance Center patient records collection for patients who used tramadol in many dosage forms. Among those, 32 cases showed Hyperhidrosis which occurred when using tramadol alone, 47 patients showed with vomiting and 67 of patients with nausea. Many recorded adverse effects found highly in Iraqi records comparing to that in globally recorded cases like chest discomfort, hyperhidrosis, headache, dyspnea and constipation. While other effects appeared lower than that globally reported like vomiting, hallucination, vertigo, respiratory depression and chills. No death appeared in all cases recorded across this duration.
Conclusion: Due to the restricting tramadol use by ministry of health and dispensing it only in public hospitals and under medical supervision, the number of reported adverse events and the percentage of major adverse events decreased.
Keywords
Tramadol, VigiBase, IC25
Introduction
Tramadol is cyclohexanolhydrochloride 2-(dimethyl amino)- methyl)-1-(3-to-methoxyphenyl). It is an analog of opioid drug codeine with 4-phenyl-piperidine group. The enantiomers (R,R) and (S,S) are now available as a racial mixture and are available mainly in hydrochloride form (ECDD, 2014).
Tramadol’s analgesic power is 10 times less than morphine but is more likely to be safe than later. Tramadol is considered safe be- cause in comparison to other opiate analgesics, it does not cause respiratory depression and addiction. In addition, tramadol has fewer potential for abuse when administered by parenteral route (Preston KL, et al., 1991).
It produces analgesic effects by showing agonistic activity of µ opioid receptors, central GABA and serotonergic receptors. It also has an antipersonnel binding property. Opioid receptors are four types of receptors coupled with G-protein. Opioid analgesia on μ opioid receptor are the most commonly used. The (+) Dextro tramadol enantiomer demonstrated an agonistic activity of the opioid receptor Mu (μ) and enhanced central dopamine activa- tion (Hennies HH, et al., 1988).
Tramadol is completely absorbed into the gut and shows bio- availability of approximately 75% of tramadol when taken orally. Tissue distribution of tramadol is about 2.6-2.9 L/kg body weight. Tramadol and its M1 metabolite reach the plasma 2-3 hours af- ter administration in healthy adults. In liver, metabolism occurs through O and N-demethylation and conjugation reactions by forming glucuronides and sulphates of tramadol. CYP2D6 en- zymes and N-demethyltramadol (M2) CYP2B6 and CYP3A4 catalyze the O-desmethyltramadol (M1) (Paar WD, et al., 1992). The M1 metabolite of tramadol as higher potency than dextro forms and has a 200-fold higher affinity. The half-life elimination for the M1 metabolite is approximately 9 hours compared to the six-hour for tramadol. The liver metabolites of the polar nature of tramadol are removed from the kidney. Approximately 30% of tramadol is excreted in the urine and 60% in metabolite form. Tramadol and its M1 metabolite are 6.3 and 7.4 hours of plasma half-lives (Black PA, et al., 2010) respectively. The Lethal Dose (LD50) is approximately 300-350 mg/kg orally for rat (Matthiesen T, et al., 1988).
Treatments of muscle pain and wound pain are primarily used for tramadol. Also in combination with non-opioid analgesics such as paracetamol is often prescribed for moderate to severe pain. The combination of tramadol with paracetamol with a fixed dose offers a brief onset and a long duration of action and multimodal analgesic effects. It alleviates the pain for a period of 8 hours com- pared with the drug itself in dental pain.
In postoperative, hydrocodone/paracetamol 10/650 mg is the same analgesic effect over 8 hours in combinations compared to tramadol (37.5 mg)+paracetamol (325 mg). Osteoarthritis pain, usually prescribed four times a day in 5 days, with one or two tramadol (37.5 mg)+paracetamol (325 mg). Usually, a maximum of 10 tablets or capsules per day are prescribed for 4 weeks in chronic back pain (37.5 mg) and paracetamol (325 mg) (Dhillon S, 2010).
Its use for children under 16 years is not recommended. The possibility of seizures when treated with tramadol is increased in patients with medical history of drug addiction, alcoholism, seiz- ure, epilepsy and headache and metabolic disturbance. It should not be used in kidney, liver, stomach, mental health, depression, and suicidal ideation patients as this may make their condition worse. Its use in women and mothers who have been pregnant is restricted as it could lead to birth defects and damage the fetus and nursing babies. In American Food an d Drug Administration, Tramadol is classified as category C in the pregnancy risk drug (Källén B and Reis M, 2015).
Tramadol’s adverse medicinal interactions are primarily caused by the overdose and abrupt combination of medicines that interact. Tramadol mainly interacts with the drugs such as MAO inhibitors, antidepressants, carbamazepine, blood thinners, digoxin, ketoconazole, rifampin, eryth- romycin, quinidine and medications that cause drowsiness. It has been shown to interact with 761 drugs; it causes major drug interactions with 446 drugs, moderate drug interactions with 311 drugs and minor drug interactions with 4 drugs. Tramadol should not be taken with alcohol, sedatives, narcotic pain relievers, antidepressants, anti-anxiety medica- tions and should be avoided for the treatment of mental illness, bipolar disorder and schizophrenia (Subedi M, et al., 2019). Tramadol injection by IM reduces gastric acid secretion during anesthesia (FDA, 2008).
Tramadol overdose increases the risk of seizures, therefore caution must be taken in patients with a history of seizure and other neurological abnor- malities during tramadol use (head trauma, brain damage or CNS tumor). Naloxone may also increase the risk of convulsions with tramadol over- dose (Kahn LH, et al., 1997). Tramadol is also contraindicated. Respira- tory depression and death can occur sometimes. In respiratory depression caused by Tramadol, naloxone is used as an antidote (Vogel W, et al., 1978).
Tramadol usage for a long time has a variety of common psychiatric ef- fects, such as CNS stimulation, anxiety, euphoria, nervousness, sleep disor- der, insomnia, depression, agitation, apathy and in rare cases nightmares, dependency and withdrawal syndrome. Also it causes menopause symp- toms and urinary tract infection in the genital system, and micturition, hematuria, dysuria, and cystitis.
In rare cases, Tramadol may cause Stevens-Johnson syndrome and hair disorder. Pruritus, swelling, skin rashes are common skin disorders. Also it may cause cellulite, piloting and urticaria.
Materials and Methods
Studydesign
This was a descriptive assessment of Tramadol and its possible disorders during its use by VigiBase mining data, the largest database of more than 20 million ICSRs submitted by members of the WHO Program for Inter- national Drug monitoring in 1968, and the risk of many disorders occur- ring simultaneously. The Uppsala Surveillance Center (UMC), Sweden, develops and maintains this database. On the 3rd February 2021, VigiBasis data mining with search criteria was conducted: “Tramadol” as medicinal substance.
During this study, several adverse events that occurred in Iraq were scanned during the duration of 7 years starting from 2014 till January 2021. Reac- tion outcome, disproportionality measure (Information Component-IC value) and other relevant variables were retrieved using VigiLyze. VigiLyze is a data mining and analytics tool developed for VigiBase. Drug adverse reaction (ADR) pair disproportionality is measured by IC.
IC used to evaluate strength of association between drug and ADR. It is mathematically expressed as-
IC=log2 p(x,y)/p(x), p(y)
Where p(x) is probability of specific drug ‘x’ being listed in a case report; p(y) is probability of specific ADR ‘Y’ being listed in a case report and p(x,y) is probability of specific drug- ADR combination being listed in a case report.
Positive IC value indicates that certain drug-adverse reaction is reported with a higher rate than the expected rate based on the rest of the reports in the database. IC value of zero indicates no quantitative dependency, while negative IC value indicates the combination is reported less commonly than statistically expected in the database.
this article include all adverse events with high IC value that appear as Iraqi IC25/global IC25>1 value which can be defined as “traditional thresh- old that indicate a correlation between drug-ADR more than expected globally based on all reported cases found in VigiBase”.
Exclusion criteria includes all adverse events that have same or lower IC value compared to that of globally found in VigiBase.
Literature search on this article include the following keywords in Goo- gle scholar, PubMed, search engine: “Tramadol”, ”Hyperhidrosis”, “chest discomfort”, “constipation”, “dizziness”, “vomiting”, “hallucination”,” Head- ache”, “respiratory depression”, “vertigo” and “dyspnea”.
Results
During last 7 years, 184 cases was surveyed and documented in VigiBase from Iraqi Pharmacovigilance center. All cases screened quantitively and compare them with adverse events that are globally reported in the same database. The most common adverse drug reaction that are labelled in the summary of Product characteristics are scheduled in Table 1 with compar- ing Iraqi IC25/Global IC25 ratio in Figure 1, gender, seriousness ,patient age, concomitant drug use, multiple adverse drug reaction.
| Adverse Drug Reaction (ADR) | No. of cases | Iraqi IC25/Global IC25 ratio | Gender M(Male) F(Female) |
Seriousness | Patient age (18-44 yrs) | Concomitant drug use (monotherapy) | Co-reported reaction term |
|---|---|---|---|---|---|---|---|
| Hyperhidrosis | 32 | 3.7/2.3 | M=15(46.9%) F=17(53.1%) |
8(25%) | 40% | 100% | 90% with sweating |
| Chest discomfort | 8 | 1.6/0.4 | M=4(50%) F=4(50%) |
6(75%) | 62.5% | 100% | 62.5% with hyperhidrosis |
| Constipation | 14 | 1.5/1 | M=5(35.7%) F=9(64.3%) |
3(21.4%) | 64.3% | 78% | 28.6% with sweating |
| Headache | 17 | 0.7/-0.4 | M=5(29.4%) F=12(70.6%) |
3(17.6%) | 70.6% | 70.50% | 47% with nausea |
| Nausea | 67 | 2.4/2.7 | M=23(65.7%) F=44(34.3%) |
16(23.9%) | 68.7% | 65.70% | 40% with vomiting |
| Gastric disorder | 8 | 1.5/-1.8 | M=4(50%) F=4(50%) |
1(12.5%) | 25% | 100% | 100% alone |
| Constipation | 14 | 1.5/1.0 | M=9(64.3%) F=5(35.7%) |
3(21.4%) | 64.3% | 78.70% | 7% with absent bowel movement |
| Dizziness | 20 | 1.5/1.7 | M=8(40%) F=12(60%) |
3(15%) | 75% | 100% | 55% with nausea |
| Vomiting | 47 | 1.3/2.7 | M=13(27.7%) F=32(68.1%) |
13(27.7%) | 68.1% | 70.20% | 57.4% with nausea |
| Hallucination | 4 | 0.7/1.6 | M=1(25%) F=3(75%) |
4(100%) | 50% | 100% | 50% with nausea |
| Dyspnea | 20 | 0.3/-0.4 | M=4(20%) F=15(75%) |
14(70%) | 55% | 80% | 25% with nausea |
| Vertigo | 3 | -0.2/1.5 | M=2 (66.7%) F=1(33.3%) |
2(66.7%) | 33.3% | 100% | 66.7% with nausea, dizziness, constipation |
| Respiratory depression | 2 | -0.5/2.4 | F=2 (100%) | 1(50%) | 50% | 100% | 100% with nausea and dizeness |
| Chills | 3 | -0.9/-1.4 | M=2(66.7%) F=1(33.3%) |
1(33.3%) | 66.7% | 100% | 100% with hyperhidrosis and 66.7% with nausea |
Table 1: Most common adverse drug reaction reported by Iraqi pharmacovigilance center
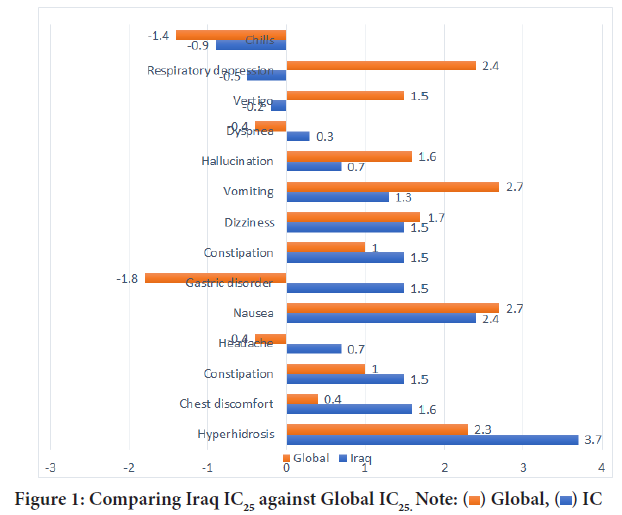
Figure 1: Comparing Iraq IC25 against Global IC25. Note: 
Sex was documented in all cases and females constitute about 59% of total cases. Age was also reported in those and the majority of them is belong to 18-44 years old with 58.7%. Tramadol was concomitantly used in 19% of cases; the most common used concomitant drugs are ceftriaxone, meto- clopramide, paracetamol, acetyl salicylic acid, clopidogrel. Moreover, So- dium Chloride, Heparin, Amikacin, Atorvastatin, Isosorbide dinitrate are also co-reported use with Tramadol. Reports also shows that Nausea is the most common adverse event that appeared in total cases which constitute about 36.4% while vomiting have 25.5% incidence in those reports. Serious cases appeared in 31% with one fatal report (0.5%) and 10.3% of cases are lives threatening and same percentages have prolonged hospitalization.
Discussion
The cases that reported through Iraq Pharmacovigilance center are con- tain several concomitant drug usage which cannot not ensure whether Tramadol is responsible for this Adverse effects. In contrast to that many reported cases showed that tramadol is the only used medicine (as in hyperhidrosis, chest discomfort, gastric disorder, dizziness, hallucination, vertigo, respiratory depression and chills) and this confirm that tramadol is responsible for those effects (Drugs.com, 2021).
Globally the majority of those effects have small differences between their percentages and some of them showed negative correlation which indi- cate that the expected reports are higher than that actually reported. Some of adverse effects reported showed great difference in IC25 between cases reported in Iraq from that globally recorded as in hyperhidrosis, chest dis- comfort, headache and gastric disorder.
During surveying these reports, reported adverse events that use tramadol as a monotherapy are being focused more that those used concomitant- ly with other drugs. Hyperhidrosis is one of these reports that tramadol being used as sole therapy and showing higher reports when compared to globally reports. Hyperhidrosis is appeared commonly in about 17% of cases which occurred through monoaminergic effect of tramadol and rarely affect their usage, but this side effect may affect patient adherence especially in hot climate like Iraq.
Chest discomfort is one of less common adverse effects of tramadol and it was appeared in only 8 patients (4%) out of 184 cases and its IC25 does not reflect the real number when compared to globally reported cases.
Nausea and Vomiting appeared commonly with Tramadol usage and they appeared in about 37% and 25% respectively, Iraqi reported cases IC25 for nausea is comparable to that globally reported while for vomiting it is less that that recorded by WHO. This may be due to less cases reported from Healthcare professional from Iraq or due to low dose usage of tramadol which cannot be approved through those reports.
Conclusion
Dizziness was appeared in about 10% of reported cases and more than half of them accompanied by nausea and comparable IC25 between Iraqi and globally reported cases. In contrast to those mentioned before, serious adverse events which are accompanied with higher dose of Tramadol are much less in Iraq than that reported globally like hallucination, respira- tory depression. Also, severe adverse events that reported globally are not recorded in Iraq. These may be due to restricted use of Tramadol in Iraq for hospitalized patient in governmental sector and prohibited their use in private sector which leads to decrease tramadol poisoning cases reported through Iraq pharmacovigilance center.
References
- WHO update review report on tramadol. (Expert Committee on Drug Dependence) ECDD. 2014.
- Preston KL, Donald RJ, Margaret T. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991; 27(1): 7-17.
[Crossref] [Google scholar] [Pubmed]
- Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988; 38(7): 877-880.
[Google scholar] [Pubmed]
- Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig. 1992; 70(8): 708-710.
[Crossref] [Google scholar] [Pubmed]
- Black PA, Cox SK, Macek M, Tieber A, Junge RE. Pharmacokinetics of tramadol hydrochloride and its metabolite O-desmethyltramadol in peafowl (Pavocristatus). J Zoo Wildl Med. 2010; 4: 671-676.
[Crossref] [Google scholar] [Pubmed]
- Matthiesen T, Wöhrmann T, Coogan TP, Uragg H. The experimental toxicology of tramadol: An overview. Toxicol Lett. 1998; 95(1): 63-71.
[Crossref] [Google scholar] [Pubmed]
- Dhillon S. Tramadol/paracetamol fixed-dose combination. Clin Drug Investig. 2010; 30(10): 711-738.
[Crossref] [Google scholar] [Pubmed]
- Källén B, Reis M. Use of tramadol in early pregnancy and congenital malformation risk. Reprod Toxicol. 2015; 58: 246-251.
[Crossref] [Google scholar] [Pubmed]
- Subedi M, Bajaj S, Kumar MS, Mayur YC. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019; 111: 443-451.
[Crossref] [Google scholar] [Pubmed]
- Ultram (tramadol) full prescribing information. FDA. 2008.
- Kahn LH, Alderfer RJ, Graham DJ. Seizures reported with tramadol. JAMA. 1997; 278: 1661.
- Vogel W, Burchardi H, Sihler K, Valic L. The effect of tramadol, a new analgesic, on respiration and cardiovascular function. Arzneimittelforschung. 1978; 28(1a): 183-186.
[Google scholar] [Pubmed]
- Tramadol. Drugs.com. 2021.
Author Info
Samer Shukur Mohammed1*, Wael Walid Mustafa1 and Manal Mohammed Younis22Department of Pharmacovigilance, Iraqi Pharmacovigilance Center, Baghdad, Iraq
Citation: Mohammed SS: Consequences behind Tramadol Dispensing Ban in Iraqi Private Health Sectors
Received: 30-Jun-2022 Accepted: 22-Jul-2022 Published: 29-Jul-2022, DOI: 10.31858/0975-8453.13.7.439-442
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






