Research Article - (2021) Volume 12, Issue 12
Design, Synthesis and Pharmacological Screening of Novel Flavone Derivatives
Sabale Prafulla*, Potey Lata, Sayyad Nusrat and Rahangdale PriyaAbstract
Flavonoids are the natural phytoconstituents widely distributed in plants originate in fruits, vegetables, grains, bark, roots, stems, flowers, tea and wine. Flavonoids have been recognized as secondary metabolites of plant, with marked biological significance such as Anti-inflammatory, Antioxidant, Anticancer and Antimicrobial activity. Hence, flavonoids are considered as an indispensable component in a variety of nutraceutical, pharmaceutical, medicinal and cosmetic applications with versatile health benefits.
Research on flavonoids received an added impulse with the discovery on Anti-inflammatory by several mechanisms, but the most important mechanism is the inhibition of eicosanoids generating enzyme. In present research a novel series of synthetic flavones (1a-l) have been synthesized after Molecular Docking studies. Synthesis of novel flavones was carried out using 2, 4-dihydroxyacetophenone as starting material through Baker-Venkataraman Reaction and then finally condensed with substituted acyl chloride. All the synthesized compounds was confirmed by their physicochemical properties and spectral studies. Novel flavone derivatives were assessed for anti-inflammatory activity by using Carrageenan induced rat paw edema method. Novel flavone derivatives were performed to establish correlation between biological activity and molecular properties. Among the synthesized compounds (1b, 1g, 1i, 1j 1l) showed good anti-inflammatory activity, whereas compounds (1c, 1e, 1f, 1h, 1k) showed moderate anti-inflammatory activity comparable to the reference drug Indomethacin. Thus, the conclusion can be made that the flavone moiety may exhibit a good anti-inflammatory activity.
Keywords
Flavonoid, Synthetic flavones, Baker-Venkataraman Reaction, Anti-inflammatory activity, Molecular docking
Introduction
Medicinal chemistry concerns with the discovery, development, identification and interpretation of the mode of action of biologically active compounds at the molecular level. It is also concerned with the study, identification, and synthesis of the metabolic products of drugs and related compounds (Narayana KR, et al., 2001). Flavonoids (from the Latin word flavus meaning yellow colour in nature) are a group of polyphenolic compounds having benzopyrone heterocyclic ring system, with a group of low molecular weight compounds (Singh M, et al., 2014). It is a class of plant secondary metabolite. Flavonoids were referred to as Vitamin P from the mid-1930s to early 50s (Yao LH, et al., 2004). Flavone is a class of flavonoids based on the backbone of 2-phenyl-4H-chromen-4-one (2-phenyl-1-benzopyran-4-one) (Verma AK and Pratap R, 2012). The molecular formula of flavone molecule is C15H10O2. It has a three-ring skeletons, C6-C3-C6, and the rings are referred to as A, C, and B rings, respectively (Harborne JB and Williams CA, 2000). They are found in seeds, citrus fruits, olive oil, tea and red wine, vegetables, nuts, stems and flowers, honey and are commonly consumed with the human diet (Chacko BK, et al., 2005).
Synthesis of novel flavones are carried out using 2, 4-dihydroxyacetophenone as starting material through Baker-Venkataraman Reaction and then finally condensed with substituted acyl chloride. All the synthesized compounds was confirmed by their physicochemical properties and spectral studies (Cushnie TT and Lamb AJ, 2011). Flavonoids exhibit a broad spectrum of biological activities, such as antibacterial, antiviral, antioxidant, anti-inflammatory, antiallergic, antitumor, antihypertensive, antidiabetic activies (Agrawal AD, 2011). Novel flavone derivatives were assessed for anti-inflammatory activity by using carrageenan induced rat paw edema method. The molecular docking tool, V-Life MDS (Molecular Design Suit) 4.3 software was used for ligand docking studies into the COX-2 enzyme, with PDB (Protein Data Bank) code (3LN1) (Lemmen C and Lengauer T, 2000).
Materials and Methods
Melting points was determined using a Veego make microprocessor based melting point apparatus having silicone oil bath and are uncorrected. IR spectra (wave numbers in cm−1) were recorded on a Bruker Alpha FT-IR (Fourier Transform Infrared Spectroscopy) spectrophotometer using Potassium bromide discs. NMR (Nuclear Magnetic Resonance) spectra were recorded on Bruk Bruker Avance II 400 MHz instrument in CDCl3 with TMS (Tetramethylsilane) as internal standard for 1H NMR. Chemical shift values are mentioned in δ, ppm. Mass spectra were recorded on Advion Expression, CMS (Compact Mass Spectrometer), USA at SynZeal Research Solutions, Gandhinagar. Chromatographic separations were performed on columns using silica gel 100-200 mesh. The progress of all reactions was monitored by TLC on 2 cm × 5 cm pre-coated silica gel 60 F254 (Merck) plates of thickness ness of 0.25 mm. The chromatograms were visualized under UV 254 nm and/or exposure to iodine vapours. All reagents used were of analytical grade, obtained from Loba chemicals, SD fine chemical limited and Spectrochem. Chemicals and solvents were purified by general laboratory techniques before use. All moisture free operations were performed in oven dried glasswares and under nitrogen atmosphere.
Experimental Work
Molecular docking
Molecular docking studies and conformational analysis of all compound were performed using the molecular design suite (V-Life MDS software package, version 4.3; from V-Life Sciences, Pune, India). Structures of compounds were sketched using the 2D structure draw application, (V-life 2D draw) and converted into 3D structures. Structures of compounds were minimized and optimized with the MMFF (Merck Molecular Force Field) method taking the Root Mean Square gradient (RMS) of 0.01 kcal/mol A° and the iteration limit to 10,000. Conformers for each structure were generated using Monte Carlo by applying MM force field method and least energy conformer was selected for further study. The molecules chosen from docking studies were used for synthesis purpose. Eleven compounds (1a- 1l) showed significant dock score. Structures of the 2-phenyl-1-benzopyran-4-one ligands or ester derivatives of 7-hydroxy 2-phenyl-1-benzopyran-4-one ligands were sketch using chem sketch 2D draw and converted into 3D structure taken in .mol format. The 3D structure in .mol format are then optimized and saved in .mol2 format. MMFF force field with default settings were used for the ligand optimization. The PDB structure [3LN1] were downloaded from protein data bank. All the bound water molecules, ligands and other moieties were removed from the proteins and saved in PDB format. Cavity #1 selected for COX-2 receptor.
Chemistry
Synthesis 7-hydroxy-2-phenyl-4H-chromen-4-one: 2,4-dihydroxyacetophenone was allowed to react with benzoyl chloride in the presence of anhydrous pyridine, and subsequent heating the mixture in the presence of KOH yielded 2,4-dihydroxydibenzoylmethane. 2, 4-dihydroxydibenzoylmethanewas further cyclized in the presence of glacial acetic acid and concentrated H2SO4 at 100°C to afford the flavone. Synthesized flavone was recrystallized from large volume of petroleum ether (60°C-80°C) (Kabalka GW and Mereddy AR, 2005).
Synthesis of substituted benzoyl chloride: The various substituted benzoic acids were heated with excess of thionyl chloride for two hours in the presence of dry Dimethylformamide on a water bath and then the excess of thionyl chloride was distilled off. Formation of acyl chloride was monitored by converting into esters with methanol using TLC (Thin Layer Chropmatography) technique. This prepared acyl chlorides were used as such without further purification for preparation of ester derivatives of flavone (Maitera ON, et al., 2018).
Reaction of acyl chloride with flavone: The various substituted acyl chloride were condensed with 7-hydroxyflavone in basic media to afford various substituted flavone derivatives. Flavone (0.5 g) and a freshly prepared substituted benzoyl chloride was dissolved in dry pyridine (2.5 ml) with stirring. Reaction mixture was heated on water bath at 50°C in dry condition using calcium chloride guard tube for more than 22 hrs. The progress of reaction was monitored by TLC. After completion of reaction, reaction mixture was cooled to room temperature and poured into ice crushed water (50 ml). The crude product precipated out was obtained by filtration, dried at room temperature. Various substituted Benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl ester (1a-l) was recrystallized from ethanol, Where, R shown in Table 1 and Figure 1 (Vh ES, et al., 2012).
| Compounds | Radical (R) |
|---|---|
| 1a | H |
| 1b | p-chloro |
| 1c | m-chloro |
| 1d | m-amino |
| 1e | p-nitro |
| 1f | m-nitro |
| 1g | m-hydroxy |
| 1h | p-hydroxy |
| 1i | 3,5-dinitro |
| 1j | 2-chloro, 5-nitro |
| 1k | p-bromomethyl |
| 1l | p-amino |
Table 1: Compounds and their radicals
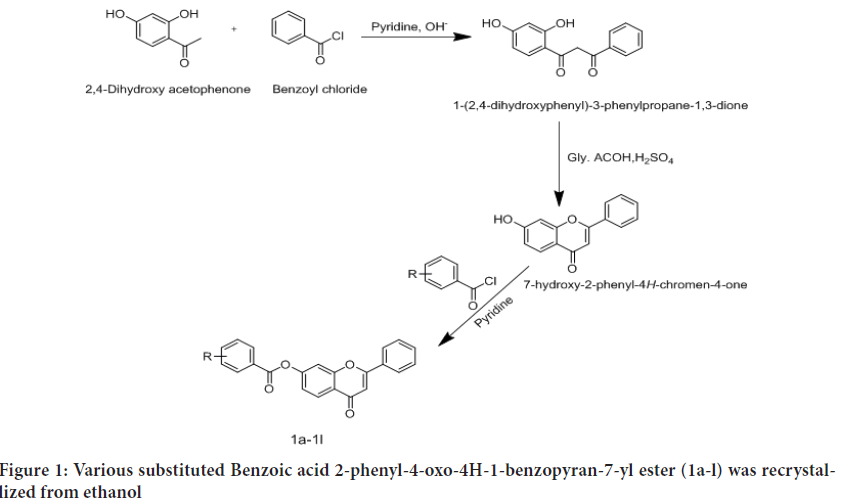
Figure 1: Various substituted Benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl ester (1a-l) was recrystallized from ethanol
Biological activity
Anti-inflammatory activity of all synthesized compounds were quantified, in vivo by Carrageenan induced rat paw edema method using Plethysmometer. All the test compounds were suspended in 0.5% of CMC and administered orally. The albino sprague dowly rats were treated orally with the newly synthesized derivatives (10 mg/kg) and standard drug Indomethacin (10 mg/kg), 1 hr prior to the 1% (w/v) solution injection of 0.1 ml Carrageenan into plantar region of right hind paw (subcutaneously). The relative paw volume was measured at an interval of 0 h, 1 h, 2 h, and 3 h in the individual animal of the control, test and the standard group (Bano S, et al., 2013; Do TH, et al., 2009; Gomes A, et al., 2012; García-Lafuente A, et al., 2009). The percent inhibition of paw edema was calculated using following formula,
%Inhibition (%I)=[1-(Vt/Vc)] × 100
Where,
Vt=Mean increase in paw volume of test.
Vc=Mean increase in paw volume of control.
Results and Discussion
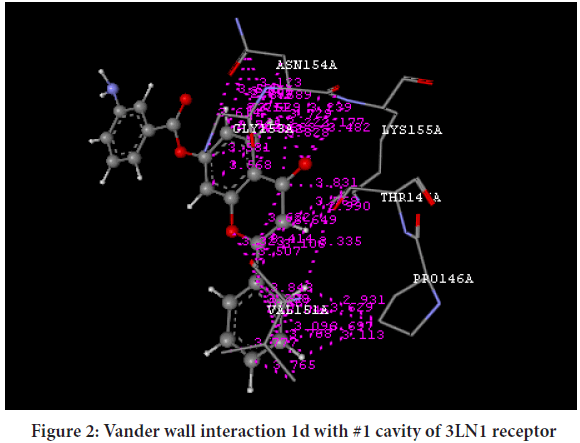
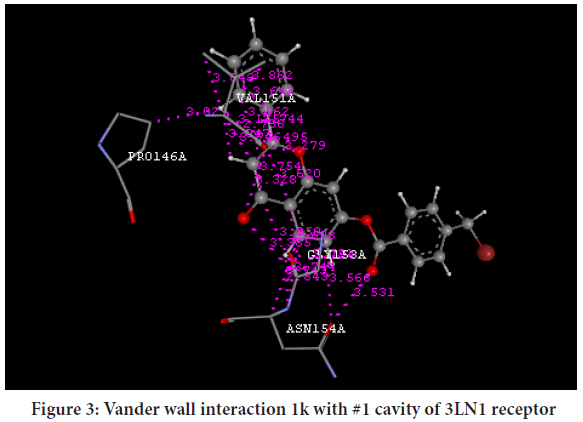
Molecular docking was performed by V-Life MDS 4.3 software was used for ligand docking studies into the COX-2 enzyme, with PDB code (3LN1). Standard drug used for docking is selective COX-2 inhibitor i.e. Celecoxib. The PDB used for docking was 3NL1 which is Celecoxib bound COX-2 receptor. The more negative value of Dock score indicated that the compound may be more potent and indicated the good binding potential, synthesized compounds were docked with 3LN1 (COX-2 enzyme). The docking score of Celecoxib and synthesized compounds were shown in Tables 2 and 3 Vander walls interactions of the Celecoxib and synthesized compound 1d, 1k at cavity 1 of COX-2 [3LN1] receptor is shown in Figures 2 and 3.
| SN | Conformer | R | Dock score |
|---|---|---|---|
| 1 | 1a | -H | -4.268206 |
| 2 | 1b | p-chloro | -4.724953 |
| 3 | 1c | m-chloro | -4.255926 |
| 4 | 1d | m-amino | -4.482858 |
| 5 | 1e | p-nitro | -4.762733 |
| 6 | 1f | m-nitro | -4.87621 |
| 7 | 1g | m-hydroxy | -4.435845 |
| 8 | 1h | p-hydroxy | -4.764583 |
| 9 | 1i | 3,5-dinitro | -3.854839 |
| 10 | 1j | 2-chloro,5-nitro | -4.134919 |
| 11 | 1k | p-bromo methyl | -3.958826 |
| 12 | 1l | p-fluoro | -4.630565 |
| 13 | Celecoxib | - | -4.585231 |
Table 2: Result of the docking study
Figure 2: Vander wall interaction 1d with #1 cavity of 3LN1 receptor
Figure 3: Vander wall interaction 1k with #1 cavity of 3LN1 receptor
| SN | Group | Dose (mg/kg) | % Inhibition of paw edema | |||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | |||
| 1. | Indomethacin | 10 | - | 33.34 | 32.15 | 50.91 |
| 2. | 1a | 10 | - | 15.79 | 17.86 | 16.37 |
| 3. | 1b | 10 | - | 36.85 | 39.29 | 41.82 |
| 4. | 1c | 10 | - | 28.08 | 26.79 | 38.19 |
| 5. | 1d | 10 | - | 17.55 | 17.86 | 18.19 |
| 6. | 1e | 10 | - | 35.09 | 35.72 | 36.37 |
| 7. | 1f | 10 | - | 24.57 | 25 | 27.28 |
| 8. | 1g | 10 | - | 42.11 | 46.43 | 45.46 |
| 9. | 1h | 10 | - | 24.57 | 25 | 25.46 |
| 10. | 1i | 10 | - | 40.36 | 46.43 | 43.64 |
| 11. | 1j | 10 | - | 36.85 | 46.43 | 63.64 |
| 12. | 1k | 10 | - | 19.3 | 25 | 27.28 |
| 13. | 1l | 10 | - | 24.57 | 32.15 | 45.46 |
Table 3: Result of anti-inflammatory activity by carrageenan induced rat paw edema method
On the basis of result of molecular docking compound 1b, 1e, 1f, 1h showed significant interaction with COX-2 receptor compared to Celecoxib which is a known selective COX-2 inhibitor, from the result it is found that electron accepting groups are more strongly interact with receptor active site. It means that binding affinity with receptor site increases with electronegativity. Top rank compounds were synthesized as per the designed scheme (Figure 1) which has been started from 2, 4-dihydroxyacetophenone and substituted acyl chloride condensed with 7-hydroxyflavone in basic media to afford various substituted flavone derivatives. Flavone (0.5 g) and a freshly prepared substituted benzoyl chloride was dissolved in dry pyridine (2.5 ml) with stirring. Reaction mixture was heated on water bath at 50°C in dry condition using calcium chloride guard tube for more than 22 hrs. The progress of reaction was monitored by TLC. After completion of reaction, reaction mixture was cooled to room temperature and poured into ice crushed water (50 ml). The crude product precipated out was obtained by filtration, dried d by physicochemical and spectral study and evaluated for anti-inflammatory activity by Carrageenan induced rat paw edema method by taking a standard drug Celecoxib.
Physicochemical and spectral characterization
• Benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1a) Yield (%): 74.85, MP (Melting Point) (°C): 120-128, Rf (Retention factor): 0.55 (Chloroform: Hexane, 8:2), λ max (nm): 227, IR (KBr, cm-1): 1621.83, 1451.44, 1289.70, 931.67, 705.71.
• 4-chloro benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1b) Yield (%): 71.5, MP (°C): 200-205, Rf: 0.77 (Chloroform: Hexane, 8:2), λ max (nm): 235, IR (KBr, cm-1):2914.95, 1738.49, 1678.85, 1589.68, 1127.37, 1089.73, 923.78, 758.75, 699.64.
• 3-chloro benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1c) Yield (%): 40.75, MP (°C): 130-138, Rf: 0.82 (Chloroform: Hexane, 8:2), λ max (nm): 230, IR (KBr, cm-1): 2931.03, 1692.60, 1551.63, 502.67, 1261.88, 1057.26, 932.87, 702.67, 663.93.
• 3-amino benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1d) Yield (%): 80.39, MP (°C): 81-89, Rf: 0.34 (Chloroform: Hexane, 8:2), λ max (nm): 251, IR (KBr, cm-1): 3067.87, 1600.85, 1566.12, 1492.47, 463.75, 1127.63, 849.34, 766.00, 699.80.
• 4-nitro benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1e) Yield (%): 51.62, MP (°C): 100-102, Rf: 0.77 (Chloroform: Hexane, 8:2), λ max (nm): 255, IR (KBr, cm-1): 2847.73, 1736.33, 1635.66, 1601.54, 493.56, 1227.83, 714.35, 697.11.
• 3-nitro benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1f) Yield (%): 89.25, MP (°C): 100-110, Rf: 0.8 (Chloroform: Hexane, 8:2), λ max (nm): 253, IR (KBr, cm-1): 3085.74, 2916.56, 1722.61, 1612.57, 523.73, 1233.49, 714.48, 680.55, NMR (δ, ppm) 6.7916 (s,1H;-CH), 7.729 (m,5H;-Ar-H), 8.0728 (s,1H;-Ar-H), 8.317 (d, 2H;-Ar-H), 8.53 (d,2H;- Ar-H), 8.61 (d,2H;-Ar-H).
• 3-hydroxy benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1g) Yield (%): 68.125, MP (°C): 80-89, Rf: 0.43 (Chloroform: Hexane, 8:2), λ max (nm): 250, IR (KBr, cm-1): 3600.12, 1734.13, 1640.26, 125.79, 846.59,750.76, 684.12., NMR (δ, ppm) 6.97 (s,1H;-OH), 7.45 (m,5H;- Ar-H), 7.44 (d, 2H;-Ar-H), 7.537 (d,2H;-Ar-H), 7.782 (s,1H;-Ar-H), 7.69 (s,1H;-CH), 8.03 (m,3H; -Ar-H).
• 4-hydroxy benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1h) Yield (%): 66.25, MP (°C): 85-90, Rf: 0.37 (Chloroform: Hexane, 8:2), λ max (nm): 250, IR (KBr, cm-1): 3630.07, 1697.86, 1553.57, 155.95, 807.32, 751.61, 592.87.
• 3, 5-dinitro benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1i) Yield (%): 64.55, MP (°C): 79-82, Rf: 0.41 (Chloroform: Hexane, 8:2), λ max (nm): 251, IR (KBr, cm-1): 3600.12, 3070.53, 1600.50, 564.04, 1539.21, 1489.32, 1371.12, 1126.15, 750.77, 667.84.
• 2-chloro, 5-nitro benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl- ester (1j) Yield (%): 75.66, MP (°C): 95-100, Rf: 0.41 (Chloroform: Hexane, 8:2), λ max (nm): 250, IR (KBr,cm-1): 1679.23, 1585.72, 1553.21, 507.87, 1268.98,1086.98, 755.01, 675.44.
• 4-bromomethyl benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl- ester (1k) Yield (%): 51.66, MP (°C): 90-98, Rf: 0.35 (Chloroform: Hexane, 8:2), λ max (nm): 250, IR (KBr, cm-1): 1691.83, 1506.25, 1425.70, 121.44, 807.49, 753.62,695.29.
• 4-amino benzoic acid 2-phenyl-4-oxo-4H-1-benzopyran-7-yl-ester (1l) Yield (%): 78, MP (°C): 150-155, Rf: 0.37 (Chloroform: Hexane, 8:2), λ max (nm): 257, IR (KBr, cm-1): 2915.22, 1624.28, 1569.37, 1465.89, 221.34,766.82, 723.14, 674.69.
The anti-inflammatory results revealed that, the compounds 1b, 1g, 1i, 1j and 1l exhibited good anti-inflammatory activity whereas compounds 1c, 1e, 1f, and 1h, 1k showed moderate activity and compounds 1a, 1d showed ow activity when compared with standard drug Indomethacin.
Conclusion
A series of substituted 2-phenyl-4-oxo-4H-1-benzopyran-7-yl ester (3a-l) were synthesized and the anti-inflammatory result revealed that, 1b, 1g, 1i, 1j and 1lexhibited good anti-inflammatory activity whereas compounds 1c, 1e, 1f, and 1h, 1k showed moderate activity when compared with standard drug Indomethacin. The electron withdrawing groups like Cl in 1b, 1c and NO2 in 1e, 1f substituted analogues at m and p position exhibited moderate activity while the o-Cl and m-NO2 substituted analogue 1j exhibited good activity.
This indicates that the compounds having electron withdrawing groups may enhanced anti-inflammatory activity and electron releasing groups diminished the activity. So, p-substituted derivatives favours good activity than m-substituted derivatives.
Acknowledgment
Authors gratefully acknowledge to University Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur for providing laboratory facilities. Authors are also thankful to V-Life Sciences, Pune, India for providing V-Life MDS Software package, version.
References
- Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol. 2001; 33(1): 2-16.
- Singh M, Kaur M, Silakari O. Flavones: An important scaffold for medicinal chemistry. Eur J Med Chem. 2014; 84: 206-239.
- Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004; 59(3): 113-122.
- Verma AK, Pratap R. Chemistry of biologically important flavones. Tetrahedron. 2012; 68(41): 8523-8538.
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000; 55(6): 481-504.
- Chacko BK, Chandler RT, Mundhekar A, Khoo N, Pruitt HM, Kucik DF, et al. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: Modulation of leukocyte-endothelial cell interactions. American J Physiol Heart Circ Physiol. 2005; 289(2): 908-915.
- Cushnie TT, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011; 38(2): 99-107.
- Agrawal AD. Pharmacological activities of flavonoids: A review. International journal of pharmaceutical sciences and nanotechnology. 2011; 4(2): 1394-1398.
- Lemmen C, Lengauer T. Computational methods for the structural alignment of molecules. J Comput Aided Mol Des. 2000; 14(3): 215-232.
- Kabalka GW, Mereddy AR. Microwave-assisted synthesis of functionalized flavones and chromones. Tetrahedron Let. 2005; 46(37): 6315-6317.
- Maitera ON, Louis H, Barminas JT, Akakuru OU, Boro G. Synthesis and characterization of some metal complexes using herbal flavonoids. Nat Prod Chem Res. 2018; 6(314): 10-4172.
- Vh ES, Matsjeh S, Wahyuningsih TD, Mustofa M, Redjeki T. Synthesis, characterization and antioxidant activity of 7-hydroxy-3', 4'-dimethoxyflavone. Indo J Chem. 2012; 12(2): 146-151.
- Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Chaitanya M, et al. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur J Med Chem. 2013; 65: 51-59.
- Do TH, Vo PN, Tran TD. Synthesis and comparison of anti-inflammatory activity of chrysin derivatives. 13th international electronic conference on synthetic organic chemistry. 2009; 1: 1.
- Gomes A, Couto D, Alves A, Dias I, Freitas M, Porto G, et al. Trihydroxyflavones with antioxidant and anti‐inflammatory efficacy. Biofactors. 2012; 38(5): 378-386.
- García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm Res. 2009; 58(9): 537-552.
Author Info
Sabale Prafulla*, Potey Lata, Sayyad Nusrat and Rahangdale PriyaReceived: 04-Aug-2021 Accepted: 18-Aug-2021 Published: 25-Aug-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3