Research Article - (2023) Volume 14, Issue 5
Abstract
TET1 (Ten-eleven translocation methylcytosine dioxygenase 1) is the enzyme methylcytosine dioxygenase of DNA demethylation in the nervous system. TET1 controls and mediates gene transcription, memory formation, and extinction. However, little is known about TET1 in Prefrontal Cortex (PFC) functions especially in the medial Prefrontal Cortex (mPFC), which controls cortex flexibility and emotional reactivity in the Central Nervous System (CNS). This study conducted behavioral tests including an open field test, sociability and social novelty preference tests, social dominance, and Prepulse Inhibition (PPI) test to examine brain functions, especially PFC functions after the deletion of TET1. The mPFC from TET1 KO mice and WT adult mice was analyzed using Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) to assess neuron growth-related genes, including GSK3β, PI3K, CRX4, FGFR1, FGFR2, EGFR, DBN1, AKT2, VEGF, VEGFR, and AKT3. Subsequently, primary PFC neuronal cells were administered shTET1 to Knockdown (KD) the TET1 gene and function. We found that the deletion of TET1 in the mouse brains impaired social interaction, novelty, and Prepulse Inhibition (PPI) in the mice. Knockdown of the TET1 gene influenced the growth and complexity of neurons. The increase in NGF and BDNF by Western blotting was found in TET1 deficient mice. The results support and complement the view that TET1 deficiency may be related to schizophrenia.
Keywords
Ten-eleven translocation methylcytosine dioxygenase 1 (TET1), medial Prefrontal Cortex (mPFC), Nerve Growth Factor (NGF), Sociability
Abbrevations
CNS: Central Nervous System; PFC: Prefrontal Cortex; mPFC: medial Prefrontal Cortex; DA: Dopamine; 5-HT: Serotonin; GABA: Gamma-Aminobutyric Acid; Glu: Glutamic acid; NE: Norepinephrine; Ach: Acetylcholine; TET1: Ten-eleven translocation methylcytosine dioxygenase 1; 5mC: 5-methylcytosine; 5hmC: 5-hydroxymethylcytosine; CRS: Chronic Restraint Stress; NAc: Nucleus Accumbens; Dnmt3a: DNA methyltransferases; SSE: Stressful Social Experience; PPI: Prepulse Inhibition; ASDs: Autism Spectrum Disorders; PTSDs: Posttraumatic Stress Disorders; WT: Wild-Type; KO: Knockout; KD: Knockdown.
Introduction
TET1 mediates the conversion of 5-methylcytosine (5mC) to 5-hydroxycytosine (5hmC) in CpG dinucleotides and plays a very important role in the process of DNA demethylation (Kaas GA, et al., 2013). TET1 and its two family member transcripts (TET2/ TET3) exist in the brain. expresses itself higher than in the cerebellum, cortex, and hippocampus than either TET2 or TET1 (Kaas GA, et al., 2013). TET1 is the first enzyme that can catalyze the conversion of 5mC into 5hmC (Szwagierczak A, et al., 2010). The transcript level of TET1 shows a decreasing trend in vitro and in vivo and is regulated by neural activity (Tahiliani M, et al., 2009). Deletion of TET1 leads to a significant decrease in TET1 mRNA levels; however, its other two TET family members, TET2 and TET3 transcripts, do not continuously respond to TET1 deletion (Kaas GA, et al., 2013).
Chronic Restraint Stress (CRS) can induce depression-like behaviors in mice, especially in the Prefrontal Cortex (Chiba S, et al., 2012). Researchers found that the loss of TET1 and TET2 has a contracted influence on the susceptibility of mice to CRS (Antunes C, et al., 2019). Under social defeat stress conditions, the selective Knockout of TET1 in the Nucleus Accumbens (NAc) of adult mice can lead to antidepressant-like effects (Feng J, et al., 2017). The amygdala, hippocampus, and PFC are the three main brain regions related to stress response and sociability. TET3 and DNA methyltransferases (Dnmt3a) in the amygdala increased the occurrence of early-life Stressful Social Experience (SSE) compared with the control group (Moy SS, et al., 2004). Social behavior dysfunction appears in various mental illnesses, such as schizophrenia, depression, and autism (Karen C and Rajan KE, 2019; Chevallier C, et al., 2012). Several brain regions are related to social cognition, especially the amygdala (Charernboon T and Patumanond J, 2017), NAc (Adolphs R, 2009), hypothalamic nuclei (Dölen G, et al., 2013), midbrain (McHenry JA, et al., 2017; Molas S, et al., 2017), hippocampus (Franklin TB, et al., 2017), and Prefrontal Cortex (Okuyama T, et al., 2016; Bicks LK, et al., 2015). In these brain regions, the Prefrontal Cortex is a critical region involved in high-order cognitive functions and emotional processing (Kim Y, et al., 2015; Zhong S, et al., 2018). Damage to the Prefrontal Cortex can cause impaired transmission of excitatory neurons, thus disrupting sociability (Finn ES, et al., 2019). Patients with a damaged frontal cortex suffer huge personal and social difficulties (Yizhar O, et al., 2011; Moss H and Damasio AR, 2001). In the social interaction, elevated plus-maze, and shock- probe tests, lesions of the mPFC in rats cause anxiolytic-like effects (Moss H and Damasio AR, 2001). Prefrontal dysfunction can lead to numerous complex cognitive deficits associated with diseases (e.g., schizophrenia (Shah AA and Treit D, 2003; Weinberger DR and Berman KF, 1996), Alzheimer’s disease (Frith C, 1996), and Parkinson’s disease (Akbarian S, et al., 1995)).
Recently, Müller U, et al., 2000, reported that TET1 mRNA, protein expression, and 5hmC levels were elevated in psychotic patients’ parietal cortexes compared with non-psychiatric subjects. TET1 has been expressed in the Prefrontal Cortex of mice (Dong E, et al., 2012). In Towers AJ, et al., 2018, TET1 regulated OXTR expression and hyper methylated CpG islands of OXTR. In addition, TET1 deletion impaired social and maternal care behaviors (Towers AJ, et al., 2018), however, it is unclear whether TET1 regulates PFC functions including social interactions and social dominance. In the present study, we selected TET1 KO mice as the research subjects and initially investigated the TET gene family and 5hmC in the Prefrontal Cortex of TET1 KO mice. We investigated whether social interaction, social dominance, and Prepulse Inhibition underlie the mechanism by which TET1 regulates PFC functions in the Prefrontal Cortex.
Materials and Methods
Animals
TET1 KO mice were generated as described in previous research and were obtained from Zhejiang University (Zhang RR, et al., 2013). Most studies adopted nine adult male WT mice and nine adult male KO mice (all were aged 8-12 weeks old, weighing 15-20 g). We divided mice in each group into two cages (4 or 5 per cage). All TET1 KO mice (10) and WT mice (10) were housed and kept in constant conditions (temperature 20°C ± 2°C, humidity 40%-60%, 12-hour shift of the light-dark cycle) and were given the standard diet and water. All mice lived in 12-hour shifts of light-dark cycles. Before the behavioral tests, all mice were moved into the test room for one week to adapt to the environment. After the behavioral tests, we sacrificed all mice humanely. Then, the mice’s brains were extracted. Later, the cortex, mPFC, hippocampus, and cerebellum were dissected and frozen immediately. The study was approved by the ethics review committee of experimental animal welfare of Zhejiang University (ZJU20170949). All animal procedures for behavioral tests and neuronal cell cultures were conducted in accordance with Zhejiang University. The study was performed in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Immunofluorescence staining and fluorescence microscopy
The experimental steps for making the brain slices were as follows. As noted in previous research (Song TT, et al., 2016), tissue sections and neuronal cells were fixed in 4% formaldehyde. Then we removed the brain tissue, and dehydrated it with 30% sucrose for three days. After three days, we used a cryotome (HM 505E, Germany) to cut the brain tissues into 20 μm slices. The brain slices were then removed for brain cortex immunofluorescence staining.
The experimental steps for staining brain slices and neurons were as follows: The brain slices and the transfected neurons were washed with 0.01 M Phosphate-Buffered Saline (PBS) three times for 5 minutes each time, and then were blocked with blocking solution (PBS, pH 7.4, containing 10% goat serum). Primary antibody was then added and stained overnight in a refrigerator at 4°C. On the second day, the neurons were removed and shaken at room temperature for 1 hour and then washed with 0.01 M PBS three times for 5 minutes each time. After washing, the samples were incubated with the second antibody for 1 hour and then washed with 0.01 M PBS three times for 5 minutes each time. After washing, we sealed the samples and collected images with a Zeiss microscope using image acquisition software (LSM 510; Carl Zeiss, Germany). The samples were then incubated with appropriate primary antibodies (GFAP, 1:500, Cell signaling, Ms, 3671, USA; NeuN, 1:500, Millipore, Ms, MAB377, USA) and secondary antibodies (Alexa Fluor 545-tagged, 1:500, Invitrogen, goat anti-mouse, A11031, USA) in 0.1% saponin. Image collection was conducted on a Zeiss scanning microscope (LSM 510; Carl Zeiss, Germany). Then images were analyzed using LSM 510 software (LSM 510; Carl Zeiss, Germany).
Behavioral tests
The behavioral tests adopted double-blind experiments. In other words, the researchers performed testing on the TET1 KO and WT mice, and they were blind to which group of mice they were testing. Adult male TET1 KO mice and littermate male WT mice (all were aged 8 weeks old, weighing 15-20 g) were used for the behavioral tests. All behavioral parameters were measured by t-tests/Analysis of Variance (ANOVA) for two genotypes (P<0.05 was regarded as statistically significant). The outcomes of this research are expressed as mean ± Standard Error of Mean (SEM). As indicated in previous studies (Zhang RR, et al., 2013; Song TT, et al., 2016), an open field test and sociability and social novelty preference tests were conducted. The experiments are described as follows.
Open field test
The open field test was investigated in the early literature (Carlen M, et al., 2012). The two types of genotype mice were put into a 45 cm × 45 cm × 45 cm chamber separately and allowed to move freely for 5 minutes. We used TET1 KO mice (6) and WT mice (7) for open field test. Then, each TET1 KO and WT mouse was gently placed into the chamber for 30 minutes. Compared to staying in the central area, the mice usually spent more time moving along the edges of the chamber. If the mice spent more time moving in the central area, it showed that the mice may have anxiety-like behaviors (Feng J, et al., 2017; Kraeuter AK, et al., 2019).
Sociability and social novelty preference tests
Sociability and social novelty preference tests have been utilized in previous research (Moy SS, et al., 2004). Briefly, TET1 KO (8) and WT (8) mice were put into the experimental room to adapt for one week. Then on the test day, mice were put into the test room to adapt for one hour. Before the tests, the subject mice were put into the middle room of the social experiment device, where they could move freely to the other two rooms on both sides for 5 minutes. After the mice explored, they were put back into separate cages, and an empty metal cup Object (O) was placed in a small room on one side of the social experiment device, where Strange mouse 1 (S1) was placed on the other side. Male C57/BL6J mice were purchased from Shanghai Slack Laboratories and used as ‘strangers’. In the sociability test, an unfamiliar male mouse (Stranger 1) that had no contact with the subject mouse before was placed in one of the side chambers. The subject mice were put into the middle room and the doors of the other two rooms on both sides were opened. The subject mice were free to move for 10 minutes. The contacts between the limbs and nose wings of stranger 1 mouse and the metal cup were calculated as the actual contact.
In the social novelty preference test, the subject mice were gently put back into the middle room. Then we removed the Object (O) from one side of the cup, placed a new unfamiliar mouse (Stranger 2) in the previous object cup, opened the doors on both sides, and allowed the subject mice to move freely in the rooms on both sides for 10 minutes. The time spent by stranger 1 and stranger 2 was measured.
All videos and analyses were collected and analyzed with Noldus Observer software (Noldus Information Technology, Wageningen, Netherlands). During the experiments, the frequency and duration of mouse activities were recorded. When two mice-maintained vibrissae or nose-to-nose contact for one or more seconds, their activities were recorded. Data and videos were analyzed by our researchers, who were blinded to the mouse genotypes.
Social dominance
This research employed the tube test to explore social dominance (Moy SS, et al., 2004; Carlen M, et al., 2012). The tube test apparatus, a kind of plexiglass tube, has a length of 30 cm and an inside diameter of 3.5 cm. TET1 KO (4) and WT (4) mice (all were 8 weeks old, weighing 15-20 g) were divided into 2 groups and were placed in the test room for at least one week for adaptation. Before the test, all mice had undergone 5 trials for tube training. On the test day, pairs of mice were released at two ends of the tube and met in the middle area of the tube. The two mice met in the middle of the tube. The mouse that drove the other mouse out of the tube was considered the winner. There were two mice in each group with no difference in body weight. Four mice between the two groups were tested against each other. The winning rate of each group was recorded.
Prepulse Inhibition (PPI) test
Prepulse Inhibition (PPI) reflects sensory-motor gating and appropriate forebrain function (Zhou T, et al., 2017; Park MJ, et al., 2018). TET1 KO mice (10) and littermate WT (10) mice (all were 8 weeks old, weighing 15- 20 g) were used in a single testing session. The test started with a 5-minute acclimatization period, followed by 64 recorded trials. All stimuli were performed in a pseudorandom order within the background of 65 dB. According to equation (1) (Swerdlow NR, et al., 2001), the percentage of PPI of each prepulse stimulus intensity (75, 80 and 85 decibels) was calculated, where the individual score of the pulse was the mean value of the motion amplitude of the individual test. Startle amplitude and PPI value were obtained through the Startle Response System, which reflects the animal’s sensorimotor gating function. When this function is normal, the animal has an obvious PPI phenomenon. When this function is damaged, the PPI value will increase or decrease.
%PPI=100 × ((pulse alone score)-(prepulse+pulse score)) ∕ pulse alone score (1)
Primary neuronal cell cultures
Primary PFC neuronal cultures were obtained from the Prefrontal Cortex of embryonic E13.5 C57BL/6J mice brains (Karacay B, et al., 2015). Briefly, the mouse Prefrontal Cortex was dissociated by mechanical trituration before removing meninges and blood vessels. The isolated cells were incubated in trypsin/EDTA solution (Thermo Fisher, USA) for 20 minutes in a humidified atmosphere of 5% CO2/95% air at 37°C. Then, these cells were suspended in Neurobasal medium (NB) within 2% B27 (Thermo Fisher, USA), 1% penicillin/streptomycin, and 1% GlutaMAX (Thermo Fisher, USA). We plated the detached cells on PDL coated coverslips in 35-mm dishes at a density of 30,000 cells/cm2. After incubating for 2 hours at 37°C, we detached the nonadherent cells and cultured the adherent cells with 2% B27, 1% penicillin/streptomycin, and 1% GlutaMAX. The cell medium was changed over 3 days with 2% B27, 1% penicillin/streptomycin, and 1% GlutaMAX present.
Transfections
To Knockdown the TET1 gene, this study used TET1 short hairpin RNAs (shRNAs). The sequence of the shRNA sequence was CCGGCAACTTGCATCCACGATTAATCTCGAGATTAATCGTGGATGCAAGTTGTTTTG. TET1 shRNAs were utilized for transfection into HEK293 cells as indicated by the manufacturer. The control samples were transfected with a lenti-control plasmid. Based on the instructions of the kit, we extracted plasmid DNA with a Qiagen plasmid kit (Qiagen, Hilden, Germany). The purity and concentration and of plasmid DNA were tested by a NanoDrop and then stored at -20°C. We added 2.5 μgs plasmid DNA and 3.3 μl of Lipofectamine 2000 (Thermo Fisher, USA) in 200 μl of NB to prepare the transfection solution. After incubating at room temperature for 5 minutes, the transfection solution was transferred to neuronal cells in 1 ml of fresh Neurobasal medium within L-glutamine. After being placed in the cell incubator at 37°C for 45 minutes, we washed neuronal cells in warm Neurobasal medium and cultured them back with the original conditioned medium. After 48 hours, we fixed neuronal cells and prepared them for immunocytochemistry, imaging, and Sholl analysis.
Sholl analysis
Sholl analysis was employed to calculate dendritic length using ImageJ (He W, et al., 2019). Neuron complexity, neurite number, and dendritic length were assessed using Sholl analysis on a Simple Neurite Tracer and are represented in full. Dendritic intersections were counted using ImageJ software. Forty-one neuronal cells in the control group and shTET1 group were included in the Sholl analysis. This study analyzed at least 41 representative neurons.
Gene correlation analysis
Gene correlation analysis was performed using GeneMANIA software (http://genemania.org) (He W, et al., 2019). GeneMANIA is a multifunctional website for researchers to make hypotheses with gene functions, and gene lists and to conduct gene function analysis. First, the relevant information was entered on the website. Then, we used the advanced options pane to expand the initial query screen. We then selected the required network. The network weighting method was then adopted. Finally, the GeneMANIA results were collected.
RNA isolation and qRT-qPCR
We extracted total RNA from the brain cortex, mPFC, hippocampus, and cerebellum of TET1 WT and KO mice using TRIzol reagent (Ambion, USA). Total RNA was isolated, and then the concentration was quantified by using a NanoDrop Spectrophotometer 2000. Reverse Transcription (RT) was conducted on a total of 400 ng RNA by using an RT reagent kit (Vazyme, China) according to the manufacturer’s guidelines. Standard real-time qPCR was conducted using SYBR Green mix (Vazyme, China). All real-time PCRs were performed three times. The analysis of the results was conducted according to the ΔΔCt method. The target genes’ relative expression levels were calculated by normalization to 18S. Data were obtained from an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems, USA).
Western blot analysis
Total protein was extracted from mouse mPFC tissue by using Radioimmunoprecipitation Assay (RIPA) buffer. After measuring the protein concentration, electrophoresis on a sodium dodecyl sulfate-polyacrylamide gel was performed. Then, we transferred the proteins to a polyvinylidene difluoride membrane after electrophoresis. The following primary antibodies were used: TRKA (diluted concentration 1:1000, ABclonal, A2098, China), NGF (diluted concentration 1:1000, ABclonal, A14216, China), Gskβ (diluted concentration 1: 1000, Cell Signaling, 9322, USA), p-AKT (diluted concentration 1:1000, Cell Signaling, 4691p, USA), and GAPDH (diluted concentration 1:10000, Ambion, AM4300, USA). The film was colored by adding ECL solution and detected it by the Western blotting Detection System (Amersham Bioscience, UK). The intensity of images and bands were analyzed with Adobe Photoshop software.
Statistical analysis
All values are displayed as the means ± SEM. The unpaired t-test and ANOVA (GraphPad Prim 5.0 Program Software, San Diego, CA) was employed for data analysis. A value of P<0.05 was regarded as statistically significant.
Results
TET1 is expressed in Prefrontal Cortex neurons, and 5hmC is present in neurons. It is involved in regulating the activities of neurons and synapses; however, its localization within the Prefrontal Cortex remains unclear. First, this study aimed to confirm the expression changes of the transcript levels of TET1 and its family members TET2 and TET3 in the cortex, hippocampus, and cerebellum and in the medial Prefrontal Cortex of WT mice (Figure 1a). Then TET1-TET3 levels were verified in the mPFC of WT mice. We disrupted the TET1 gene using the deletion of exons 11-13, encoding the critical catalytic domain in deoxygenates (Zhang RR, et al., 2013). Second, this study aimed to confirm the expression changes of the transcript levels of TET1 and its family members TET2 and TET3 in the Prefrontal Cortex. As such, we used quantitative Reverse-Transcription PCR for TETs in the medial Prefrontal Cortex. TET2 and TET3 were more abundantly expressed than TET1, especially TET3 (Figures 1b-1e). TET1- TET3 have been researched in brain development and neurogenesis; however, little research on TETs has been conducted concerning social behaviors. The medial Prefrontal Cortex is one of the critical regions regulating social behavior and emotions in humans and mice. Additionally, TET1 is one of the important enzymes responsible for converting 5mC to 5hmC. Therefore, we conducted immunofluorescence staining (5hmC) in the medial Prefrontal Cortex of TET1 KO and WT mice. We found that 5hmC is abundantly expressed in the nucleus, especially in neurons. Then we double-labeled the Prefrontal Cortex sections with the neuronal marker NeuN and an antibody against 5 hmC. Immunohistochemical analysis was conducted later in neurons throughout the Prefrontal Cortex (Figure 1f). In neurons, we discovered that 5-hydroxymethylcytosine appeared in the nucleus and soma (Figure 1f). These results suggested that TET1 is abundantly expressed in Prefrontal Cortex neurons and may play an important role in regulating cortex flexibility and emotion.
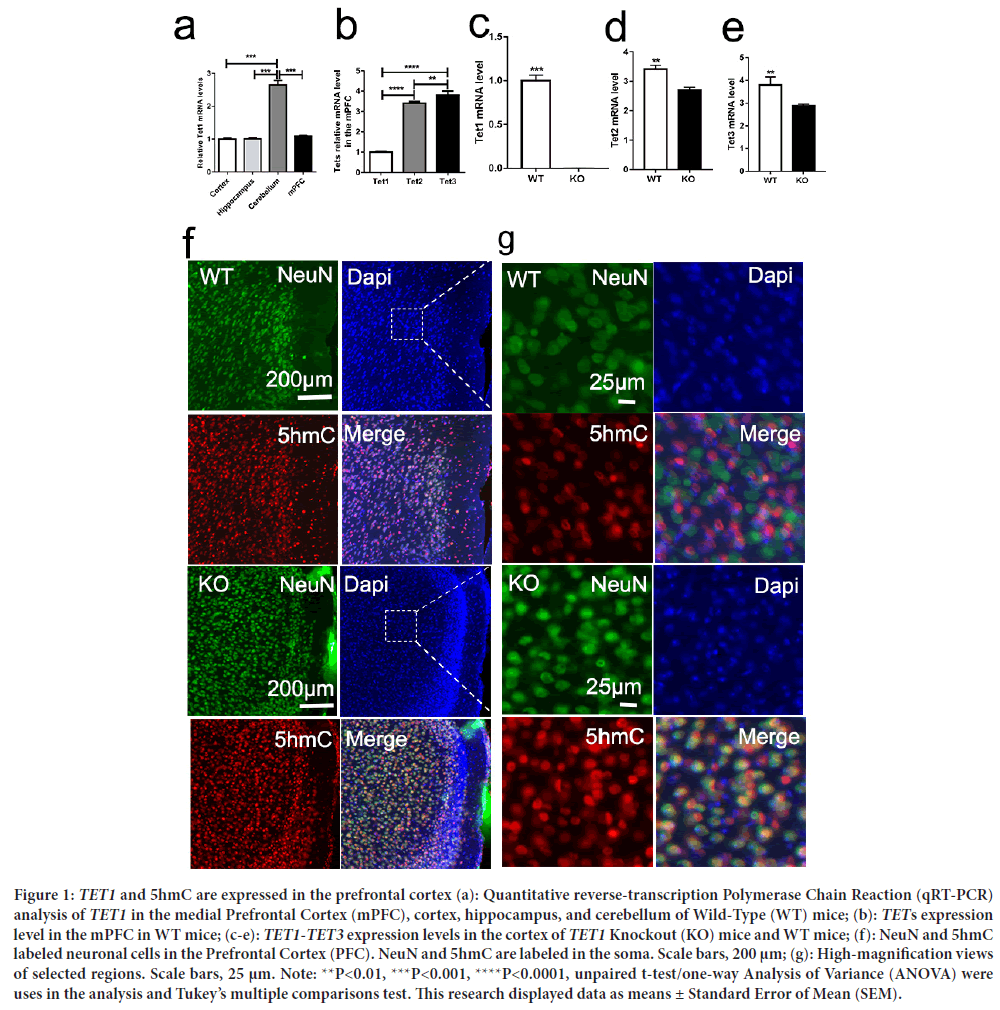
Figure 1: TET1 and 5hmC are expressed in the prefrontal cortex (a): Quantitative reverse-transcription Polymerase Chain Reaction (qRT-PCR) analysis of TET1 in the medial Prefrontal Cortex (mPFC), cortex, hippocampus, and cerebellum of Wild-Type (WT) mice; (b): TETs expression level in the mPFC in WT mice; (c-e): TET1-TET3 expression levels in the cortex of TET1 Knockout (KO) mice and WT mice; (f): NeuN and 5hmC labeled neuronal cells in the Prefrontal Cortex (PFC). NeuN and 5hmC are labeled in the soma. Scale bars, 200 μm; (g): High-magnification views of selected regions. Scale bars, 25 μm. Note: **P<0.01, ***P<0.001, ****P<0.0001, unpaired t-test/one-way Analysis of Variance (ANOVA) were uses in the analysis and Tukey’s multiple comparisons test. This research displayed data as means ± Standard Error of Mean (SEM).
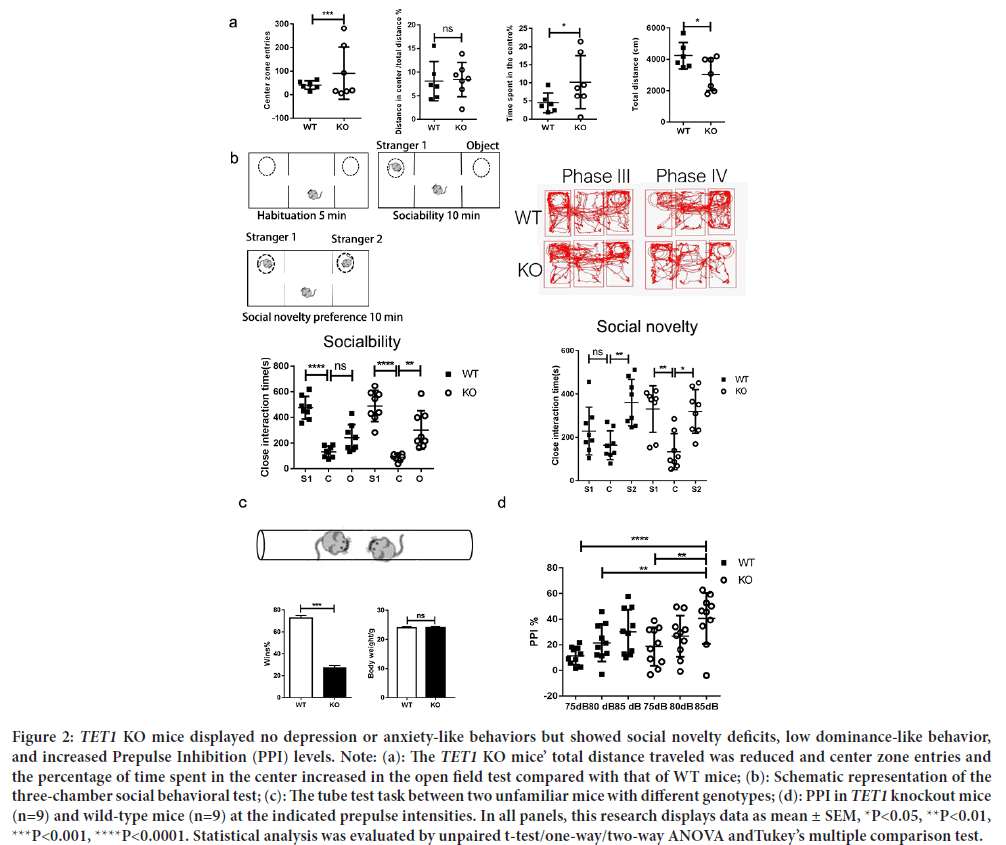
Figure 2: TET1 KO mice displayed no depression or anxiety-like behaviors but showed social novelty deficits, low dominance-like behavior, and increased Prepulse Inhibition (PPI) levels. Note: (a): The TET1 KO mice’ total distance traveled was reduced and center zone entries and the percentage of time spent in the center increased in the open field test compared with that of WT mice; (b): Schematic representation of the three-chamber social behavioral test; (c): The tube test task between two unfamiliar mice with different genotypes; (d): PPI in TET1 knockout mice (n=9) and wild-type mice (n=9) at the indicated prepulse intensities. In all panels, this research displays data as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Statistical analysis was evaluated by unpaired t-test/one-way/two-way ANOVA andTukey’s multiple comparison test.
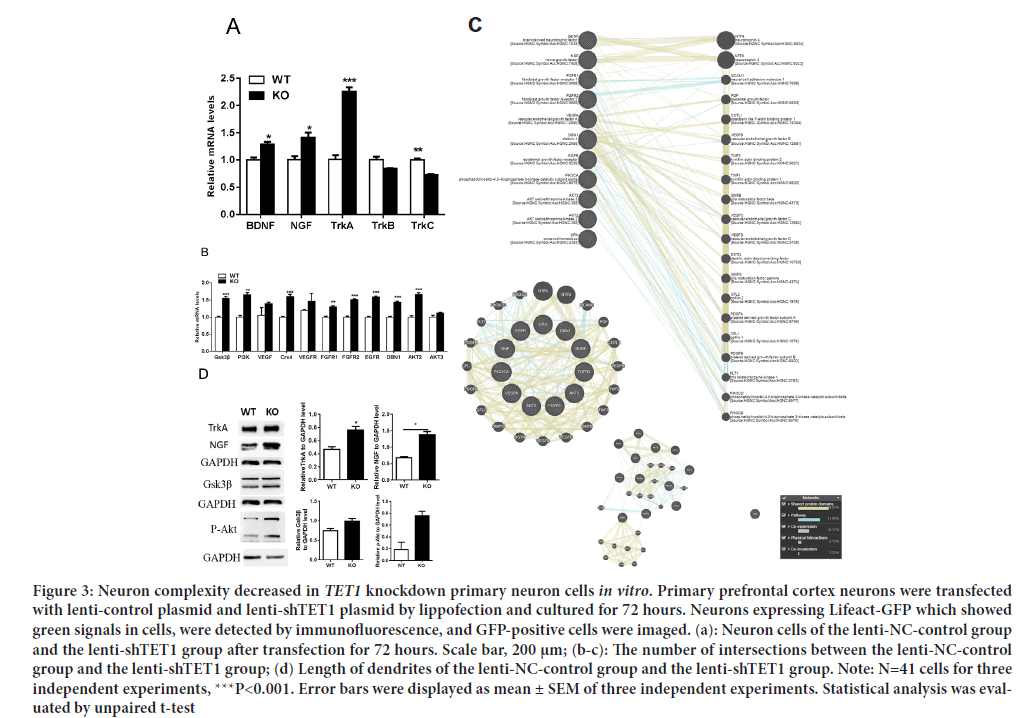
Figure 3: The expression level of neuron growth genes was increased significantly (a): qRT-PCR of BDNF, NGF, TRKA, TRKB and TRKC in 8-W adult Wild-Type (WT) and TET1 Knockout (KO) mPFC; (b): qRT-PCR of the neuron growth genes GSK3β, PI3K, CRX4, FGFR1, FGFR2, EGFR, DBN1, AKT2and AKT3 in 8-W adult Wild-Type (WT) and TET1 Knockout (KO) mPFC; (c): Gene correlation analysis by GeneMANIA; (d): Western blotting of NGF, TRKA, GSK3β, and p-Akt in 8-W adult Wild-Type (WT) and TET1 Knockout (KO) mPFCs. Note: Error bars are displayed as mean ± SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; Statistical analysis was evaluated by unpaired t-test/one-way/two-way ANOVA and Tukey’s multiple comparison test.
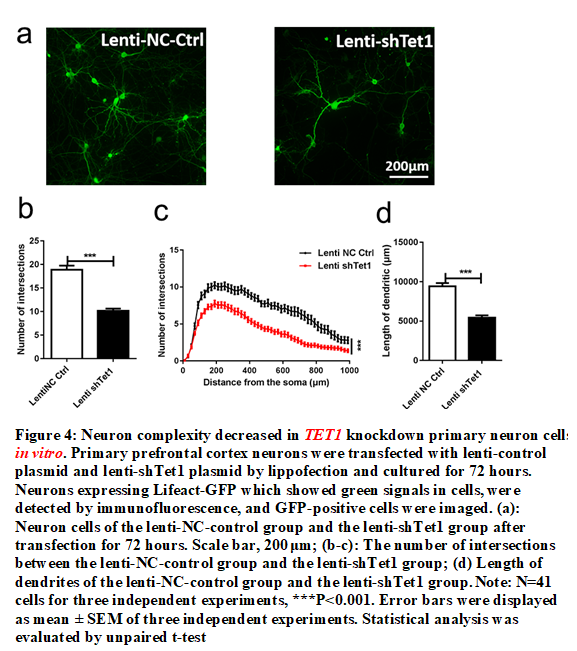
Figure 4: Neuron complexity decreased in TET1 knockdown primary neuron cells in vitro. Primary prefrontal cortex neurons were transfected with lenti-control plasmid and lenti-shTet1 plasmid by lippofection and cultured for 72 hours. Neurons expressing Lifeact-GFP which showed green signals in cells, were detected by immunofluorescence, and GFP-positive cells were imaged. (a): Neuron cells of the lenti-NC-control group and the lenti-shTet1 group after transfection for 72 hours. Scale bar, 200 μm; (b-c): The number of intersections between the lenti-NC-control group and the lenti-shTet1 group; (d) Length of dendrites of the lenti-NC-control group and the lenti-shTet1 group. Note: N=41 cells for three independent experiments, ***P<0.001. Error bars were displayed as mean ± SEM of three independent experiments. Statistical analysis was evaluated by unpaired t-test.
TET1 ablation does not affect anxiety-like behaviors, but it reduces the total distances traveled. To evaluate the effects of TET1 ablation on cortex flexibility and emotional behaviors of adult mice, an open field test was conducted using 8-week-old TET1 KO and WT male littermate mice. None of the animals used in the tests had any overt anatomical or developmental abnormalities. No significant difference existed between the two groups in distance for the center/total distance. TET1 KO mice spent more time entering the central zone than WT mice, showing that anxiety-like behaviors were reduced (P>0.05). However, the TET1 KO mice showed a lower total distance traveled and more central zone entries than the WT mice (P<0.05) (Figure 2a). TET1 KO mice showed reduced ambulatory ability to WT mice.
TET1 KO mice present social deficits and social processing impairment. DNA (de)methylation in the cortex is essential for cortex flexibility and emotion (Ferreira TA, et al., 2014). To evaluate social interactions, we conducted a three-chamber social interaction experiment. Time spent in close interaction with an object (O) versus stranger mouse (S1) in the phase II social interaction test. Time spent in close interaction with a familiar mouse (S1) versus the second stranger mouse (S2) in the phase III social interaction test. TET1 KO mice showed no difference in close interaction time between stranger mice (S1) and stranger mice (S2). TET1 KO mice spend more time with object compared with WT littermates in terms of sociability (P<0.01) (Figure 2b). However, a significant difference existed in social novelty between the two groups (P<0.05) (Figure 2b). Preference for social novelty refers to the significant propensity that a mouse spends more time in contact with the second stranger (Stranger 2) or the first stranger (Stranger 1) (Moy SS, et al., 2004). Usually, normal mice spend more time in contact with Stranger 2. However, mice with social novelty dysfunction spend more time choosing Stranger 1. In our research TET1 KO group mice showed no difference in interest between Stranger 1 and Stranger 2. However, WT group mice showed a higher interest in Stranger 2 than Stranger 1. These findings indicated that deletion of TET1 impairs social cognition and social memory. Furthermore, social cognition and social memory play an important role in interpreting social signals, which can influence social behavior and identification of other mice met before. Briefly, TET1 KO mice did not prefer social novelty between Stranger 1 and Stranger 2 nor could they distinguish strangers they had never met previously.
Social dominance of mice depends on their experiences of winning in social contests, and its effects are mediated by neuronal projections from the thalamus to the dorsomedial prefrontal cortex (Warde-Farley D, et al., 2010). Social dominance is associated with aggressive behaviors and the attainment of resources, such as food, mates, and territory (Bicks LK, et al., 2015). Additionally, social hierarchies have important consequences for adaptation to living. This study employed the tube test to assess social dominance to check Prefrontal Cortex function. A standard tube test was conducted between TET1 KO and WT mice (Figure 2c). Previous research found that if two stranger mice met in the middle of a test tube, the dominant mouse would move forward and would force the other mouse to leave the test tube (Sui L, et al., 2012). TET1 KO mice had a lower probability of winning the competition with WT mice (P<0.001) (Figure 2c). Winning percentage in test pairs between indicated genotypes, 11/15 (73%) of TET1 Wild-Type (WT) and Knockout (KO), Students t-test, ***P<0.001, indicates that the results were significantly different from an expected chance (50:50 win-lose outcome). There was no difference in body weight between the two groups.
PPI is an experiment that can test the function of sensory-motor gating. Our research showed that the PPI of TET1 KO mice was higher than that of WT mice under 85 dB auditory stimulation (P<0.01) (Figure 2d), indicating that the sensory gating function of TET1 KO mice was impaired. Additionally, PPI increased slightly with 75 dB auditory stimulus and 80 dB auditory stimulus but the increase was not statistically significant.
TET1 is critical for neuronal growth to maintain normal NGF-TRKA signaling in the mPFC. To understand TET1 expression in the mPFC, we performed qRT-PCR and Western blotting tests of the mPFC in 8-week- old TET1 KO and WT mice. We found that TET1 KO upregulated BDNF, NGF, and TRKA but downregulated TRKC (Figure 3a). The NGF-TRKA signaling pathway is essential in neuron growth. We then performed qRT- PCR on genes related to neuron growth. The GSK3β, PI3K, CRX4, FGFR1, FGFR2, EGFR, DBN1 and AKT2 genes were significantly upregulated, while the VEGF, VEGFR, and AKT3 genes were not significantly increased (Figure 3b). We conducted tests of the gene-gene interaction network for these genes using GeneMANIA and found that these genes influenced neuron growth (Figure 3c). We observed strong genetic correlations for neuron growth between these genes. NGF and BDNF act as important functions in regulating the survival, growth, development, and differentiation of neuronal cells in the central and peripheral nervous systems (Lu AT, et al., 2004). Next, we performed Western blot analysis and found that NGF levels, NGF receptor (TRKA), and downregulation signals (GSK3β and p-Akt) were significantly increased in the mPFC of KO mice (Figure 3d).
Because the NGF-TRKA signaling pathway is essential for regulating neuron growth, we subsequently analyzed the growth and complexity of neurons in E13.5 mice. PFC neurons were detached and cultured in vitro. We used lenti-shTET1 RNA to Knockdown TET1 to understand the growth and complexity of PFC neurons. The results of this study indicated that the length of dendrites and axons of TET1 Knockdown neurons was reduced and that the complexity became simplistic (Figures 4a-4d). These results suggested that TET1 Knockdown influenced the growth and complexity of neurons.
Discussion
The links between TETs, learning, and memory, as well as neurodevelopment and neurogenesis, have been well explored (Dinçel N, et al., 2013; Bryn V, et al., 2015; Sun Z, et al., 2019; Kim H, et al., 2016). Epigenetic changes in neuronal development are obvious, but the effects of TET enzymes on social behaviors and information processing remain vague. Our research found that deletion of the TET1 gene can impair social behaviors, including social novelty, social dominance, and information processing by activating NGF/TRKA signaling pathways in the Prefrontal Cortex. Additionally, the Knockdown of the TET1 gene impairs neuron cell complexity in vitro and in vivo in the Prefrontal Cortex. Other research findings have shown that the mPFC is a primary site of the pathophysiology of depression, Posttraumatic Stress Disorders (PTSDs), schizophrenia, anxiety, and attention-deficit hyperactivity disorder (Zhu X, et al., 2016). Social stress is related to psychiatric illness that is characterized by impaired cognitive functions mediated by the medial Prefrontal Cortex (mPFC) (Zhang RR, et al., 2013). Recent studies also discovered that children with autism have elevated levels of BDNF and NGF (Lu AT, et al., 2004; Zhou T, et al., 2017; Wang F, et al., 2011). Moreover, these research examined socially related genes, including NGF (Veeraiah P, et al., 2014), BDNF (Urban KR and Valentino RJ, 2017), FGFR (Sloan EK, et al., 2008; Li A, et al., 2021), VEGF (Mierzwa AJ, et al., 2013), and EGFR (Rudenko O, et al., 2010) in the mPFC to determine mPFC function. The results showed that NGF and downstream signaling pathway genes were abnormally upregulated in the mPFC. In particular, BDNF, NGF, CRX4, FGFR1, FGFR2, VEGF, DBN1, and EGFR are abnormally expressed and are related to the growth, survival, and differentiation of neuronal cells (Nowacka MM, et al., 2015). Cholinergic neurons in the basal forebrain secrete NGF to support neuron growth and development (Futamura T, et al., 2003). The signaling system regulates feeding and social behaviors (Song M, et al., 2017). These results suggested that the deletion of the TET1 gene influences mPFC neuronal growth, thereby affecting social behaviors and PPI, which may have a connection with schizophrenia (Kraeuter AK, et al., 2020; Sampedro-Viana D, et al., 2021).
Previous studies focused on TET1 controlling CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, memory formation, and memory extinction (Isaev NK, et al., 2017; Liu Y, et al., 2011). TET1 regulates hippocampal neurogenesis, cognition, and development in the brain (Kaas GA, et al., 2013; Rudenko A, et al., 2013). However, we found that TET1 regulates neuronal cell growth by knocking down the TET1 gene in primary neuronal cells
In summary, our research contributes to the literature by identifying that the deletion of TET1 disrupts PFC function, resulting in impaired social behaviors and neuronal cell growth and complexity. We proposed that TET1 influences social interaction and information processing by disrupting neuronal growth factors and socially related genes. It is very important to understand the pathogenesis of social behavior defects in mental illness. Future research may explore other mouse models to study psychiatric diseases.
Conclusion
This study found the effects of TET1 gene deletion on mouse social behavior and information processing in the mPFC. This study first proposed that the TET1 gene is related to social behavior, information processing with mPFC neuron growth, and dysfunction. The findings underscore that the TET1 gene is a special factor that affects social behavior by influencing the expression of NGF and BDNF in the mPFC.
Limitations of the Study
Although we found that deletion of the TET1 gene in mice neurons affected the neuron growth, information processing and social behavior. Our study contains some limitations. First, although this study involved experiments on mice, PFC samples from psychiatric disease patients were not obtained to verify the experimental results. Thus, our research is not sufficient to establish a definitive mechanism about TET1 and social behaviors. Future research should obtain data from encephalon organoids of psychiatric disease patients for further investigation. Second, molecular players can be potential therapeutic targets to treat psychiatric disease patients. However, side effects or syndromes can occur during the treatment, which indicates that further exploration is needed.
Declarations
Ethics approval and consent to participate
All animal procedures for behavioral tests and neuronal cell cultures were conducted in accordance with Zhejiang University. The study was approved by ethics review committee of experimental animal welfare of Zhejiang University (ZJU20170949). The study was performed in accordance with ARRIVE guidelines.
Availability of data and materials
The datasets and materials used and/or analyzed during the research are available from the corresponding author (Yanhua Bi) upon responsible request.
Competing interests
The authors declare that they have no competing interests.
Funding
Our work was funded by Zhejiang basic public welfare research project, LGD19H090007.
Acknowledgment
We would like to thank Professor Xuekun Li for kindly providing the TET1 Knockout mice, Wild-Type mice and technical guidance and support.
Authors’ contributions
Yanhua Bi conceived and designed the project. Yanhua Bi and Hui Gao performed the experiments. Yanhua Bi wrote the manuscript. Yahua Bi revised the manuscript. All authors have read and approved of the final manuscript.
References
- Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, et al. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron. 2013; 79(6): 1086-1093.
[Crossref] [Google Scholar] [Pubmed]
- Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 2010; 38(19): e181.
[Crossref] [Google Scholar] [Pubmed]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009; 324(5929): 930-935.
[Crossref] [Google Scholar] [Pubmed]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety-and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39(1): 112-119.
[Crossref] [Google Scholar] [Pubmed]
- Antunes C, Sousa N, Pinto L, Marques CJ. TET enzymes in neurophysiology and brain function. Neurosci Biobehav Rev. 2019; 102: 337-344.
[Crossref] [Google Scholar] [Pubmed]
- Feng J, Pena CJ, Purushothaman I, Engmann O, Walker D, Brown AN, et al. TET1 in nucleus accumbens opposes depression-and anxiety-like behaviors. Neuropsychopharmacology. 2017; 42(8): 1657-1669.
[Crossref] [Google Scholar] [Pubmed]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic‐like behavior in mice. Genes Brain Behav. 2004; 3(5): 287-302.
[Crossref] [Google Scholar] [Pubmed]
- Karen C, Rajan KE. Social behaviour and epigenetic status in adolescent and adult rats: The contribution of early-life stressful social experience. Cell Mol Neurobiol. 2019; 39: 371-385.
[Crossref] [Google Scholar] [Pubmed]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012; 16(4): 231-239.
[Crossref] [Google Scholar] [Pubmed]
- Charernboon T, Patumanond J. Social cognition in schizophrenia. Mental Illn. 2017; 9(1): 7054.
[Crossref] [Google Scholar] [Pubmed]
- Adolphs R. The social brain: Neural basis of social knowledge. Annu Rev Psychol. 2009; 60: 693-716.
[Crossref] [Google Scholar] [Pubmed]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013; 501(7466): 179-184.
[Crossref] [Google Scholar] [Pubmed]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017; 20(3): 449-458.
[Crossref] [Google Scholar] [Pubmed]
- Molas S, Zhao-Shea R, Liu L, de Groot SR, Gardner PD, Tapper AR. A circuit-based mechanism underlying familiarity signaling and the preference for novelty. Nat Neurosci. 2017; 20(9): 1260-1268.
[Crossref] [Google Scholar] [Pubmed]
- Franklin TB, Silva BA, Perova Z, Marrone L, Masferrer ME, Zhan Y, et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat Neurosci. 2017; 20(2): 260-270.
[Crossref] [Google Scholar] [Pubmed]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016; 353(6307): 1536-1541.
[Crossref] [Google Scholar] [Pubmed]
- Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015; 6: 1805.
[Crossref] [Google Scholar] [Pubmed]
- Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, et al. Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep. 2015; 10(2): 292-305.
[Crossref] [Google Scholar] [Pubmed]
- Zhong S, Zhang S, Fan X, Wu Q, Yan L, Dong J, et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature. 2018; 555(7697): 524-528.
[Crossref] [Google Scholar] [Pubmed]
- Finn ES, Huber L, Jangraw DC, Molfese PJ, Bandettini PA. Layer-dependent activity in human prefrontal cortex during working memory. Nat Neurosci. 2019; 22(10): 1687-1695.
[Crossref] [Google Scholar] [Pubmed]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011; 477(7363): 171-178.
[Crossref] [Google Scholar] [Pubmed]
- Moss H, Damasio AR. Emotion, cognition, and the human brain. Ann N Y Acad Sci. 2001; 935: 98-100.
[Crossref] [Google Scholar] [Pubmed]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003; 969(1-2): 183-194.
[Crossref] [Google Scholar] [Pubmed]
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: Confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996; 351(1346): 1495-1503.
[Crossref] [Google Scholar] [Pubmed]
- Frith C. The role of the prefrontal cortex in self-consciousness: The case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 1996; 351(1346): 1505-1512.
[Crossref] [Google Scholar] [Pubmed]
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer's disease, Huntington's disease and schizophrenia. Brain Res. 1995; 699(2): 297-304.
[Crossref] [Google Scholar] [Pubmed]
- Müller U, Wächter T, Barthel H, Reuter M, von Cramon DY. Striatal [123 I] β-CIT SPECT and prefrontal cognitive functions in Parkinson's disease. J Neural Transm. 2000; 107: 303-319.
[Crossref] [Google Scholar] [Pubmed]
- Dong E, Gavin DP, Chen Y, Davis J. Upregulation of TET1 and downregulation of APOBEC3A and APOBEC3C in the parietal cortex of psychotic patients. Transl Psychiatry. 2012; 2(9): e159.
[Crossref] [Google Scholar] [Pubmed]
- Towers AJ, Tremblay MW, Chung L, Li XL, Bey AL, Zhang W, et al. Epigenetic dysregulation of Oxtr in TET1-deficient mice has implications for neuropsychiatric disorders. JCI Insight. 2018; 3(23): e120592.
[Crossref] [Google Scholar] [Pubmed]
- Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, et al. TET1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 2013; 13(2): 237-245.
[Crossref] [Google Scholar] [Pubmed]
- Song TT, Bi YH, Gao YQ, Huang R, Hao K, Xu G, et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J Neuroinflammation. 2016; 13: 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012; 17(5): 537-548.
[Crossref] [Google Scholar] [Pubmed]
- Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019; 1916: 99-103.
[Crossref] [Google Scholar] [Pubmed]
- Park MJ, Seo BA, Lee B, Shin HS, Kang MG. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci Rep. 2018; 8(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology. 2001; 156: 194-215. [Crossref]
[Google Scholar] [Pubmed]
- Karacay B, Mahoney J, Plume J, Bonthius DJ. Genetic absence of nNOS worsens fetal alcohol effects in mice II: Microencephaly and neuronal losses. Alcohol Clin Exp Res. 2015; 39(2): 221-231.
[Crossref] [Google Scholar] [Pubmed]
- He W, Tian X, Yuan B, Chu B, Gao F, Wang H. Rosuvastatin improves neurite extension in cortical neurons through the Notch 1/BDNF pathway. Neurol Res. 2019; 41(7): 658-664.
[Crossref] [Google Scholar] [Pubmed]
- Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, et al. Neuronal morphometry directly from bitmap images. Nat Methods. 2014; 11(10): 982-984.
[Crossref] [Google Scholar] [Pubmed]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010; 38(2): W214-W220.
[Crossref] [Google Scholar] [Pubmed]
- Sui L, Wang Y, Ju LH, Chen M. Epigenetic regulation of reelin and brain-derived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex. Neurobiol Learn Mem. 2012; 97(4): 425-440.
[Crossref] [Google Scholar] [Pubmed]
- Lu AT, Yoon J, Geschwind DH, Cantor RM. QTL replication and targeted association highlight the nerve growth factor gene for nonverbal communication deficits in autism spectrum disorders. Mol Psychiatry. 2013; 18(2): 226-235.
[Crossref] [Google Scholar] [Pubmed]
- Dinçel N, Ünalp A, Kutlu A, Öztürk A, Uran N, Ulusoy S. Serum nerve growth factor levels in autistic children in Turkish population: A preliminary study. Indian J Med Res. 2013; 138(6): 900-903.
[Google Scholar] [Pubmed]
- Bryn V, Halvorsen B, Ueland T, Isaksen J, Kolkova K, Ravn K, et al. Brain Derived Neurotrophic Factor (BDNF) and Autism Spectrum Disorders (ASD) in childhood. Eur J Paediatr Neurol. 2015; 19(4): 411-414.
[Crossref] [Google Scholar] [Pubmed]
- Sun Z, Xu X, He J, Murray A, Sun MA, Wei X, et al. EGR1 recruits TET1 to shape the brain methylome during development and upon neuronal activity. Nat Commun. 2019; 10(1): 3892.
[Crossref] [Google Scholar] [Pubmed]
- Kim H, Jang WY, Kang MC, Jeong J, Choi M, Sung Y, et al. TET1 contributes to neurogenesis onset time during fetal brain development in mice. Biochem Biophys Res Commun. 2016; 471(4): 437-443.
[Crossref] [Google Scholar] [Pubmed]
- Zhu X, Girardo D, Govek EE, John K, Mellén M, Tamayo P, et al. Role of TET1/3 genes and chromatin remodeling genes in cerebellar circuit formation. Neuron. 2016; 89(1): 100-112.
[Crossref] [Google Scholar] [Pubmed]
- Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 2017; 357(6347): 162-168.
[Crossref] [Google Scholar] [Pubmed]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011; 334(6056): 693-697.
[Crossref] [Google Scholar] [Pubmed]
- Veeraiah P, Noronha JM, Maitra S, Bagga P, Khandelwal N, Chakravarty S, et al. Dysfunctional glutamatergic and γ-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry. 2014; 76(3): 231-238.
[Crossref] [Google Scholar] [Pubmed]
- Urban KR, Valentino RJ. Age-and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cereb Cortex. 2017; 27(1): 244-253.
[Crossref] [Google Scholar] [Pubmed]
- Sloan EK, Capitanio JP, Tarara RP, Cole SW. Social temperament and lymph node innervation. Brain Behav Immun. 2008; 22(5): 717-726.
[Crossref] [Google Scholar] [Pubmed]
- Li A, Jing D, Dellarco DV, Hall BS, Yang R, Heilberg RT, et al. Role of BDNF in the development of an OFC-amygdala circuit regulating sociability in mouse and human. Mol Psychiatry. 2021; 26(3): 955-973.
[Crossref] [Google Scholar] [Pubmed]
- Mierzwa AJ, Zhou YX, Hibbits N, Vana AC, Armstrong RC. FGF2 and FGFR1 signaling regulate functional recovery following cuprizone demyelination. Neurosci Lett. 2013; 548: 280-285.
[Crossref] [Google Scholar] [Pubmed]
- Rudenko O, Tkach V, Berezin V, Bock E. Effects of FGF receptor peptide agonists on animal behavior under normal and pathological conditions. Neurosci Res. 2010; 68(1): 35-43.
[Crossref] [Google Scholar] [Pubmed]
- Nowacka MM, Paul-Samojedny M, Bielecka AM, Plewka D, Czekaj P, Obuchowicz E. LPS reduces BDNF and VEGF expression in the structures of the HPA axis of chronic social stressed female rats. Neuropeptides. 2015; 54: 17-27.
[Crossref] [Google Scholar] [Pubmed]
- Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, Nawa H. Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: Implication for epidermal growth factor in cognitive development. Mol Psychiatry. 2003; 8(1): 19-29.
[Crossref] [Google Scholar] [Pubmed]
- Song M, Martinowich K, Lee F. BDNF at the synapse: Why location matters. Mol Psychiatry. 2017; 22(10): 1370-1375.
[Crossref] [Google Scholar] [Pubmed]
- Kraeuter AK, Mashavave T, Suvarna A, van den Buuse M, Sarnyai Z. Effects of beta-hydroxybutyrate administration on MK-801-induced schizophrenia-like behaviour in mice. Psychopharmacology. 2020; 237: 1397-1405.
[Crossref] [Google Scholar] [Pubmed]
- Sampedro-Viana D, Cañete T, Sanna F, Soley B, Giorgi O, Corda MG, et al.. Decreased social interaction in the RHA rat model of schizophrenia-relevant features: Modulation by neonatal handling. Behav Processes. 2021; 188: 104397.
[Crossref] [Google Scholar] [Pubmed]
- Isaev NK, Stelmashook EV, Genrikhs EE. Role of nerve growth factor in plasticity of forebrain cholinergic neurons. Biochemistry. 2017; 82: 291-300.
[Crossref] [Google Scholar] [Pubmed]
- Liu Y, Jiang YA, Si Y, Kim JY, Chen ZF, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011; 472(7341): 95-99.
[Crossref] [Google Scholar] [Pubmed]
- Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, et al. TET1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013; 79(6): 1109-1122.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Yanhua Bi1*, Hui Gao2 and Yahua Bi32Department of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China
3Department of Tourism and Convention, Pusan National University, Busan, South Korea
Citation: Bi Y: Deletion of TET1 in Mice with Impaired Prefrontal Cortex Functions by Activating the NGF/TRKA Signaling Pathway
Received: 03-Apr-2023 Accepted: 26-Apr-2023 Published: 03-May-2023, DOI: 10.31858/0975-8453.14.5.315-324
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






