Research Article - (2022) Volume 13, Issue 8
Abstract
As a type I Interferon’s, Human leukocyte Interferonalfa (HuIFN-αN3) is a complex pleiotropic molecule composed from several natural subtypes, showing: Antiviral, proor anti-inflammatory and antiproliferative activity in vitro. The present work was aimed to detect, analyze and compare the natural subtype’s composition of HuIFN-αN3 from EGIS (Budapest, Hungary) or IMZ (Zagreb, Croatia) and to measure theirs proor anti-inflammatory, antiproliferative and cytocidal activity in vitro. HuIFN-αN3 from EGIS, (Budapest, Hungary) or IMZ, (Zagreb, Croatia) contains seven “minor” (a-g) and eight “major” (1-8) subtypes with the isoelectric points: 8.12, 7.40, 7.02, 6.90, 6.45, 6.06, 4.45, and 5.80, 5.65, 5.50, 5.30, 5.05, 4.88, 4.72, 4.53. Subtypes show different pro and anti-inflammatory effects. Those separated in the range of pI 8.0 to 6.0 show pro-inflammatory activity. Those separated in the range 5.80 to 3.50 show anti-inflammatory activity. The subtypes isolated from HuIFN-αN3 (IMZ, Zagreb, Croatia) show very weak pro and strong anti-inflammatory activity. The strongest antiinflammatory activity has subtype 3. The antiproliferative assays on Human Embryonic Fibroblasts (HEF) or Human amnion cells line (FL) cells shows, that the total antiproliferative activity of HuIFN-αN3 is bigger than this from different subtypes, with the exception of subtype a. The cytocidal activity measured as IU/ml needed to get the 50% cytocidal effect on the Adult pig kidney cell line (PLA) cells shows: In the subtype a with 78.1, 1 with 312.5, 2 with 625, 3 with 0, 4 with 1.250, 5 with 0, 6 with 2.500, 7 with 625 and 8 with 0. When different HuIFN-αN3’s were tested, this from IMZ (Zagreb, Croatia) and SAV (Bratislava, Slovakia) has 156.2. HuIFN-αN3 from EGIS, (Budapest, Hungary) has 312.5, HuIFN-γ has 156.2, rHuIFN-α1 (recombinant Interferon's) has 2500 and rHuIFN-α2 has 5000. The results show, that all of the subtypes (a-g, 1-8) can be neutralized with the polyclonal anti-IFN-αN3. The values of NI were in the range from: -1.15 till -2.21. It can be concluded that this is the picture of different natural subtypes’ content in both preparations from EGIS or IMZ because of different technology. EGIS use concentrated purified preparation, while IMZ use concentrated non purified one.
Keywords
Human Interferon alfa, Natural subtypes, Antiproliferative activity, Cytocidal activity, Chromatofocusing, Reverse Phase High Performance Liquid Chromatography (RP-HPLC) analysis
Introduction
Interferon family represents a widely expressed group of cytokines. It includes three main classes, designated as type I Interferons (IFNs), type II IFN and type III IFNs. The two main type I IFNs includes IFN-α (further classified into 13 different subtypes such as HuIFN(-α1, -α2, -α4, -α5, -α6, -α7, -α8, -α10, -α13, -α14, -α16, -α17 and -α21)), and IFN-β. Human Interferon-α (HuIFN-αN3) is a pleiotropic complex molecule composed from several natural subtypes. Until now, eight “major” subtypes designated as 1-8 and seven “minor” subtypes designated as a-g were recognized. They show antiviral, proor anti-inflammatory (Platanias L, 2005; Billiau A, 2006), antiproliferative, immune-modulatory and antitumor activity in vitro and in some cases also in vivo (Mecs I, 1986; Shirono H, et al., 1990). Increasing evidence was accumulated that Human Interferon alfa (HuIFN-αN3) can inhibit the growth of both normal and malignant cells (Billiau A, 1984; Borecký L, 1986; Taylor JL and Grossberg SE, 1998). The cell growth inhibitory activity was observed with Human IFN preparations ranging from crude Buffy-coat derived extracts to purified HuIFN-αN3 preparations (Fish EN, et al., 1983; Ito M, Buffett RF, 1980; Viscomi GC, et al., 1995) and with various HuIFN-αN3 preparations (Fuchsberger N, et al., 1993; Lidin B and Lamon EW, 1992). It was found that this antiproliferative (AP) activity is closely associated with IFN molecule per se because the purified HuIFN-αN3 possesses the ability to inhibit the cell growth to a degree similar to that of crude IFN. Variations in the cell growth inhibitory effects are seen with different HuIFN-αN3 preparations as well as with the different target cells employed (Overall ML, et al., 1992; Ravine TJ and Ledinko N, 1986). Cloning of genes for HuIFN-αN3 has revealed the existence of the family of HuIF-αN3 subtypes (Goeddel DV, et al., 1981; Nagata S, et al., 1980). The various subtypes show the distinct antiviral activities with different potency against several viruses in a range of mammalian cell lines (Todt D, et al., 2016). The same antiproliferative activity against human cells was demonstrated for two subtypes, IFN-α A and D (HuIFNα-2 and HuIF-Nα-1). The C-terminal part of HuIFNα-2 molecule contributes to the antiproliferative activity possesses by IFN, N-and C-terminal part together contribute into IFN’s antiviral activity. However, to date the purified subtypes generally shows the antiproliferative activity lower than that of Buffy coat HuIFN-αN3 preparations, which are the mixtures of various natural subtypes (Harper MS, et al., 2015; Slimmer S, et al., 1981; Lavoie TB, et al., 2011). Among the other activities, the cytotoxicity in vitro of HuIFN-αN3 and its subtypes should be mentioned (Fent K and Zbinden G, 1987; Ito M and Buffett RF, 1981). In this respect the nephro toxicity by renal injury indicated by the rise in urinary protein excretion can be seen. In some cases, during the treatment also a nephritic syndrome could be found. Some of these side effects can be caused by one or more cytokines, HuIFN-αN3 or its isolated natural subtypes.
The present work was aimed to detect, analyze and compare the natural subtypes composition of HuIFN-αN3 originated from EGIS (Budapest, Hungary) or IMZ (Zagreb, Croatia), and to measure theirs pro or anti-inflammatory activity, antiproliferative and cytocidal activity in vitro.
Materials and Methods
Materials
Interferon: In the performed experiments, the following Human Interferon’s were used: (i) Natural leukocyte Sendai virus induced HuIFN-αN3 (EGIS, Budapest, Hungary; IMZ, Zagreb, Croatia; Slovak Academy of Sciences (SAV), Bratislava, Slovakia); HuIFN-αβ (Welcome-Glaxo, England) with the specific activity of 105-107 IU/mg of proteins; (ii) Isolated and partially purified HuIFN-αN3 subtypes a, (1-8) (Institute of Biotechnology, JATE-University of Szeged, Szeged, Hungary) with the specific activity of 106-107 IU/ml; (iii) Recombinant Interferon’s: rHuIFN-α1(recombinant Interferon's) (Protein, Moscow, Russia), rHuIFN-α2 (Schering, USA) with the specific activity of 106-108 IU/ml. All of the Interferon’s were used in the initial concentration of 1.000.000 IU/ml.
Antisera: Antiserums containing polyclonal antibodies to HuIFN-αN3 (Rabbit-Anti-HuIFN-αN3, Sigma, St.Luis, USA) were used during the experiments. The monoclonal antibodies against rHuIFN-α1, rHuIFN-α2 and against acidolabile IFN were obtained from Institute of Virology, Slovak Academy of Sciences, Bratislava, Slovakia). Monoclonal antibodies against HuIFN-γ were from Beringer, Mannheim, Germany. Monoclonal antibodies against PoIFN-γ (Procine Interferon-γ) were from Dr. Claude LaBonnardiere (INRA-VIM, Jouy-en-Josas, Cedex, France).
Cell cultures: The Adult pig kidney cell line (PLA) and Human Embryonic Fibroblasts (HEF) were prepared on the Virological department at the Institute of Microbiology and Immunology, Medical Faculty in Ljubljana, Slovenia (Malaise EP, et al., 1985). The transformed Human amnion cells line (FL) and its no transformed counterpart (WISH) were obtained from the Institute of Biotechnology, JATE-University of Szeged, Szeged, Hungary. Bovine Kidney cell line (MDBK) was obtained from Dr. Claude LaBonnardiere, INRA-VIM, Youy-en-Josas, Paris-Cedex, France. All the cells were grown in Eagle's medium supplemented with 10% of FCS (Fetal Calf Serum) (Sigma, St. Luis, USA) and antibiotics (Penicillin, Streptomycin, Gentamycin) (Sigma-Aldrich, EU).
Vesicular Stomatitis Virus (VSV): The Vesicular Stomatitis Virus (VSV) (serotype Indiana) was multiplied and purified by the method developed by Prevec L and Whitmore GF (Prevec L and Whitmore GF, 1963). The mouse L cells in Minimal Essential Medium (MEM)+5% FCS were cultivated to get the monolayer. After, the cells were infected with 2 ml of stock VSV virus (approximately 108 PFU/ml) diluted 1:1 with MEM. The infected monolayers were incubated at 37°C in a 5% CO2 for 0.5 hours to allow VSV adsorption. The infected cultures were incubated for a 12-16 hours. At this time there was pronounced cell destruction and the virus titer in the supernatant was approximately 2 × 108 PFU/ml. The virus plaque assay was used to measure the Plaque-Forming Unit (PFU). Briefly, 0.1 ml of a virus suspension, was adsorbed for 30 minutes on a monolayer of L cells, after which 10 ml of overlay medium, consisting of medium MEM plus 2% FCS together with 0.9% washed agar was added. After 36 hours, 3 ml of a solution of neutral red in medium MEM (1/20,000) (W/V) was added to the overlay and the plaques were counted 10 hours later. The VSV purification procedure was as follows: 14 hours after virus infection, the supernatant of infected L cell monolayer were pooled and spun at 600 g for 15 minutes to remove large cell particles. The supernatants were centrifuged at 37,000 g for 2 hours to sediment the virus. The pellet was resuspended in Phosphate Buffer Saline (PBS) containing 0.1 mg of both ribonuclease and deoxyribonuclease, and the suspension was incubated at 37°C for 1.5 hours. The virus suspension was then sediment by centrifugation at 50,000 g for 30 minutes. The final virus pellet was resuspended in PBS and stored at -80°C.
Methods
Antiviral assay of HuIFN-αN3: The antiviral assay of HuIFN-αN3 was performed by the method developed by Voigt E, et al. (Voigt E, et al. 2013). The WISH cells were seeded into 96-well micro-titer plates at a density of 2.5 × 105 cells/ml and cultured for 24 h before antiviral treatment. Interferon was diluted serially 1:2 in Roswell Park Memorial Institute (RPMI) media supplemented with 2% FBS to final concentrations of 1:512 IU/ ml to 0.5 U/ml. Culture media was vacuum aspirated from 96-well plates with confluent cell minelayers, 67 μl/well of antiviral dilution or control media was added, and plates were again incubated under culture conditions for 24 hours. After 24-hour incubation, cells were challenged with VSV virus in 30 μl RPMI media+2% FBS per well added to the antiviral dilution for a final Multiplicity of Infection (MOI) of 5.0 Pfu/cell. In the standard antiviral assay with VSV infection, the infection was allowed to progress until cytopathic effects were readily apparent in unprotected control cells (16-28 hours post infection, as indicated). The cell medium was discarded, and cells were fixed with a solution of 4% para formaldehyde (w/v) and 5% sucrose (w/v) in PBS for 20 minutes. The cells were rinsed twice with PBS and stained with crystal violet (0.1% w/v) in 20% ethanol overnight. Crystal violet staining was measured with a Synergy H4 hybrid multi-mode micro-plate reader (BioTek, USA) reading absorbance at 570 nm, and scanned using a desktop scanner to obtain reference images. The IC50 value calculations for each dilution series were found by linear leastsquares regression through the three data points in the linear range of the dose-response curves closest to half-maximum intensity. Subsequent interpolation determined the standard Interferon dilution corresponding to a 50% decrease in signal above background with respect to the positive (infected, untreated) and negative (uninfected, untreated) control wells. The limit of detection was defined as the minimum interferon concentration that resulted in an IC50 curve that included the 50% viral inhibition point.
RP-HPLC analysis of the HuIFN-αN3: HuIFN-α Interferon species in different IFN compositions were separated according to theirs relative hydrophobicity using RP-HPLC column, as it was stated by Punainen S, et al. (Punainen S, et al., 1999). The HuIFN-αN3 subtype composition was analyzed by Reverse Phase High Performance Liquid Chromatography (RP-HPLC). HPLC column was Phenomenex, Aeris Peptide column 3.6 μm XB-C18, 250 × 4.6 mm. Different HuIFN-αN3 samples (natural and recombinant) approximately 1 million IU/ml in a volume of 20-40 μl were applied to the column and eluted with the linear gradient of Solvent A=water+0.1% of Trifluoroacetic Acid (TFA) and Solvent C=Acetonitril+0.1% TFA for 20 minutes with a flow rate of 0.8 ml/min. and pressure of 139-140 bar. The course of RP-HPLC chromatography of different IFN samples is shown in Table 1. Temperature of the column was 40°C. The absorbance was monitored at 214 and 280 nm.
Chromatofocusing: Chromatofocusing is a technique that employs ion-exchange chromatography using a pH gradient (usually linear) to separate biomolecules with acid/base functionalities (Anderson D, 2005). It is used in the analysis and purification of proteins. It was developed with the hope to become a liquid chromatographic version of Isoelectric Focusing (IEF), which performs both a separation role based on the pI values of a protein and a characterization role in determining the pI values. Irrespective of the ways in which the pH gradient is generated, there are two modes of chromatofocusing: (1) Anion chromatofocusing, (2) Cation chromatofocusing. Most conventional chromatofocusing techniques utilize elution buffer components from Amersham Biosciences (Amersham, 1987). During the experiments, the chromatofocusing was performed in the 50 × 1 cm3 Bio-Rad chromatographic columns filled with Polybuffer Exchanger (Mécs I, Koltai M, 1985; Toth S and Mecs I, 1986; Toth S and Mecs I, 1986). The column was equilibrated to pH 7.4 with the 25 mM imidazol/Hcl buffer and then the IFN samples were added onto the column in phosphate buffer saline solution (pH=7.4) containing a total antiviral activity of 1.000.000 IU/ml units. So loaded column was eluted with 8-times diluted pH=3.5 polybuffer 74 and 2 ml fractions were collected. In each fraction the antiviral, antiproliferative and cytocidal activity were determined.
Pro-or anti-inflammatory assay: The inflammatory assay was performed according to Mecs and Koltai (Toth S and Mecs I, 1986) as follows: CFIuP (Complement Factor I Precursor) mice’s weighing 28.6 ± 1.34 g fed with the commercial food pellets and tap water ad libido was used for the detection of inflammatory responses. The acute inflammatory reaction was induced either by 300 μg Carrageenan (Viscarin 402, Lot No. 203215, Marine Colloids Inc. USA) or by Human IFN-αN3 preparations in doses of 1.24 × 106 IU/ml and 3.6 × 106 IU/ml injected into the plantar region of the footpad in a volume of 0.03 mL/region. The contra lateral feet were given the same volume of isotonic NaCl. Determination of the inflammatory responses was made according to Levy (Levy L, 1969; Slimmer S, et al., 1981) three hours after Carrageenan and two hours after IFN preparations. The mice were bled; theirs’ hind paws were cut at the tarso-metatarsal joint, and the weights of the inflamed vs. paws vs. saline treated were compared, and the percentage increase produced by the philologer was calculated. The results were statistically analyzed by the unpaired Student’s t-test.
Antiproliferative assay: The antiproliferative activity of various IFNs and theirs natural subtypes was determined on HEF and FL cells (Filipic B, et al., 1991; Taylor JL, et al., 1984) as follows: Cells (HEF, FL) were seeded into the 96 well micro-titer plate (Costar, USA) in a density of 4-6 × 104/well. In addition 1/3 of the another micro-titer plate was seeded under same condition and used as “initial number” and put into the 5% CO2 filled thermostat at 37° C for three hours. Cells were fixed with 2% glutaraldehyde (100 μL/well). The wrapped plates in aluminum foil were kept for 72 hours in the refrigerator at +4°C. To the experimental plates, on next day the IFN samples were added (100 μL/well) in triplicate. They were serially diluted from 1:2 to 1:1024 and incubated for additional four days in the 5% CO2 filled thermostat at 37°C. After four days of incubation, the medium was removed and the cells were fixed with 2% Glutaraldehyde, washed with Phosphate Buffer Saline (PBS) and stained with the Methylene blue for 45 minutes at room temperature. Afterward, plates were washed and the bound color was eluted by 1 mM HCl (100 μl/well). On the same way the plates with the “initial number” of cells were managed. Finally the optical density was measured at 570 nm. The calculation of the GI (Growth Index)=OD (Optical Density) 570 nm of cells after 4 days/OD 570 nm of “initial number of cells”.
Cytocidal activity in vitro: The cytocidal activity was determined by the method similar to that described (Karayianni-Vasconcelos G, 1993). In brief: The PLA cells were seeded into the 96 well micro-titer plates (Costar, USA) in a density of 2 × 105 cells/well, and incubated. In the 5% CO2 filled thermostat at 37°C. When the cells approached a confluence, the medium was aspirated and 10.000 IU of IFN/mL in 100 μl of medium was added to the previous 100 μl of the medium in the tray. Afterward, the samples were serially diluted from 1:2 to 1:1024 and incubated for additional four days in the 5% CO2 filled thermostat at 37°C. Cells were stained with 0.05% Neutral red in PBS for 75 minutes, washed twice with Saline and added 100 μL of the mixture of 96% Ethanol:PBS in ratio 1:1 to elute the cell bound color. The cytocidal activity was determined by measuring the optical density of the eluted dye at 540 nm.
Neutralization test: Neutralization of the cytocidal activity of different IFNs by the antiserum were assayed by the “constant antibody” method (La Bonnardière C, et al., 1986) in which the fixed dilution of antiserum were applied to the PLA cells, followed by the serial 2-fold dilutions of IFNs. The Neutralization Index (NI) was calculated as follows: NI=(log2 of IFN)-(log2 of IFN control).
Results
The subtype composition of HuIFN-αN3
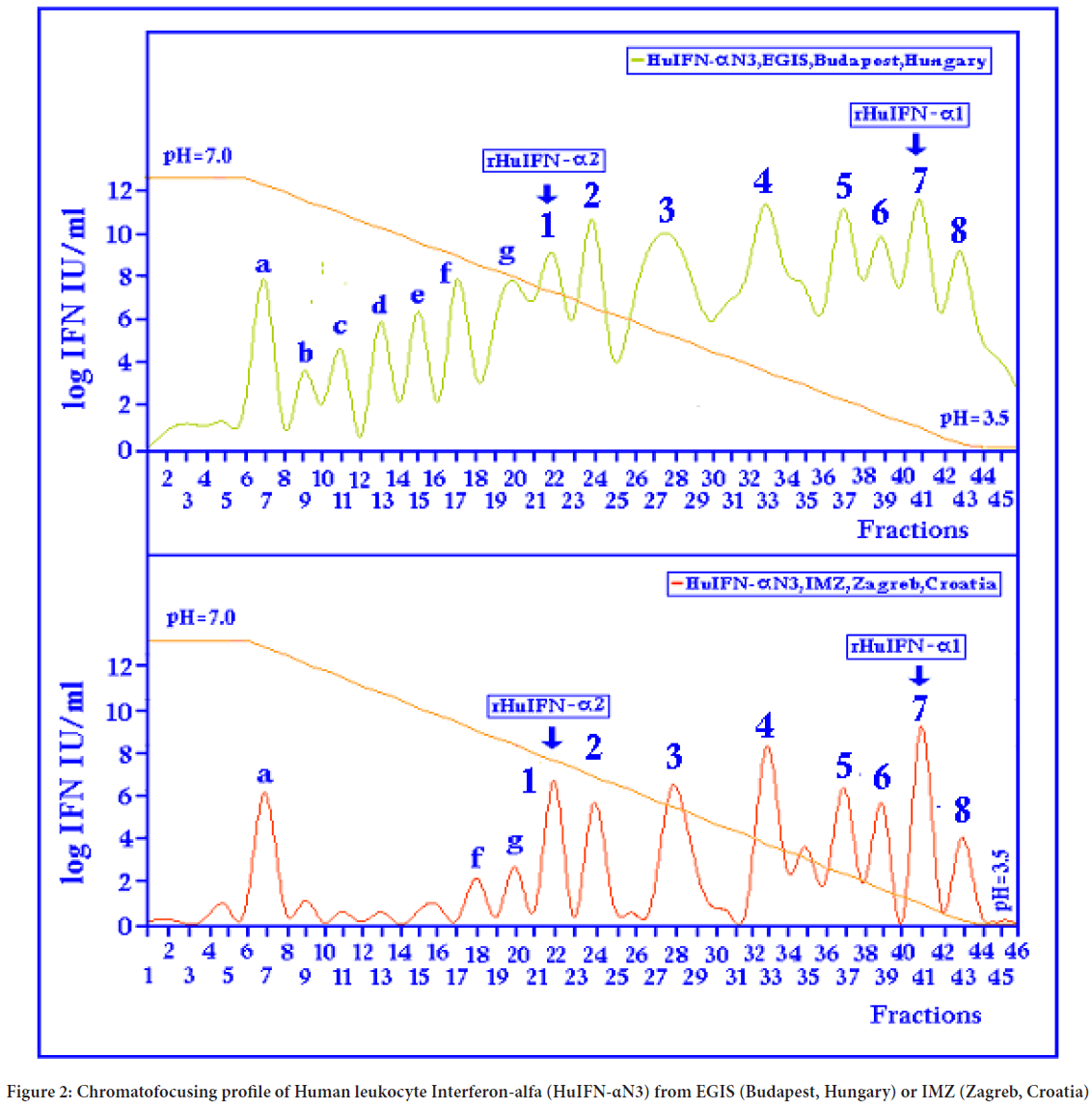
Through the RP-HPLC analysis (Figure 1 and Table 1) it was found, that the natural RP-HPLC type I. correspond to IFN subtype α14, RP-HPLC type II to IFN subtype α2, RP-HPLC type III to IFN subtypes α21 and α4, RP-HPLC type IV corresponds to IFN subtype α10, RP-HPLC type V corresponds to IFN subtypes α17 and α7, RP-HPLC type VI correspond to IFN subtype α8; RP-HPLC type VII to IFN subtype α1 and RP-HPLC type VIII to IFN subtype α a. In the native HuIFN-αN3 from EGIS, Budapest, Hungary or IMZ, Zagreb, Croatia (Figure 2) generally with seven “minor” (a-g) and eight “major” (1-8) subtypes of antiviral activities can be determined by chromatofocusing on WISH cells. They have the following pI values (Isoelectric points): 8.12, 7.40, 7.02, 6.90, 6.45, 6.06, 4.45, and 5.80, 5.65, 5.50, 5.30, 5.05, 4.88, 4.72, 4.50 (Table 2). The highly purified rHuIFN-α2 and rHuIFN-α1 has pIs of 5.80 and 4.70. It can be assumed that a natural chromatofocusing subtype 1 and 7 corresponds to rHuIFN-α2 and rHuIFN-α1.
| Step | Time (min) | Solvent A (%) | Solvent C (%) |
|---|---|---|---|
| 0 | 0 | 91 | 9 |
| 1 | 3 | 80 | 20 |
| 2 | 6 | 50 | 50 |
| 3 | 12 | 50 | 50 |
| 4 | 15 | 91 | 9 |
| 5 | 20 | 91 | 9 |
Table 1: The course of Reverse Phase High Performance Liquid (RP-HPLC) chromatography of different Interferons (IFN) samples
| IFN type/ subtype | Polyclonal anti-IFN-α | Monoclonal anti-IFN-α1 | Monoclonal anti-IFN-α2 | Monoclonal anti-Acidolabile IFN | Monoclonal anti-HuIFN-γ | Monoclonal anti-PoIFN-γ |
|---|---|---|---|---|---|---|
| a | -1.15* | 0 | 0 | -0.98* | 0 | 0 |
| 1 | -1.92* | -2.43* | 0 | 0 | 0 | 0 |
| 2 | -1.22* | 0 | 0 | 0 | 0 | 0 |
| 3 | -1.77* | 0 | 0 | 0 | 0 | 0 |
| 4 | -2.01* | 0 | 0 | 0 | 0 | 0 |
| 5 | -1.88* | 0 | 0 | 0 | 0 | 0 |
| 6 | -2.12* | 0 | 0 | 0 | 0 | 0 |
| 7 | -2.21* | 0 | -2.84* | 0 | 0 | 0 |
| 8 | -1.77* | 0 | 0 | 0 | 0 | 0 |
| HuIFN-αN3 | -3.11* | -1.11* | -1.97* | -1.43* | -0.22* | -0.11* |
| rHuIFN-α1 | -1.53* | -3.72* | 0 | 0 | 0 | 0 |
| rHuIFN-α2 | -2.01* | 0 | -2.75* | 0 | 0 | 0 |
| HuIFN-αβ | -1.91* | -0.95* | -2.11* | -0.21* | 0 | 0 |
| HuIFN-γ | -1.05* | 0 | 0 | -1.15* | -2.23* | 0 |
Note: *Values of NI (Neutralization Index) were calculated as follows: NI=(log2 of IFN titer)-(log2 of IFN control). IFN: Interferon; HuIFN-αN3: Human leukocyte Interferon-alfa; rHuIFN: recombinant Interferons; PoIFN: Procine Interferon
Table 2: Neutralizations of Interferon’s cytocidal activity
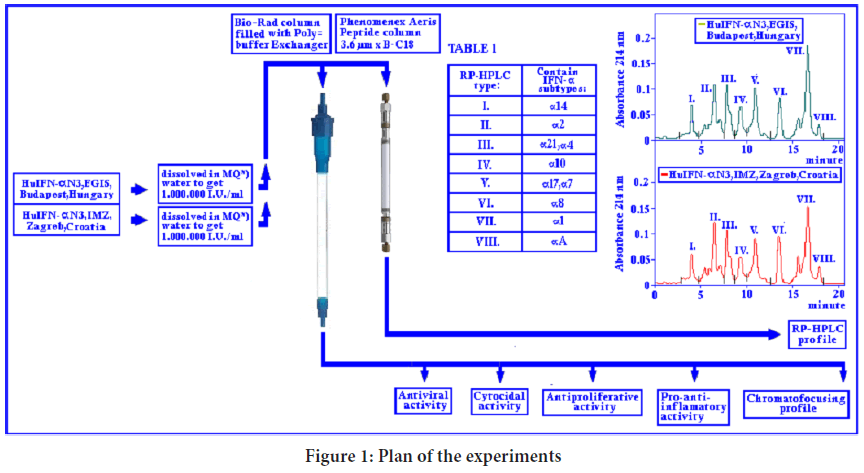
Figure 1: Plan of the experiments
Figure 2: Chromatofocusing profile of Human leukocyte Interferon-alfa (HuIFN-αN3) from EGIS (Budapest, Hungary) or IMZ (Zagreb, Croatia)
Proand anti-Inflammatory activity of different HuIFN-αN3 types
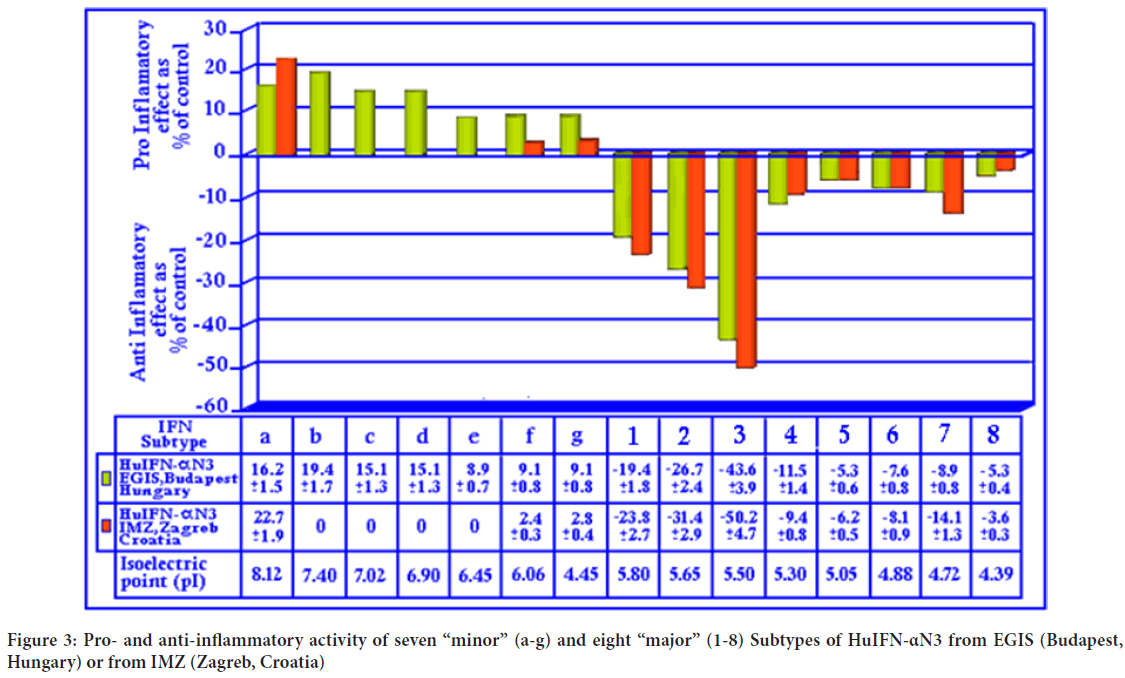
The natural subtypes isolated from HuIFN-αN3 from EGIS, Budapest, Hungary or IMZ, Zagreb, Croatia show (Figure 3) different pro and anti-inflammatory effects. The natural subtypes separated in the range of pI 8.0 to 6.0 show proinflammatory activity. Those, chromatofocused in the range 5.80 to 3.50 show anti-inflammatory activity. It is interesting, that the type isolated from HuIFN-αN3 (IMZ, Zagreb, Croatia) (Figure 3) show very “weak” pro and “strong” anti-inflammatory effects. The strongest anti-inflammatory activity can be found in the isolated natural HuIFN-α subtype 3. It is important, that this from IMZ is stronger than those from EGIS. Additionally, this is the picture of different natural subtypes’ content in both preparations as EGIS or IMZ has different technology. EGIS use concentrated purified preparation, while IMZ use concentrated non purified one.
Figure 3: Pro- and anti-inflammatory activity of seven “minor” (a-g) and eight “major” (1-8) Subtypes of HuIFN-αN3 from EGIS (Budapest, Hungary) or from IMZ (Zagreb, Croatia)
Antiproliferative activity
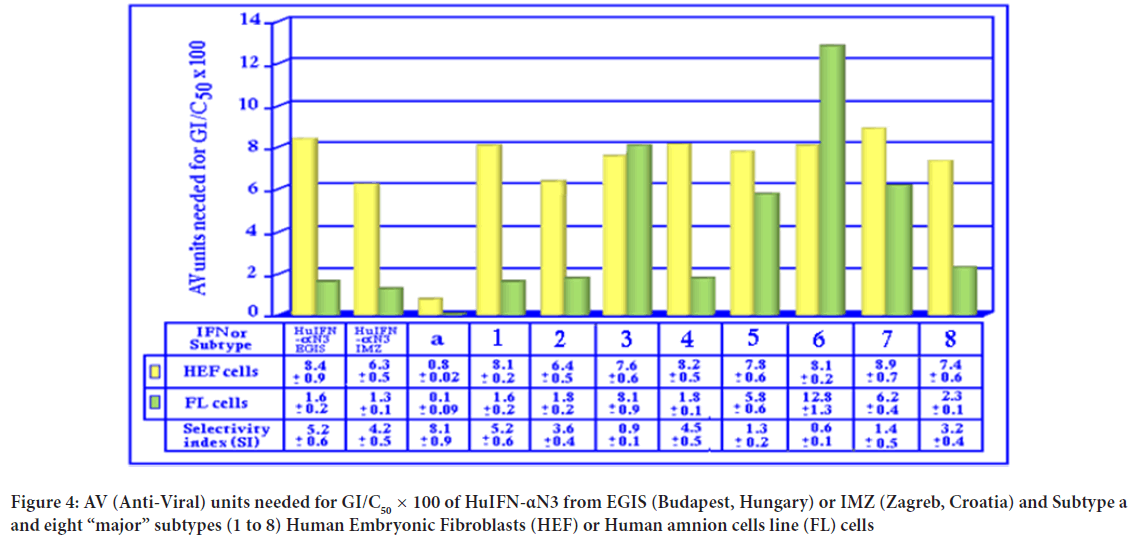
The data about the antiproliferative assays performed on HEF or FL cells shows (Figure 4) that the total antiproliferative activity of HuIFN-αN3 is bigger from the different isolated subtypes, with the exception of the isolated natural subtype a. From the isolated subtypes, the most interesting are subtype 3 with a lower sensitivity for FL cells, and subtype 6 with the lowest sensitivity for FL cells. The recombinant IFNs has similar antiproliferative activity as natural subtypes 1 and 7. In this respect, the subtype ‘a’ is somehow similar to IFN-γ and IFN-ω, even it is different according to the pI value and biological properties. In general, the FL (=transformed) cells are more sensitive for the antiproliferative activity than HEF (=no transformed) cells.
Figure 4: AV (Anti-Viral) units needed for GI/C50 × 100 of HuIFN-αN3 from EGIS (Budapest, Hungary) or IMZ (Zagreb, Croatia) and Subtype a and eight “major” subtypes (1 to 8) Human Embryonic Fibroblasts (HEF) or Human amnion cells line (FL) cells
IFN’s cytocidal activity in vitro
Quantification of cytocidal activity: The cytocidal activity of different natural and recombinant HuIFNs, together with the isolated natural subtypes (a, 1-8) were assayed on the PLA (Adult pig kidney cell line), HEF or FL cells (Table 3). They are sensitive for the cytocidal activity of HuIFN-αN3 similarly as it was described for the sensitivity of embryonic and new born pig kidney cells for PoIFN-α (Laude H and Bonnardiere CL, 1984). Treatment with HuIFN-αN3 show the morphological changes, similar to the cytopathic effects in a dose dependent manner. When different natural subtypes of HuIFN-αN3 were tested on the PLA cells, the cytocidal activity was found only in some of them as IU/ml needed to get the 50% cytocidal effect: In the subtype a with 78.1, subtype 1 with 312.5, subtype 2 with 625, subtype 3 with 0, subtype 4 with 1250, subtype 5 with 0, subtype 6 with 2500, subtype 7 with 625 and subtype 8 with 0. When different natural preparations of HuIFN-αN3 were tested the preparation from IMZ, Zagreb, Croatia has 156.2 as it was found for the preparation from SAV, Bratislava, Slovakia. The HuIFN-αN3 from EGIS, Budapest, Hungary has 312.5. Relatively high cytocidal activity was found when HuIFN-γ was tested. It was 156.2. The recombinant rHuIFN-α1 shows the very low cytocidal activity at 2.500, the rHuIFN-α2 at 5.000.
Neutralization of cytocidal activity: The cytocidal activity of various forms of HuIFN-αN3 and its natural subtypes can be neutralized by adding either polyclonal or monoclonal antibodies. The results in the Table 3 show, that all of the subtypes (a, 1-8) can be neutralized with the polyclonal anti-IFN-αN3. The values of NI were in the range from: -1.15 till -2.21. The HuIFN-αN3 from EGIS had NI of -3.11. The rHuIFN-α1 has NI of -1.53; the rHuIFN-α2 had NI of -2.01. When the HuIFN-αβ was tested with polyclonal anti-HuIFN-αN3, the NI was -1.91. With the monoclonal anti-rHuIFN-α1 the subtype I can be neutralized with the NI -2.43. The HuIFN-αN3 has the NI-1.11; rHuIFN-α1 has the NI-3.72 and HuIFN-αβ with the NI -0.95. With the monoclonal anti-rHuIFN-α2 the subtype VII can be neutralized with the NI of -2.84. The HuIFN-αN3 has the NI of -1.97, and the rHuIFN-α2 has the NI of -2.75. Surprisingly the HuIFN-αβ has the NI of -2.11. The monoclonal anti-acidolabile IFN can neutralize the isolated natural subtype ‘a’ with the NI -0.98, and HuIFN-αβ with NI of -0.21. With the monoclonal anti-HuIFN-γ the HuIFN-αN3 can be neutralized with the NI of -0.22. With the monoclonal anti-PoIFN-γ the HuIFN-αN3 can be neutralized with the NI of -0.11.
| IFN type/subtype | PLA** | HEF*** | FL**** |
|---|---|---|---|
| a | 78.1* | 0 | 0 |
| 1 | 312.5* | 0 | 0 |
| 2 | 625* | 0 | 0 |
| 3 | 0* | 0 | 0 |
| 4 | 1250* | 0 | 0 |
| 5 | 0* | 0 | 0 |
| 6 | 2500* | 0 | 0 |
| 7 | 625* | 0 | 0 |
| 8 | 0* | 0 | 0 |
| HuIFN-α (EGIS) | 312.5* | 0 | 0 |
| HuIFN-α (IMZ) | 156.2* | 0 | 0 |
| HuIFN-α (SAV) | 156.2* | 0 | 0 |
| HuIFN-αβ (Wellcome) | 625.2* | 0 | 0 |
| rHuIFN-α1 (Roche) | 2500* | 0 | 0 |
| rHuIFN-α2 (Schering) | 5000* | 0 | 0 |
| HuIFN-γ (Genen-tech) | 156.2* | 0 | 0 |
Note: *Antiviral units (IU/mL) needed to get the 50% cytocidal effect; **PLA=Adult pig kidney cell line; ***HEF=Human Embryonic Fibroblasts; ****FL=Human amniotic cell line
Table 3: Quantification of cytocidal effects of various preparations of HuIFN-αN3 and theirs natural subtypes on PLA, HEF and FL cells
Discussion
The natural Human leukocyte Interferon (HuIFN-αN3) is a complex molecule consisting of at least 15 natural subtypes. They were characterized as seven “minor” designed as a-g and eight “major” designed as 1-8. For the subtypes 1 and 7 it is known that they have the recombinant counterparts’ rHuIFN-α1 and rHuIFN-α2 (Platanias LC, 2005). Recently, thirteen rHuIFN-α subtype were expressed in E. coli and purified by affinity chromatography (Kuruganti S, et al., 2014). They are comparable with the natural subtypes by isoelectric points (pI). Today it is known the binding kinetics and activity of all thirteen HuIFN-α subtype. The mechanism by which HuIFN-αN3 either as natural mixture or as an individual subtype affect some other cytokines is not completely understood. A key report has identified, in HuIFN-αN3 subtypes, a correlation with stronger inhibition of virion’s infectivity forged the higher relative potency of HuIFN-α8. Interestingly, both potent (HuIFN-α8) and weak (HuIFN-α1) subtypes significantly induced hyper mutation of GG-to-AG in HIV-1, which revealed strong implications for HIV-1 mucosal immunity, viral evolution and HuIFN-αN3 based functional cure strategies described. The results unraveled non-redundant functions of the HuIFN-αN3 subtypes against HIV-1 infection, more broadly, the existence of subtypes virtually showed a potential advantage for HuIFN-αN3 gene family to allow the infected host to differentially express HuIFN-αN3 genes in response to diverse antigens. It seems, that for this are mainly responsible some of the natural subtypes. The detailed analyses found (Crow MK and Ronnblom L, 2019), that some of them act anti and some proinflamatory. In this contest, the subtype a (probably IFN-ω) (Kontsek P, et al., 1991) is the most interesting concerning the cytocidal and antiproliferative activity. It was also found that this is the most important acid-labile component of the HuIFN-αN3. Regarding the antiproliferative and cytocidal activity it was found, that it is not necessary that they are always in correlation. The used experimental cell system (PLA) for testing of the Interferon's cytocidal activity show the major differences between crude or purified HuIFN-α isolated subtypes and recombinant HuIFN-α1 and HuIFN-α2 having very little of cytocidal activity. The cytocidal activity of all tested natural or recombinant HuIFN-α can be neutralized by polyclonal anti-HuIFN-α rabbit's antiserum (Aranson BG and Dianzani F, 1998). Susceptibilities of PLA, HEF and FL cell lines for the cytocidal, antiproliferative and antiviral activity of various interferons were tested. The PLA cells were highly sensitive for cytocidal, moderately for antiproliferative and nearly insensitive for antiviral activity. The FL cells were insensitive for cytocidal, highly sensitive for antiproliferative and antiviral activity. On contrary, the HEF cells were insensitive for cytocidal, moderately sensitive for the antiproliferative and highly sensitive for antiviral activity. Cell's sensitivity for cytocidal, antiproliferative and antiviral effects of IFNs appear to be distinct suggesting that cell membrane receptors for the antiviral activity of IFN-α may not be a major factor in the response of PLA cells, being highly susceptible for the cytocidal activity. The HEF cells, which are most susceptible for the antiviral effect of HuIFN-αN3, were insensitive for the cytocidal effect. The cytocidal effect of IFNs does not appear to be a general phenomenon. As it is recognized (Hannun YA and Linardic CM, 1993; Hannun Y, 1993; Blitterswijk WJ, et al., 2003) some Interferon’s can utilize another novel signal transduction pathway related to the production of ceramid serving within the cell as a second messenger controlling downstream event. Further experiments will show if the cytocidal activity of HuIFN-α or some of its natural subtype is somehow connected with this pathway.
Conclusion
The present work was aimed to detect, analyze and compare the natural subtype’s composition of HuIFN-αN3 from EGIS (Budapest, Hungary) or IMZ (Zagreb, Croatia) and to measure theirs proor anti-inflammatory, antiproliferative and cytocidal activity in vitro. It can be concluded that this is the picture of different natural subtypes’ content in both preparations from EGIS or IMZ because of different technology. EGIS use concentrated purified preparation, while IMZ use concentrated non purified one.
Funding
The research was performed in the frame of CIETO (Croatian Institute for Experimental and Translational Oncology, Koledinečka 03, 10040 Zagreb, Croatia). It was included into the Project: “Oncolytic Newcastle Disease Virus in the Veterinary Medicine”. The research was partially supported by Ivan Čermak and by Deltagrupa, Kaptol 19, 10000 Zagreb, Croatia.
Acknowledgments
Authors are indebted to Tomaž Velnar for English suggestions. This article is devoted to the kind memory on Prof. Dr. Imre Mecs and Doc. Dr. Eugen Šooš
References
- Platanias LC. Mechanisms of type-I-and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005; 5(5): 375-386.
[Crossref] [Google Scholar] [Pubmed]
- Billiau A. Anti-inflammatory properties of Type I interferons. Antiviral Res. 2006; 71(2-3): 108-116.
[Crossref] [Google Scholar] [Pubmed]
- Mecs I. Comparison of some physicochemical, biological and physiological properties of natural, recombinant and synthetic subtypes of Human Alpha Interferon. Yugoslave Colloquium on Interferon. 1986; 23-28.
- Shirono H, Ito C, Koga J. Studies on subtype composition in natural leukocyte interferon preparations. J Virol Methods. 1990; 27(1): 1-9.
[Crossref] [Google Scholar] [Pubmed]
- Billiau A. The clinical application of fibroblast interferon: An overview. Med Oncol Tumor Pharmacother. 1984; 1(2): 87-96.
[Crossref] [Google Scholar] [Pubmed]
- Borecký L. Current view on the perspectives of interferon therapy. Acta Virol. 1986; 30(2): 161-169.
[Google Scholar] [Pubmed]
- Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998; 25(1): 23-29.
[Google Scholar] [Pubmed]
- Fish EN, Banerjee K, Stebbing N. Human leukocyte interferon subtypes have different antiproliferative and antiviral activities on human cells. Biophys Res Comm. 1983; 112(2): 537-546.
[Crossref] [Google Scholar] [Pubmed]
- Ito M, Buffett RF. Exogenous interferon: Use in humans for treatment of malignancies. Interferon and interferon inducers: Clinical applications. 1980; 89-112.
- Viscomi GC, Grimaldi M, Palazzini E, Silvestri S. Human leukocyte interferon alpha: Structure, pharmacology, and therapeutic applications. Med Res Rev. 1995; 15(5): 445-478.
[Crossref] [Google Scholar] [Pubmed]
- Fuchsberger N, Kubes M, Kontsek P, Borecký L, Hornak M, Godal A, et al. In vitro antiproliferative effect of interferon alpha in solid tumors: A potential predictive test. Neoplasma. 1993; 40(5): 293-296.
[Google Scholar] [Pubmed]
- Lidin B, Lamon EW. Differential effects of human recombinant interferons on the expression of two early gene products of Epstein-Barr virus. Antiviral Res. 1992; 17(1): 79-89.
[Crossref] [Google Scholar] Pubmed]
- Overall ML, Chambers P, Hertzog PJ. Different interactions of interferon-α subtypes at the surface of epithelial and lymphoid cells. J Interferon Res. 1992; 12(4): 281-288.
[Crossref] [Google Scholar] [Pubmed]
- Ravine TJ, Ledinko N. Treatment with human recombinant leukocyte interferons inhibits in vitro invasive ability of human lung carcinoma cells. Clin Exp Metastasis. 1986; 4(3): 191-203.
[Crossref] [Google Scholar] [Pubmed]
- Goeddel DV, Leung DW, Dull TJ, Gross M, Lawn RM, McCandliss R, et al. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981; 290(5801): 20-26.
[Crossref] [Google Scholar] [Pubmed]
- Nagata S, Taira H, Hall A, Johnsrud L, Streuli M, Ecsödi J, et al. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980; 284(5754): 316-320.
[Crossref] [Google Scholar] [Pubmed]
- Todt D, François C, Anggakusuma, Behrendt P, Engelmann M, Knegendorf L, et al. Antiviral activities of different interferon types and subtypes against hepatitis E virus replication. Antimicrob Agents Chemother. 2016; 60(4): 2132-2139.
[Crossref] [Google Scholar] [Pubmed]
- Harper MS, Guo K, Gibbert K, Lee EJ, Dillon SM, Barrett BS, et al. Interferon-α subtypes in an ex vivo model of acute HIV-1 infection: Expression, potency and effector mechanisms. PLoS Pathog. 2015; 11(11): 1005254.
[Crossref] [Google Scholar] [Pubmed]
- Slimmer S, Masui H, Kaplan NO. [50] Antiproliferative assay for human interferons. Methods in Enzymology. 1981; 79: 419-422.
[Crossref] [Google Scholar] [Pubmed]
- Lavoie TB, Kalie E, Crisafulli-Cabatu S, Abramovich R, DiGioia G, Moolchan K, et al. Binding and activity of all human alpha interferon subtypes. Cytokine. 2011; 56(2): 282-289.
[Crossref] [Google Scholar] [Pubmed]
- Fent K, Zbinden G. Toxicity of interferon and interleukin. Trends Pharmacol Sci. 1987; 8(3): 100-105.
- Ito M, Buffett RF. Cytocidal effect of purified human fibroblast interferon on tumor cells in vitro. J Natl Cancer Inst. 1981; 66(5): 819-825.
[Google Scholar] [Pubmed]
- Malaise EP, Guichard M, Deschavanne PJ. Primary cultures from lung and kidney. Cell Clones: Manual of Mammalian Cell Techniques. 1985: 175-183.
- Prevec L, Whitmore GF. Purification of vesicular stomatitis virus and the analysis of P32-labeled viral components. Virology. 1963; 20(3): 464-471.
[Crossref] [Google Scholar] [Pubmed]
- Voigt E, İnankur B, Baltes A, Yin J. A quantitative infection assay for human type I, II, and III interferon antiviral activities. Virol J. 2013; 10(1): 1.
[Crossref] [Google Scholar] [Pubmed]
- Punainen S, Veripavel UR, Tőlő H, Parkkinen J. Alpha Interferon manufacturing process using immunoadsorbtion and virus removal filtration.Patent No WO 99/6444. 1999.
- Anderson D. 12 Chromatofocusing. Separation Science and Technology. 2005; 7: 265-296.
- Amersham. Chromatofocusing with Polybuffer and PBE. Amersham Pharmacia Biotechnology. 1987.
- Mécs I, Koltai M. Direct evidence for the anti-inflammatory effect of human interferon-α in CFLP mice. Arch Virol. 1985; 85(1): 151-155.
[Crossref] [Google Scholar] [Pubmed]
- Toth S, Mecs I. Effects of amino-acids on antiviral action of interferons. Yugoslave Colloquium on Interferon. 1986: 29-34.
- Toth S, Mecs I. Separation of human interferon subtypes by chromatofocusing and comparison of their sensitivities to amino acid effects. Yugoslave Colloquium on Interferon. 1986: 35-39.
- Levy L. Carrageenan paw edema in the mouse. Life Sci. 1969; 8(11): 601-606.
[Crossref] [Google Scholar] [Pubmed]
- Filipic B, Golob A, Toth S, Mecs I, Beladi I, Likar M. Interactions between human and porcine interferons. Acta Virol. 1991; 35(1): 19-26.
[Google Scholar] [Pubmed]
- Taylor JL, Papadimitrou J. Effects of interferons on cell growth and function. Interferon: General and Applied Aspects. 1984; 1: 140.
- Karayianni-Vasconcelos G, Borecký L, Kontsek P. Porcine leukocyte interferon exhibits close antigenic relatedness to human interferon alpha 2, but not to human interferon alpha 1. Vet Immunol Immunopathol. 1993; 38(3-4): 359-365.
[Crossref] [Google Scholar] [Pubmed]
- La Bonnardière C, Laude H, Berg K. Biological and antigenic relationships between virus-induced porcine and human interferons. Ann Inst Pasteur Virol. 1986; 137: 171-180.
[Crossref] [Google Scholar] [Pubmed]
- Laude H, Bonnardiere CL. Cytocidal effect of interferons on porcine renal cells. J Interferon Res. 1984; 4(1): 101-110.
[Crossref] [Google Scholar] [Pubmed]
- Kuruganti S, Accavitti-Loper MA, Walter MR. Production and characterization of thirteen human type-I interferon-α subtypes. Protein Expr Purif. 2014; 103: 75-83.
[Crossref] [Google Scholar] [Pubmed]
- Crow MK, Ronnblom L. Type I interferons in host defence and inflammatory diseases. Lupus Sci Med. 2019; 6(1): 336.
[Crossref] [Google Scholar] [Pubmed]
- Kontsek P, Borecký L, Novák M. Are the acid-labile interferon α and interferon ω-1 identical? Virology. 1991; 181(1): 416-418.
[Crossref] [Google Scholar] [Pubmed]
- Aranson BG, Dianzani F. Correlation of the appearance of anti-interferon antibodies during treatment and diminution of efficacy: Summary of an international workshop on anti-interferon antibodies. J Interferon and Cytokine Res. 1998; 18(8): 639-644.
[Crossref] [Google Scholar] [Pubmed]
- Hannun YA, Linardic CM. Sphingolipid breakdown products: Anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993; 1154(3-4): 223-236.
[Crossref] [Google Scholar] [Pubmed]
- Hannun Y. The novel second messenger ceramide identification, mechanism of action and cellular activity. Adv Lipid Res. 1993; 25: 23.
[Google Scholar] [Pubmed]
- Blitterswijk WJ, Luit AH, Veldman RJ, Verheij M, Borst J. Ceramide: Second messenger or modulator of membrane structure and dynamics? Biochem J. 2003; 369(2): 199-211.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Bratko Filipič1*, Sandor Toth2, Lidija Gradišnik3, Adriana Pereyra4 and Hrvoje Mazija12Department of Biomedical Sciences, TEWA Consultant, Szeged, Hungary
3Department of Cell Culture, Institute of Biomedical Sciences, University of Maribor, Maribor, Slovenia
4Department of Biomedical Sciences, MEDEX D.O.O. Company, Ljubljana, Slovenia
Citation: Filipic B: Determining of the Human Interferon-Alfa and its Natural Subtypes' Pro- or Anti-Inflammatory, Antiproliferative and Cytocidal Activity In Vitro
Received: 01-Jul-2022 Accepted: 25-Jul-2022 Published: 01-Aug-2022, DOI: 10.31858/0975-8453.13.8.531-538
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3