Research Article - (2023) Volume 14, Issue 4
Abstract
Background: Dopamine 2 receptor agonists, Bromocriptine and Cabergoline, were originally introduced for prolactinomas and pituitary tumors but have glucose-lowering effects. This paper studied the significance of their effects on lowering blood glucose level and conducted a comprehensive analysis to identify relevant clinical trials of dopamine 2 agonists on Glycated Hemoglobin (HbA1c) and Fasting Blood Sugar (FBS).
Methods: We conducted a study using different databases; PubMed, Google Scholar, Cochrane Library, HINARI, Registers, and Citations until December 31, 2022 using the PRISMA 2020 statement, looking for studies relevant to clinical studies on FBS and HbA1c. Jadad score were used to assess the study quality. The study included studies with full abstracts, predefined garlic doses, clear interventions, and blood glucose measurements.
Results: Data were synthesized from 23 clinical studies that recruited 6125 study subjects. The pooled effect analysis of the trials revealed that dopamine 2 agonists improve glycated hemoglobin (HbA1c) (SMD=-1.26; 95% CI (-1.60,-0.93), p<0.00001), and FBS (SMD=-1.84; 95% Confidence Limit (CI) (-2.61,-1.07), p<0.00001). Each drug’s pooled effect analysis indicates bromocriptine significantly improved HbA1c (SMD=-1.25; 95% CI (-1.64,-0.87), p<0.00001) and FBS (SMD=-1.90; 95% CI (-2.79,-1.01), p<0.00001) and similarly, cabergoline significantly improved HbA1c (SMD =-1.29; 95% CI (-1.96, -0.62), p<0.00001) and FBS (SMD=-1.62; 95% CI (-2.82,-0.41), p<0.00001). The data presented above demonstrated that dopamine 2 agonists have a significant ability to lower blood sugar levels in clinical studies
Conclusion: The study shows that dopamine 2 agonists have significantly reduced glycated hemoglobin and fasting blood sugar levels without major side effects. Although there are encouraging results, more data is required to determine the best anti-hyperglycemic dose and frequency of daily use, as well as side effects and possible product interactions when using dopamine 2 receptor agonists for their anti-hyperglycemic benefits.
Keywords
Bromocriptine, Cabergoline, FBS, HbA1c, Dopamine 2 agonist, Meta-analysis
Introduction
Diabetes Mellitus (DM) is a condition where blood glucose levels are not properly controlled. Hyperglycemia is a common symptom of a set of metabolic illnesses that are caused by flaws in insulin action, secretion, or both (Care D, 2022). Uncontrolled diabetes frequently results in chronic hyperglycemia, which is linked to long-term harm, dysfunction, and failure of different organs, particularly the eyes, kidneys, nerves, heart, and blood vessels (Iatcu CO, et al., 2021). Serious problems result from improper treatment, which lowers patients’ quality of life and increases the expense of their care (Molinaro R and Dauscher C, 2017). According to the IDF, there are currently 537 million diabetics worldwide between the ages of 20 and 79, with that figure expected to rise to 643 million by 2030 and 783 million by 2045 (Federation ID, 2013).
The most frequent causes of increasing diabetic cases are an increase in sedentary behavior, consumption of foods high in calories, obesity, and a longer life expectancy (Care D, 2022). The percentage of patients with DM who have seen a physician is sharply rising (Lucier J, Weinstock RS, 2023; Ingle PV, et al., 2018). Numerous complications are caused by hyperglycemia, including diabetic retinopathy, diabetic nephropathy, atherosclerosis, hypercoagulability, coronary heart disease, abdominal obesity, hypertension, hyperlipidemia, cerebrovascular disease, coronary artery disease, foot damage, skin complications, alzheimer’s disease, hearing loss, and depression (Kumar S, et al., 2017). Diabetes is a more severe illness than other diseases because of these potentially fatal complications. Though several synthetic medications have been created, none of the compounds have yet to offer a full recovery. Because certain synthetic substances have serious negative effects when used continuously, there is still a need for accessible, non-toxic medications (Padhi S, et al., 2020).
Bromocriptine and cabergoline are dopamine D2 receptor agonists originally introduced for prolactinomas and pituitary tumors. However, in 2009, the Food and Drug Administration (FDA) approved bromocriptine as a treatment for Type 2 Diabetes (T2D) and as a glucose-lowering drug (Lamos EM, et al., 2016; Mahajan R, 2009). The mechanism of action is complex but partly results from the suppression of monoamines and partly from the suppression of prolactin (Vicchi FL, et al., 2016). Bromocriptine suppresses the sympathetic nervous system and lowers noradrenaline and serotonin levels, which inhibits hepatic glucose production, slows adipose tissue breakdown, and improves insulin sensitivity (Vicchi FL, et al., 2016; deFronzo RA, 2011; Luo S, et al., 1998). A recent systematic analysis of observational studies indicated that dopamine receptor agonist treatment in individuals with prolactinomas improved metabolic variables. Dopamine agonists suppress prolactin release from lactotropic cells in the pituitary (Byberg S, et al., 2019). It has been demonstrated that using bromocriptine and metformin together has a much larger impact on improving HbA1c than using either medication alone (Schwartz SS and Zangeneh F, 2016). However, neither the lipid profile nor postprandial hyperglycemia was affected by bromocriptine administration (Liang W, et al., 2015).
Dopamine-agonist therapy as a treatment for type 2 diabetes mellitus has received a lot of attention. For people with T2DM and HbA1c readings higher than 7.5%, bromocriptine-QR is a successful add-on medication. If bromocriptine-QR is tolerated by the patient, there may be slight improvements in postprandial hyperglycemia and cardiometabolic endpoints, which could reduce the risk of serious adverse cardiovascular events (MACE) (Lamos EM, et al., 2016). The Cycloset Safety Trial, a significant randomized placebo-controlled trial assessing the efficacy and safety of bromocriptine on T2D, found a 48 percent reduction in the likelihood of a composite cardiovascular endpoint problems like myocardial infarction, stroke, coronary revascularization, or hospitalization for angina or congestive heart failure (Chamarthi B, et al., 2015). The most frequent side effects following bromocriptine therapy were nausea, vomiting, and dizziness (Chamarthi B, et al., 2015).
Previous studies didn’t evaluate cabergoline as an antihyperglycemic drug while evaluating dopamine agonists, except for one study that compared only three studies. Furthermore, the determination of internal and external validity has not yet been evaluated because no prior evaluations have used a bias assessment of the trials that were included or quantified the potential risk of random error (Liang W, et al., 2015; Andersen IB, et al., 2021). Furthermore, the published studies have limitation of not including all findings and additionally since the last study was published, new investigations have been done. This manuscript will explore the effects of dopamine 2 agonists as a diabetes therapeutic agent in clinical investigations when compared to a placebo or control group in order to reach comprehensive conclusions.
Materials and Methods
Search design
The present study is done by considering dopamine 2 agonists for the management of type 2 diabetes, conducted on English language articles published until December 31, 2022. This study was conducted using database searches, and the reporting adhered to the preferred reporting items (Muka T, et al., 2020; Siddaway AP, et al., 2019).
Search strategy
From conception through December 31, 2022, databases such as PubMed/ MEDLINE, Cochrane Library, and Google Scholar were evaluated. Additional studies were found by searching the website and the reference lists of all listed papers. To summarize the number of papers identified, screened, excluded, and finally included in the study, a PRISMA 2020 flow diagram was employed. The key words used in the search include: (diabetes mellitus OR diabetes mellitus type 2 OR T2DM OR diabetes type 2 OR diabetes mellitus type 2) AND (bromocriptine OR bromocriptine-QR OR dopamine agonists OR bromocriptine OR dopamine receptor agonist OR parlodel OR cabergoline OR dostinex OR bromocriptin* OR cabergolin*).
Study selection and data extraction
The study, examined relevant studies, and sequentially screened their titles and abstracts for eligibility. The full texts of potentially eligible studies were retrieved. To ensure the reliability of the selection criteria, a screening guide was used. Studies conducted to examine dopamine 2 agonists for the management of type 2 diabetes were included. Data extraction was performed in a pre-designed format for simplicity and better evidence management. The extracted data consists of author, study model, effects on blood glucose levels, sample number, and age and sex of study participants.
Data synthesis and analysis
The Standardized Mean Difference (SMD) was determined for outcomes that were continuous. Hence, SMD is the pooled standard deviation divided by the mean outcome difference between the intervention group and the control group (SD). The outcome is a unit-free effect size, with SMDs of 0.2, 0.5, and 0.8, respectively, being categorized as small, medium, and high effect sizes. The difference from the baseline was utilized to calculate the impact size in cases where the absolute values were not reported post-intervention. When a trial provided results at various time points, the observation with the longest follow-up was taken into account. When a trial included more than one intervention arm, the data were combined to boost the trial’s power. Due to the anticipated heterogeneity, effect estimates from the included trials were pooled using a random effect model. P-values less than 0.05 were regarded as significant for results in the primary analysis, which was conducted using RevMan 5.4 (Schmidt L, et al., 2019).
The I2, which measures the amount of heterogeneity not explained by stochastic fluctuation, was used to quantify heterogeneity (Migliavaca CB, et al., 2022). A funnel plot was used to evaluate the publication bias (Aisbett J, et al., 2023). The observed SD, a mean difference of the observed SD/2, an alpha of 2.5% for primary outcomes, an alpha of 5% for secondary and exploratory outcomes, and a beta of 10% for continuous outcomes were utilized for continuous outcomes in the trial sequential analysis. Each of the predetermined outcomes was used to construct a table with the summary findings (HbA1c and fasting blood sugar). For the outcomes, imprecision was evaluated using trial sequence analysis, and recommendations from the Cochrane Handbook (Higgins JP and Altman DG, 2008).
Subgroup analysis
The test for subgroup interactions in review manager was used to conduct subgroup analysis for the key outcomes (Cochrane Collaboration, 2020). Trials with a low risk of bias were contrasted with those with a high risk. In addition, factors such as the length of the intervention, the type of drug, the dosage of the drug, and HbA1c or FBS baselines were considered as potential explanations for between-trial heterogeneity. A high dose of a drug was defined as more than 2.5 mg of bromocriptine or 0.5 mg of cabergoline (Liang W, et al., 2015; Andersen IB, et al., 2021). The intervention lasted an average of 12 weeks, which was used as the mean duration, with an HbA1c of 8% and a FBS of 126 mg/dL used in the assessment. The analysis included a random effect meta-analysis with SMD (95% CI, and I2) and p-value for subgroup explaining heterogeneity (Migliavaca CB, et al., 2022; Aisbett J, et al., 2023).
Inclusion criteria
Studies having a particular measurement approach and predetermined doses of the dopamine 2 agonist, whether bromocriptine or cabergoline, utilized in the investigation are more likely to pass the inclusion requirements. Articles with treatment interventions and the original research articles were included.
Exclusion criteria
Studies without full abstracts, predefined dopamine 2 receptor agonist doses, and blood glucose measurements were disregarded. Additionally, studies in which no intervention was performed, studies with no control group, review articles, commentaries, communications or correspondences, and short communications were excluded.
Results
Characteristics of included studies
A total of 1,293 study articles were found through the electronic database, registers, and other methods of search, which were updated and done by using ShinyApp for making PRISMA 2020 flow diagrams (Haddaway NR, et al., 2022; Page MJ, et al., 2021). By deleting duplicates and unconnected entries manually and automatically by the PRISMA 2020 online application, the total number of articles was reduced to 346; after thorough abstract and title screening, 113 papers remained. Following additional full- text screening and the exclusion of 82 articles, a total of 23 articles were included in the study, with the addition of 10 previous studies.
The reasons for exclusion for both databases, registries, and other methods of data retrieval were listed accordingly. Four publications were disqualified for failing to disclose doses; nine for lacking fasting blood glucose and HbA1c readings; five for failing to indicate interventions; eight for lacking a control group; three for being only short reports; and four for having only abstracts. As a result, this paper included 10 articles from previous studies, 9 articles from database searches, and 4 articles from websites and citations; in total, 23 clinical trials are included (Figure 1).
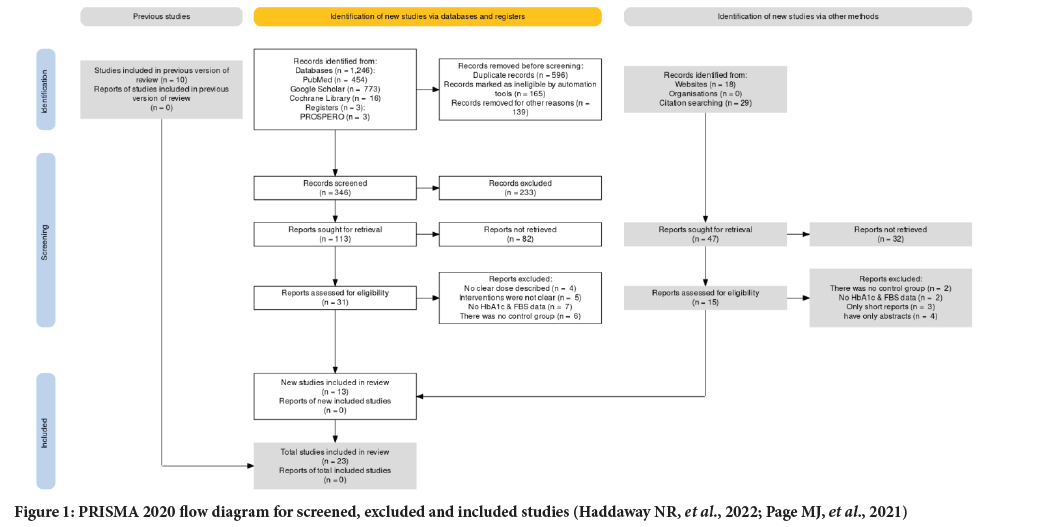
Figure 1: PRISMA 2020 flow diagram for screened, excluded and included studies (Haddaway NR, et al., 2022; Page MJ, et al., 2021)
Quality of the studies
All clinical trial articles were independently assessed for their methodological quality by using the Jadad quality rating system. The study qualities of the included trials were diverse, as eleven trials were classified as high quality with a Jadad score ≥ 4, and thirteen trials were classified as low quality with a Jadad score of 3 or 2. Allocation concealment was clearly adequate in fourteen studies. No clinical trials reported the generation of random numbers. Randomization, dropouts, and free selective reporting were all reported in all clinical trials (Percie du Sert N, et al., 2020; Kilkenny C, et al., 2010) (Tables 1 and 2).
| Subgroups | Trials, n (No. of participants) | SMD (95% CI, P, I2), random | Heterogeneity (p-value) |
|---|---|---|---|
| Risk of bias | |||
| Lesser risk of bias | 11; 2114 | -0.38 (-0.71 to -0.06; p=0.03; I2=4%) | 0.14 |
| Higher risk of bias | 12; 4011 | -0.86 (-1.54 to -0.18; p=0.008; I2=81%) | |
| Dosage range | |||
| Low dose | 13; 3628 | -0.77 (-1.23 to -0.32; p=0.0004; I2=67%) | 0.005 |
| High dose | 10; 2497 | -0.14 (-0.21 to -0.06; p<0.0001; I2=3%) | |
| Duration of intervention | |||
| ≤ 12 weeks | 14; 591 | -0.12 (-0.19 to -0.06; p<0.0001; I2=2%) | 0.006 |
| >12 weeks | 9; 5534 | -0.79 (-1.22 to -0.31; p=0.0006; I2=78%) | |
| Baseline HbA1c | |||
| <8% | 12; 5221 | -0.37 (-1.01 to 0.09; p=0.11; I2=80%) | 0.27 |
| ≥ 8% | 11; 711 | -0.81 (-1.28 to -0.26; p=0.001; I2=62%) | |
| Baseline FBS | |||
| <126 mg/dl | 9; 2129 | -0.43 (-1.12 to 0.13; p=0.11; I2=74%) | 0.32 |
| ≥ 126 mg/dl | 14; 3996 | -0.89 (-1.33 to -0.21; p=0.001; I2=58%) | |
Table 1: Heterogeneity of effect estimates for trials assessing the effect of dopamine 2 agonists on HbA1c and FBS in patients with type 2 diabetes explored by comparing subgroups.
| Allocation concealment | Blinding | Randomization | Withdraw, dropouts | Free selective reporting | Random number generation | Jadad score | Studies |
|---|---|---|---|---|---|---|---|
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Aliasgarzadeh A, et al., 2020) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Bahar A, et al., 2016) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Barnett AH, et al., 1980) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Chamarthi B, et al., 2016) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Ghosh A, et al., 2014) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Kok P, et al., 2006) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Krysiak R and Okopien B, 2015) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Meier AH, et al., 1992) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Mejía-Rodríguez O, et al., 2013) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Pijl H, et al., 2000) |
| Yes | Yes | Yes | Yes | Yes | Not clear | 4 | (Taghavi SM, et al., 2012) |
| Not clear | Yes | Yes | Yes | Yes | Not clear | 3 | (Aminorroaya A, et al., 2004) |
| Not clear | Yes | Yes | Yes | Yes | Not clear | 3 | (Assad HC, et al., 2014) |
| No | Yes | Yes | Yes | Yes | Not clear | 3 | (Chamarthi B and Cincotta AH, 2017) |
| Not clear | Yes | Yes | Yes | Yes | Not clear | 3 | (Cincotta AH and Meier AH, 1996) |
| No | Yes | Yes | Yes | Yes | Not clear | 3 | (Gaziano JM, et al., 2010) |
| Yes | No | Yes | Yes | Yes | Not clear | 3 | (Kamath V, et al., 1997) |
| No | Yes | Yes | Yes | Yes | Not clear | 3 | (Khalilzade SH, et al., 2015) |
| Yes | No | Yes | Yes | Yes | Not clear | 3 | (Morcos JA, et al., 2017) |
| Yes | No | Yes | Yes | Yes | Not clear | 3 | (Ramteke KB, et al., 2011) |
| Not clear | No | Yes | Yes | Yes | Not clear | 2 | (Roe ED, et al., 2015) |
| Not clear | Yes | Yes | Yes | Yes | Not clear | 3 | (Tell SS, et al., 2022) |
| Not clear | Yes | Yes | Yes | Yes | Not clear | 3 | (Vinik AI, et al., 2012) |
Table 2: Quality analysis of included clinical trails.
Heterogeneity and risk of bias assessment
In this study both HbA1c and FBS pooled effect analysis, funnel plot effects were estimated from individual studies were indicated to assess the potential role of publication bias and to visualize the investigated publication bias (Figures 2 and 3). The risk of bias was assessed for all twenty-three trials, twelve of which were at high risk of bias (Aliasgarzadeh A, et al., 2020; Bahar A, et al., 2016; Barnett AH, et al., 1980; Chamarthi B et al., 2016; Ghosh A, et al., 2014; Kok P, et al., 2006; Krysiak R and Okopien B, 2015; Meier AH, et al., 1992; Mejía-Rodríguez O, et al., 2013; Pijl H, et al., 2000; Taghavi SM, et al., 2012) and the other eleven of which were judged to have “some concerns” or a “low” risk of bias. The baseline for HbA1c and FBS, duration of intervention, and dosage level were assessed by including the heterogeneity test of the meta-analysis in RevMan version 5.4. (Andersen IB, et al., 2021; Winzap P, et al., 2019) (Table 1).
Figure 2: Funnel plot for clinical studies with pseudo 95% CI that indicate the graphical representation of the size of experiments plotted
against the effect size for HbA1c
Note: ( ): Subgroup-bromocriptine; (
): Subgroup-bromocriptine; ( ): Subgroup-cabergoline
): Subgroup-cabergoline
Figure 3: Funnel plot for clinical studies with pseudo 95% CI that indicate graphical representation of the size of trials plotted against the effect
size for FBS
Note: ( ): Subgroup-bromocriptine; (
): Subgroup-bromocriptine; ( ): Subgroup-cabergoline
): Subgroup-cabergoline
Primary outcomes
A total of 23 clinical trials recruiting 6125 subjects reported data on HbA1c and FBS concentrations, of which 5932 subjects were recruited for bromocriptine trials and 193 subjects were recruited for cabergoline trials. The duration of the 23 clinical studies on diabetic patients ranges from 7 days to 52 weeks, with various dose and preparation levels. For the effects of both dopamine 2 agonist drugs on blood sugar levels, a minimum dose of 0.8 mg per day and a maximum dose of 8.8 mg per day were utilized for bromocriptine, while a minimum dose of 0.25 mg per day and a maximum of 0.5 mg per day were utilized for cabergoline (Table 3).
| Intervention given | Control/Placebo | Intervention duration | References |
|---|---|---|---|
| CAB 0.25 mg-0.5 mg/day | Placebo | 12 weeks | (Aliasgarzadeh A, et al., 2020) |
| CAB 0.5 mg/day+OAD | Placebo+OAD | 12 weeks | (Bahar A, et al., 2016) |
| BRC 2.5 mg single dose | Placebo | 7 days | (Barnett AH, et al., 1980) |
| BRC QR 1.6-4.8 mg/day+Metformin 500 mg BID | Metformin 500 mg BID+Placebo | 52 weeks | (Chamarthi B, et al., 2016) |
| BRC 0.8 mg/1.6 mg+Metformin 500 mg BID | Metformin 500 mg BID | 12 weeks | (Ghosh A, et al., 2014) |
| BBC 2.5 mg/day | Placebo+diet | 4 weeks | (Kok P, et al., 2006) |
| BRC QR 1.25-8.8 mg/day+CAB 0.25-1.25 mg/day | Placebo+diet | 24 weeks | (Krysiak R and Okopien B, 2015) |
| BRC 1.5 mg 2.5 mg/day | Placebo | 8 weeks | (Meier AH, et al., 1992) |
| BRC 2.5 mg 7.5 mg/day | Placebo | 24 weeks | (Mejía-Rodríguez O, et al., 2013) |
| BRC 0.8-4.8 mg/day+diet | Placebo+diet | 16 weeks | (Pijl H, et al., 2000) |
| CAB 0.5 mg/day | Placebo | 12 weeks | (Taghavi SM, et al., 2012) |
| BRC-QR 1.25-2.5 mg/day+OAD | Placebo+OAD | 12 weeks | (Aminorroaya A, et al., 2004) |
| CAB 0.25 mg+Metformin 500 mg BID | Metformin 500 mg BID | 12 weeks | (Assad HC, et al., 2014) |
| BRC QR 2.5 mg/day+Metformin 500 mg BID | Metformin 500 mg BID | 12 weeks | (Chamarthi B and Cincotta AH, 2017) |
| BRC QR 1.6-2.4 mg/day | Placebo+diet | 18 weeks | (Cincotta AH and Meier AH, 1996) |
| BRC-QR 0.8-4.8 mg/day+diet/OAD/insulin | Placebo+diet/OAD/insulin | 52 weeks | (Gaziano JM, et al., 2010) |
| BRC 2.4 mg-3.4 mg/day | Placebo+diet | 10 weeks | (Kamath V, et al., 1997) |
| BRC 2.5 mg/day | Placebo | 12 weeks | (Khalilzade SH, et al., 2015) |
| CAB 0.25 mg × 2 weekly+Gliclazide 60-120 mg/daily | Placebo+OAD | 16 weeks | (Morcos JA, et al., 2017) |
| BRC-QR 1.6 mg/2.4 mg+Metformin 500 mg BID | Metformin 500 mg BID | 12 weeks | (Ramteke KB, et al., 2011) |
| BRC QR 1.6-4.8 mg/day | Metformin 500 mg BID | 24 weeks | (Roe ED, et al., 2015) |
| BRC QR 0.8 mg-1.6 mg to 3.2 mg/day | Placebo | 4 weeks | (Tell SS, et al., 2022) |
| BRC QR 1.6 to 4.8 mg/day | Placebo+OAD | 24 weeks | (Vinik AI, et al., 2012) |
Table 3: Summary of interventions, control and duration of the studies
The number of randomized participants in the studies ranged from 13 to 3070. The mean age was 50.92 years; 56.38% were male while 43.62% were female; the mean duration of diabetes was 7.2 years; and the mean percentage of participants on insulin treatment was 19.4%. There was no statistically significant difference in the risk of major adverse events between the two trials (n=3123), reporting 181 (8.75%) incidents in the intervention group and 101 (9.57%) occurrences in the control group (RR=0.73; 95% CI=0.66; 1.04; p=0.221) (Table 4). The fixed effect analysis showed a reduction in HbA1c of 0.55, SMD (95% CI (-0.60, -0.49), p<0.00001; I2=95%) compared with the placebo and FBS reduction of 1.52, SMD (95% CI (-1.58, -1.45), p<0.00001; I2=99%).
| Variables | Trials, n (No. of participants) | Pooled effect (95% CI) | p-value | I2 | SMD in original units |
|---|---|---|---|---|---|
| HbA1c, SMD | 23; 6125 | -1.26 (-1.60; -0.93) | <0.00001 | 95% | -1.42% |
| FBS, SMD | 23; 6125 | -1.84 (-2.61; -1.07) | <0.00001 | 99% | -37.23 mg/dl |
| Serious adverse effects, RR | 2; 3123 | 0.73 (0.66; 1.04) | 0.221 | - | - |
| Adverse events, RR | 17; 2944 | 1.98 (0.71; 5.23) | 0.182 | 31% | - |
Table 4: Results for primary and secondary outcomes
Subgroup analysis
The pooled estimate on HbA1c was associated with considerable heterogeneity (I2=95%). The size of the effect was inversely correlated with the duration of the intervention as well as with the dosage of dopamine 2 agonists. The heterogeneity was not explained by the type of dopamine 2 agonist, the baseline HbA1c, the baseline FBS, or the risk of bias in the included trials. Furthermore, the I2 values for both the HbA1c and FBS pooled analyses show a high degree of heterogeneity among the studies (Table 1). The individual effect analysis for each drug shows they significantly improved blood glucose level. For HbA1c level; bromocriptine with (SMD=- 1.25; 95% CI (-1.64, -0.87), p<0.00001) and (SMD=-1.29; 95% CI (-1.96, -0.62), p<0.00001) as well as for FBS level; bromocriptine with (SMD=- 1.90; 95% CI (-2.79, -1.01), p<0.00001) and cabergoline with (SMD=-1.62; 95% CI (-2.82, -0.41), p<0.00001) (Figures 4 and 5).
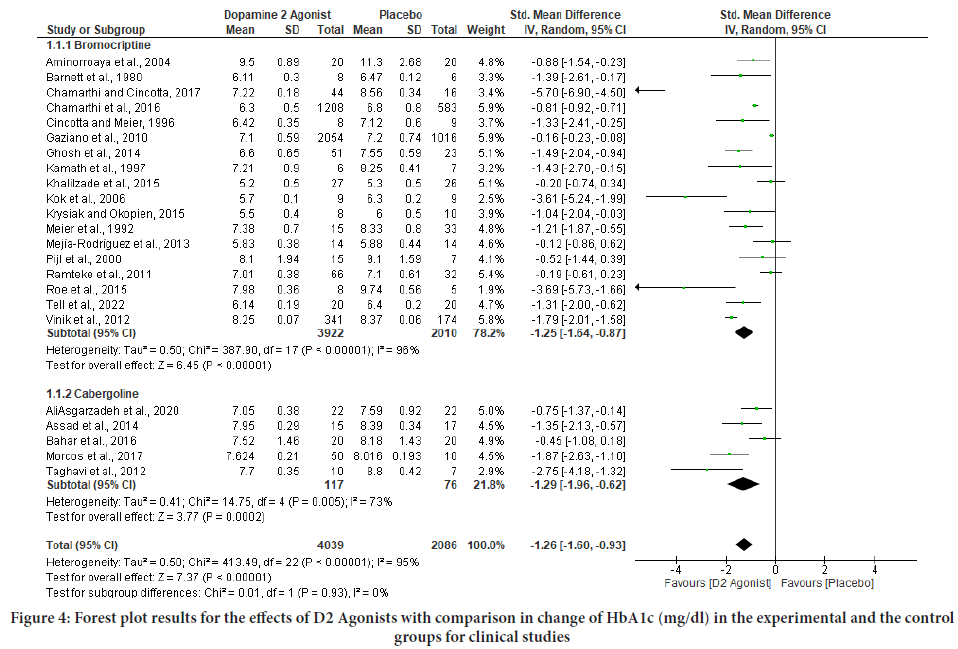
Figure 4: Forest plot results for the effects of D2 Agonists with comparison in change of HbA1c (mg/dl) in the experimental and the control groups for clinical studies
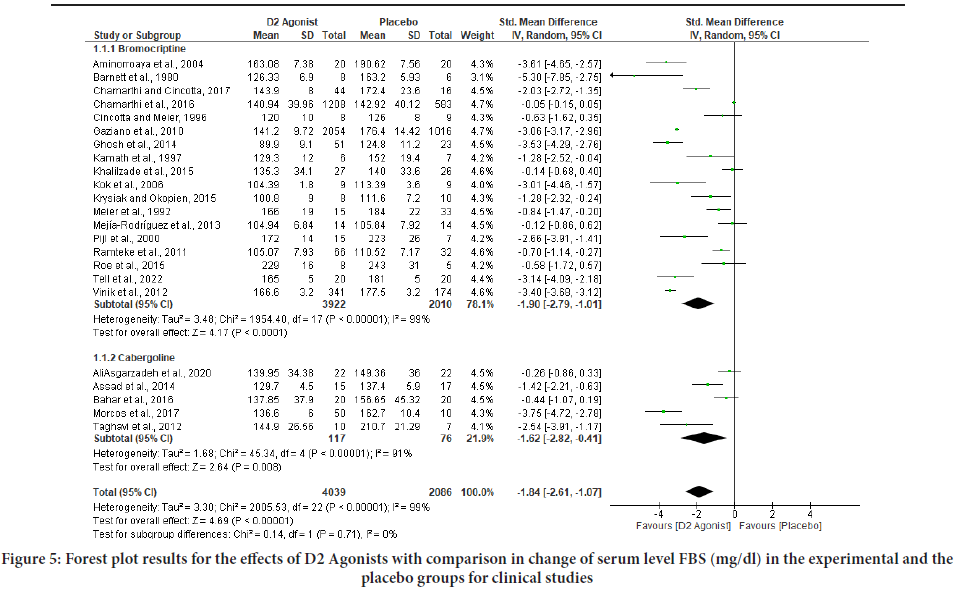
Figure 5: Forest plot results for the effects of D2 Agonists with comparison in change of serum level FBS (mg/dl) in the experimental and the placebo groups for clinical studies
Secondary outcomes
The level of heterogeneity during secondary outcome analysis among pooled studies was moderate to high when sensitivity analyses were made by eliminating outlier trials. As a result, the pooling technique was based on the random effect model. Dopamine 2 agonists (Bromocriptine and Cabergoline) both had a significant effect on the reduction of both HbA1c (SMD=-1.26; 95% CI (-1.60, -0.93), p<0.00001) and fasting blood sugar (SMD=-1.84; 95% CI (-2.61, -1.07), p<0.00001) compared with placebo. Long-term dopamine 2 agonist intervention studies revealed more pronounced benefits of the drugs on fasting blood sugar levels.
Discussion
The present study examined clinical trials that were published with dopamine 2 agonist effects on fasting blood sugar and HbA1c levels. Twenty- three Randomized Controlled Trials (RCTs) allocating 6125 study participants diagnosed with type 2 diabetes to a dopamine 2 receptor agonist or placebo were included. The findings imply that dopamine 2 agonists have a comparably better effect size on HbA1c and a large effect size on fasting blood sugar without any significant negative effects. The I2 for HbA1c was 95%, suggesting considerable heterogeneity. Part of the heterogeneity was explained by an inverse relationship between dosage and effect estimates and an inverse relationship between duration of the intervention and effect estimates. The current study was unable to determine to what extent these variables independently explain the heterogeneity because ten studies were included in the subgroup of high dose, nine studies were included in the subgroup of long duration of intervention, thirteen studies were included in the subgroup of low dose, and fourteen studies were included in the subgroup of short duration of intervention.
It is noteworthy that both the dosage and the length of the intervention are inversely correlated with the effect estimates. The lack of an additional intervention impact for HbA1c longer than three months may be explained by the fact that antidiabetic medicine only has a full effect on HbA1c after 12 weeks of starting, at which point HbA1c stabilizes (Berard LD, et al., 2018). Other than the aforementioned overlap between the categories, this study didn’t identify any other reasonable explanation for the somewhat lesser effect among patients receiving high doses of the drug. Although some prior studies, such as those by Chamarthi and Cincotta and Liang et al., indicated a higher effect in patients with poor glycemic control (high HbA1c at baseline) compared to those whose diabetes is well controlled (Liang W, et al., 2015; Andersen IB, et al., 2021; Chamarthi B and Cincotta AH, 2017). This study discovered that the heterogeneity was not explained by the HbA1c level at baseline, which is similar to prior study findings.
The heterogeneity was neither explained by the risk of bias nor the type of dopamine agonist. Prior studies, such as those by Dos Santos Nunes et al. (2011), indicated that cabergoline is less expensive and known to have fewer adverse events than bromocriptine as an antihyperglycemic agent, and cabergoline is the first choice in the treatment of hyperprolactinemia (dos Santos Nunes V, et al., 2011; Melmed S, et al., 2011). Other studies also show that bromocriptine-QR formulations have the benefits of a low tendency for hypoglycemia, a neutral effect on body weight, reassuring short- term cardiovascular safety (up to one year), and the ability to be used alone or in conjunction with other anti-diabetic medications with comparable efficacy. But a small decrease in HbA1c levels, a lack of efficacy data beyond 24 weeks, a high incidence of nausea, a high pill burden, and a high price are some of the shortcomings that have been identified (Andersen IB, et al., 2021, Gaziano JM, et al., 2010; Mikhail N, et al., 2011). Despite the fact that dopamine agonists have a moderate effect on HbA1c reduction, the observed heterogeneity needs to be explained.
Furthermore, the included trials were all judged to have “some concerns” of bias or a high risk of bias; as a result, this study has little confidence in the effect estimate due to unexplained heterogeneity and the risk of bias. In comparison to previous studies by Liang W, et al., 2015 and Andersen IB, et al., 2021 this study found that bromocriptine reduced HbA1c and fasting blood sugar. Liang W, et al., 2015 found a significant difference in HbA1c decline from baseline favoring quick-release bromocriptine over placebo with a weighted mean difference of -117.36 mg/dl (95% CI=-145.26 to -89.46 mg/dl), while Andersen et al. found a similar effect on cabergoline with a standardized mean difference of -118.53 mg/dl (95% CI=-151.42 to -89.46 mg/dl). This study included more articles and participants from newly published studies as well as articles that were not included in previous studies, and it was discovered that the effects of cabergoline were mostly comparable with a standardized mean difference of -120.81 mg/dl (95% CI=-159.70 to -102.63 mg/dl) (Table 5).
| Study design | Total (N) | Experimental (N) | Control (N) | Male (N) | Female (N) | Mean age | References |
|---|---|---|---|---|---|---|---|
| Double blind | 44 | 22 | 22 | 26 | 18 | 52 ± 7.4 | (Aliasgarzadeh A, et al., 2020) |
| Double blind | 40 | 20 | 20 | 8 | 32 | 53.9 ± 7.4 | (Bahar A, et al., 2016) |
| Single blind | 14 | 8 | 6 | 6 | 8 | 42 ± 11.8 | (Barnett AH, et al., 1980) |
| Double blind | 1791 | 1208 | 583 | 1048 | 743 | 59.65 ± 9.8 | (Chamarthi B, et al., 2016) |
| Double blind | 74 | 51 | 23 | NA | NA | 50 ± 14.3 | (Ghosh A, et al., 2014) |
| Single blind | 18 | 9 | 9 | 18 | 0 | 37.5 ± 1.7 | (Kok P, et al., 2006) |
| Double blind | 18 | 8 | 10 | NA | NA | 34 ± 5.5 | (Krysiak R and Okopien B, 2015) |
| Single blind | 48 | 15 | 33 | 48 | 0 | NA | (Meier AH, et al., 1992) |
| Double blind | 28 | 14 | 14 | 12 | 16 | 61.1 ± 8.3 | (Mejía-Rodríguez O, et al., 2013) |
| Double blind | 22 | 15 | 7 | 8 | 14 | 54 ± 2.3 | (Pijl H, et al., 2000) |
| Double blind | 17 | 10 | 7 | 6 | 13 | 52.7 ± 7.2 | (Taghavi SM, et al., 2012) |
| Double blind | 40 | 20 | 20 | 6 | 34 | 51.5 ± 2.1 | (Aminorroaya A, et al., 2004) |
| Single blind | 32 | 15 | 17 | 11 | 21 | 45.82 ± 2.65 | (Assad HC, et al., 2014) |
| Double blind | 60 | 44 | 16 | 50 | 10 | 58.5 ± 2.5 | (Chamarthi B and Cincotta AH, 2017) |
| Double blind | 17 | 8 | 9 | 10 | 7 | 47.5 ± 0.4 | (Cincotta AH and Meier AH, 1996) |
| Double blind | 3070 | 2054 | 1016 | 1739 | 1331 | 59.7 ± 10.1 | (Gaziano JM, et al., 2010) |
| Open label | 13 | 6 | 7 | 13 | 0 | 51 ± 3 | (Kamath V, et al., 1997) |
| Double blind | 53 | 27 | 26 | 14 | 39 | 48.15 ± 5.7 | (Khalilzade SH, et al., 2015) |
| Open label | 60 | 50 | 10 | 17 | 43 | 49.4 ± 2.72 | (Morcos JA, et al., 2017) |
| Open label | 98 | 66 | 32 | NA | NA | NA | (Ramteke KB, et al., 2011) |
| Open label | 13 | 8 | 5 | 4 | 9 | 50 ± 3 | (Roe ED, et al., 2015) |
| Double blind | 40 | 20 | 20 | 17 | 23 | 52.4 ± 4.3 | (Tell SS, et al., 2022) |
| Double blind | 515 | 341 | 174 | 297 | 218 | 58.5 ± 0.6 | (Vinik AI, et al., 2012) |
Table 5: Summary of design and number participants in the clinical studies
The fact that this study is based on a technique that has been published and used a thorough search approach is its strength. In this study, the potential for random error was examined and evaluated the risk of bias in the included trials. Furthermore, the Jadad or Oxford quality scoring systems were used to independently assess the methodological quality of a clinical trial. The included clinical trials were either at a high risk of bias or somewhat concerning because of the randomization procedure; all of the trials were determined to have a risk of bias (Table 1). It is debatable whether increasing effect estimates would result from only including studies with a minimal risk of bias. Although there was a trend toward a lesser benefit in studies with a reduced risk of bias, a subgroup analysis that compared the effect on HbA1c across trials with a high risk of bias and trials with a lower risk of bias found no significant differences. Furthermore, all clinical studies should collect and report data with greater certainty on safety outcomes such as serious adverse events, all-cause mortality, diabetic ketoacidosis, and hypoglycemia, as well as the quality of life in type 2 diabetes patients. The evidence at hand, according to this study, points to the possibility that treating type 2 diabetes patients with dopamine 2 receptor agonists could lower HbA1c and fasting blood sugar without having any life-threatening side effects.
Conclusion
Dopamine 2 agonists lower fasting blood glucose and HbA1c in all trials included in the study. Standard diabetes treatments can be used as antihyperglycemic, but some diabetic individuals are unable to use them due to their negative side effects. Therefore, cabergoline and bromocriptine use may be an advantageous alternative for those with slight elevations in serum glucose who cannot handle standard medications. Despite encouraging results, it has been underlined that further clinical trials, homogeneity in the approaches used, the number of participants, and the length of the intervention are still necessary to get reliable data.
Strength and Limitation of the Study
There are some strengths of the studies observed through the study-they provide the optimal means of minimizing the effect of confounding, some of them somewhat reduce bias in allocation to exposure groups; and most of them use double-blind randomized clinical trials, which is the best design for detecting small to moderate effects that may be clinically important. Because of the intervention approach, which included few patients with the implementation of trials that would not indicate the real life unlike with a long duration of follow-up, some trials did not fully provide answers to the questions raised by the investigators.
References
- Care D. Diabetes: Standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(1): S113-S24.
[Crossref] [Google Scholar] [Pubmed]
- Iatcu CO, Steen A, Covasa M. Gut microbiota and complications of type-2 diabetes. Nutrients. 2021; 14(1): 166.
[Crossref] [Google Scholar] [Pubmed]
- Molinaro R, Dauscher C. Complications resulting from uncontrolled diabetes. MLO Med Lab Obs. 2017; 49(2): 20-22.
[Google Scholar] [Pubmed]
- Federation ID. Five questions on the IDF diabetes atlas. Diabetes Res Clin Pract. 2013; 102(2): 147-148.
[Crossref] [Google Scholar] [Pubmed]
- Lucier J, Weinstock RS. Diabetes mellitus type 1. Statpearls. 2023.
- Ingle PV, Yin SB, Ying BJ, Leong BK, Xin TZ, Hwa LT, et al. Current trends in pharmacological treatment of type ii diabetes mellitus. Int J Pharm Res Rev. 2018; 7: 1-5.
- Kumar S, Mittal A, Babu D, Mittal A. Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev. 2021; 17(4): 437-456.
[Crossref] [Google Scholar] [Pubmed]
- Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed Pharmacother. 2020; 131: 110708.
[Crossref] [Google Scholar] [Pubmed]
- Lamos EM, Levitt DL, Munir KM. A review of dopamine agonist therapy in type 2 diabetes and effects on cardio-metabolic parameters. Prim Care Diabetes. 2016; 10(1): 60-65.
[Crossref] [Google Scholar] [Pubmed]
- Mahajan R. Bromocriptine mesylate: FDA-approved novel treatment for type-2 diabetes. Indian J Pharmacol. 2009; 41(4): 197.
[Crossref] [Google Scholar] [Pubmed]
- Vicchi FL, Luque GM, Brie B, Nogueira JP, Tornadu IG, Becu-Villalobos D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res. 2016; 109: 74-80.
[Crossref] [Google Scholar] [Pubmed]
- deFronzo RA. Bromocriptine: A sympatholytic, D2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011; 34(4): 789-794.
[Crossref] [Google Scholar] [Pubmed]
- Luo S, Meier AH, Cincotta AH. Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology. 1998; 68(1): 1-0.
[Crossref] [Google Scholar] [Pubmed]
- Byberg S, Futtrup J, Andreassen M, Krogh J. Metabolic effects of dopamine agonists in patients with prolactinomas: A systematic review and meta-analysis. Endocr Connect. 2019; 8(10): 1395.
[Crossref] [Google Scholar] [Pubmed]
- Schwartz SS, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med. 2016; 128(8): 828-838.
[Crossref] [Google Scholar] [Pubmed]
- Liang W, Gao L, Li N, Wang B, Wang L, Wang Y, et al. Efficacy and safety of bromocriptine-QR in type 2 diabetes: A systematic review and meta-analysis. Horm Metab Res. 2015; 47(11): 805-812.
[Crossref] [Google Scholar] [Pubmed]
- Chamarthi B, Gaziano JM, Blonde L, Vinik A, Scranton RE, Ezrokhi M, et al. Timed bromocriptine-QR therapy reduces progression of cardiovascular disease and dysglycemia in subjects with well-controlled type 2 diabetes mellitus. J Diabetes Res. 2015.
[Crossref] [Google Scholar] [Pubmed]
- Andersen IB, Andreassen M, Krogh J. The effect of dopamine agonists on metabolic variables in adults with type 2 diabetes: A systematic review with meta analysis and trial sequential analysis of randomized clinical trials. Diabetes Obes Metab. 2021; 23(1): 58-67.
[Crossref] [Google Scholar] [Pubmed]
- Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020; 35: 49-60.
[Crossref] [Google Scholar] [Pubmed]
- Siddaway AP, Wood AM, Hedges LV. How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019; 70: 747-770.
[Crossref] [Google Scholar] [Pubmed]
- Schmidt L, Shokraneh F, Steinhausen K, Adams CE. Introducing RAPTOR: RevMan parsing tool for reviewers. Syst Rev. 2019; 8(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Migliavaca CB, Stein C, Colpani V, Barker TH, Ziegelmann PK, Munn Z, et al. Meta‐analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. 2022; 13(3): 363-367.
[Crossref] [Google Scholar] [Pubmed]
- Aisbett J, Drinkwater EJ, Quarrie KL, Woodcock S. Applying generalized funnel plots to help design statistical analyses. Stat Pap. 2023; 64(1): 355-364.
- Higgins JP, Altman DG. Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions: Cochrane book series. 2008.
- Review Manager Web. Cochrane Collaboration. 2020.
- Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020‐compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022; 18(2): e1230.
[Crossref] [Google Scholar] [Pubmed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 88: 105906.
[Crossref] [Google Scholar] [Pubmed]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020; 40(9): 1769-1777.
[Crossref] [Google Scholar] [Pubmed]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 1(2): 94-9.
[Crossref] [Google Scholar] [Pubmed]
- Aliasgarzadeh A, Karimiavval S, Houshyar J, Gojazadeh M, Hadi A. Evaluating the effect of cabergoline on glycaemic control of patients with type 2 diabetes mellitus: A randomised controlled trial. J Clin Diagnostic Res. 2020; 14(2).
- Bahar A, Kashi Z, Daneshpour E, Akha O, Ala S. Effects of cabergoline on blood glucose levels in type 2 diabetic patients: A double-blind controlled clinical trial. Medicine. 2016; 95(40).
[Crossref] [Google Scholar] [Pubmed]
- Barnett AH, Chapman C, Gailer K, Hayter CJ. Effect of bromocriptine on maturity onset diabetes. Postgrad Med J. 1980; 56(651): 11-14.
[Crossref] [Google Scholar] [Pubmed]
- Chamarthi B, Ezrokhi M, Rutty D, Cincotta AH. Impact of bromocriptine-QR therapy on cardiovascular outcomes in type 2 diabetes mellitus subjects on metformin. Postgrad Med. 2016; 128(8): 761-769.
[Crossref] [Google Scholar] [Pubmed]
- Ghosh A, Sengupta N, Sahana P, Giri D, Sengupta P, Das N. Efficacy and safety of add on therapy of bromocriptine with metformin in Indian patients with type 2 diabetes mellitus: A randomized open labeled phase IV clinical trial. Indian J Pharmacol. 2014; 46(1): 24.
[Crossref] [Google Scholar] [Pubmed]
- Kok P, Roelfsema F, Frolich M, van Pelt J, Stokkel MP, Meinders AE, et al. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006; 291(5): E1038-1043.
[Crossref] [Google Scholar] [Pubmed]
- Krysiak R, Okopien B. Different effects of cabergoline and bromocriptine on metabolic and cardiovascular risk factors in patients with elevated prolactin levels. Basic Clin Pharmacol Toxicol. 2015; 116(3): 251-256.
[Crossref] [Google Scholar] [Pubmed]
- Meier AH, Cincotta AH, Lovell WC. Timed bromocriptine administration reduces body fat stores in obese subjects and hyperglycemia in type II diabetics. Experientia. 1992; 48: 248-253.
[Crossref] [Google Scholar] [Pubmed]
- Mejía-Rodríguez O, Herrera-Abarca JE, Ceballos-Reyes G, Avila-Diaz M, Prado-Uribe C, Belio-Caro F, et al. Cardiovascular and renal effects of bromocriptine in diabetic patients with stage 4 chronic kidney disease. Biomed Res Int. 2013.
[Crossref] [Google Scholar] [Pubmed]
- Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, et al. Bromocriptine: A novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000; 23(8): 1154-1161.
[Crossref] [Google Scholar] [Pubmed]
- Taghavi SM, Fatemi SS, Rokni H. Cabergoline effect on blood sugar in type 2 diabetic patients with oral agent failure. Med J Malaysia. 2012; 67(4): 390-392.
[Google Scholar] [Pubmed]
- Winzap P, Davies A, Klingenberg R, Obeid S, Roffi M, Mach F, et al. Diabetes and baseline glucose are associated with inflammation, left ventricular function and short-and long-term outcome in acute coronary syndromes: Role of the novel biomarker Cyr 61. Cardiovasc Diabetol. 2019; 18(1): 1-0.
[Crossref] [Google Scholar] [Pubmed]
- Aminorroaya A, Janghorbani M, Ramezani M, Haghighi S, Amini M. Does bromocriptine improve glycemic control of obese type-2 diabetics? Horm Res. 2004; 62(2): 55-59.
[Crossref] [Google Scholar] [Pubmed]
- Assad HC, Mosah HA, Hashim HM, Khazaal FA. Effect of cabergoline added to metformin on glycemic control, insulin resistance and beta cell function in obese type 2 diabetic patients. Global Journals Inc. 2014; 14.
- Chamarthi B, Cincotta AH. Effect of bromocriptine-QR therapy on glycemic control in subjects with type 2 diabetes mellitus whose dysglycemia is inadequately controlled on insulin. Postgrad Med. 2017; 129(4): 446-455.
[Crossref] [Google Scholar] [Pubmed]
- Cincotta AH, Meier AH. Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care. 1996; 19(6): 667-670.
[Crossref] [Google Scholar] [Pubmed]
- Gaziano JM, Cincotta AH, O'Connor CM, Ezrokhi M, Rutty D, Ma ZJ, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010; 33(7): 1503-1508.
[Crossref] [Google Scholar] [Pubmed]
- Kamath V, Jones CN, Yip JC, Varasteh BB, Cincotta AH, Reaven GM, et al. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care. 1997; 20(11): 1697-1701.
[Crossref] [Google Scholar] [Pubmed]
- Khalilzade SH, Aminorroaya A, Hovsepain S, Amini M. Efficacy of bromocriptine on glycemic and metabolic control of prediabetic patients. Adv Biomed Res. 2015;4.
[Crossref] [Google Scholar] [Pubmed]
- Morcos JA, Ebeid AM, Khodeir SA, Elazab GA. Co-administration of cabergoline and gliclazide improve glycemic parameters and lipid profile in T2DM. J Diabetes Metab. 2017;8(9): 1-9.
- Ramteke KB, Ramanand SJ, Ramanand JB, Jain SS, Raparti GT, Patwardhan MH, et al. Evaluation of the efficacy and safety of bromocriptine QR in type 2 diabetes. Indian J Endocrinol Metab. 2011; 15(Suppl1): S33.
[Crossref] [Google Scholar] [Pubmed]
- Roe ED, Chamarthi B, Raskin P. Impact of bromocriptine-QR therapy on glycemic control and daily insulin requirement in type 2 diabetes mellitus subjects whose dysglycemia is poorly controlled on high-dose insulin: A pilot study. J Diabetes Res. 2015.
[Crossref] [Google Scholar] [Pubmed]
- Tell SS, Schafer M, Vigers T, Baumgartner AD, Lyon E, Gross S, et al. Bromocriptine quick‐release as adjunct therapy in youth and adults with type 1 diabetes: A randomized, placebo‐controlled crossover study. Diabetes Obes Metab. 2022; 24(11): 2148-2158.
[Crossref] [Google Scholar] [Pubmed]
- Vinik AI, Cincotta AH, Scranton RE, Bohannon N, Ezrokhi M, Gaziano JM. Effect of bromocriptine-QR on glycemic control in subjects with uncontrolled hyperglycemia on one or two oral anti-diabetes agents. Endocr Pract. 2012; 18(6): 931-943.
[Crossref] [Google Scholar] [Pubmed]
- Berard LD, Siemens R, Woo V. Monitoring glycemic control. Can J Diabetes. 2018; 42: S47-53.
[Crossref] [Google Scholar] [Pubmed]
- dos Santos Nunes V, El Dib R, Boguszewski CL, Nogueira CR. Cabergoline versus bromocriptine in the treatment of hyperprolactinemia: A systematic review of randomized controlled trials and meta-analysis. Pituitary. 2011; 14: 259-265.
[Crossref] [Google Scholar] [Pubmed]
- Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96(2): 273-288.
[Crossref] [Google Scholar] [Pubmed]
- Mikhail N. Quick-release bromocriptine for treatment of type 2 diabetes. Curr Drug Deliv. 2011; 8(5): 511-516.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Beyene Dereje1* and Aschalew Nardos22Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Citation: Dereje B: Dopamine 2 Agonists for Identification and Management of Type 2 Diabetes
Received: 20-Mar-2023 Accepted: 04-Apr-2023 Published: 11-Apr-2023, DOI: 10.31858/0975-8453.14.4.286-295
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






