Research Article - (2022) Volume 13, Issue 9
Abstract
The liver plays an important function in the metabolism of carbohydrate-protein and fats. Now a day’s obesity and high fatty diet cigarettes increased Oxidative stress which leads to the chance of Non-Alcoholic Fatty Liver Disease (NAFLD). The main objective of this study was to evaluate the effect of Vitamin-U on a High-Fat Diet (HFD) and nicotine-induced NAFLD in rats. Female Wistar rats (n=6) that weighed 200-250 g were randomly segregated into six groups of nine animals in each group. The Group-I animals received a normal diet and the rest of the groups received HFD and nicotine (1.5 mg/kg, b.wt (body weight), I.P (Intraperitoneal)) (NAFLD control) and water ad libitum. After 28th days, blood was collected from the retro-orbital plexus under mild chloroform to ensure that all animals which received HFD and nicotine were hyperlipidemia or not. After conformation animals were differentiated into different (Negative control) normal diets (NAFLD control) where cholesterol, triglyceride, Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), Alkaline Phosphatase (ALP) level significantly increased compared with normal control. After 14 days of treatment with Vitamin U (100 mg/kg, b.wt, oral) alone, Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U (150 mg/kg, b.wt, oral), Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) and Vitamin-E (200 mg/kg, b.wt, oral) alone along with induction it has been seen that serum cholesterol, triglyceride, fasting glucose SGOT, SGPT, ALP level significantly decreased in comparison with NAFLD control. As NAFLD condition increased oxidative stress liver homogenate Superoxide Dismutase (SOD), reduced Glutathione (GSH) level was significantly decreased and Lipid Peroxidation (LPO) level significantly increased in NAFLD control compare with normal control. After treatment with Vitamin U alone (100 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral), Vitamin-U (100 mg/kg, b.wt) combined with Vitamin- E (200 mg/kg, b.wt, oral) and Vitamin-E (200 mg/kg, b.wt, oral) alone significantly increased SOD and GSH level and decreased LPO level. So, Vitamin U can be a good option for the treatment of NAFLD. It has been seen that when Vitamin-U combined with Vitamin E which is more effective than the individual drug to improve NAFLD.
Keywords
Alkaline Phosphatase (ALP), Glutathione (GSH), Lipid Peroxidation (LPO), Oxidative stress
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is characterized as the accumulation of additional fat in the liver, which is not related to alcohol consumption. This is the most significant universal problem in society. NAFLD may lead to different diseases Non-Alcoholic Steatohepatitis (NASH), cirrhosis, and possibly Hepatocellular Carcinoma (HCC). Non-Alcoholic Steatohepatitis (NASH) is a fatty liver disease categorized by the accumulation of fat in liver cells with chronic inflammation in the liver, that‘s differentiated NASH from NAFLD, where the absence of inflammation. NASH may progressively convert to more thoughtful chronic liver disease. NASH typically occurs in those people who are overweight and diabetic, with high blood cholesterol and triglyceride levels. For the patients having NAFLD, the capability of insulin to usually defeat the production of glucose and Very Low Density Lipoproteins (VLDL) is decreased (Seppälä-Lindroos A, et al., 2002). The liver, when fatty, also increases the production of cardiovascular markers like C-Reactive Protein (CRP), fibrinogen, and coagulation factors (Anstee QM, et al., 2013; Targher G, et al., 2009). The prevalence is tantamount to overcome NAFLD as low as NAFLD does not diagnose properly and is asymptomatic at the initial level. Aged people are more impacted by NAFLD. The NAFLD is a global health issue. It has been seen that 10%-24% of common residents, and 57%-74% of obese persons affected with NAFLD (Duseja A, 2010). Vitamin U as of late increased significant consideration during the 1950s when Dr. Garnett Cheney, a professor of medicine at the Stanford University School of Medicine, coined S-methyl methionine as Vitamin-U found its mending properties through different tests. In one of his better-known examinations, a quart of crude cabbage juice was directed to 100 patients with a peptic ulcer.
The previously different activity of Vitamin-U was reported like a novel free radical scavenger, prevents lens injury in rats administered with valproic acid (Tunali SE, et al., 2015) effects of Vitamin U (S-methyl methionine sulphonium chloride) on valproic acid-induced liver injury in rats (Sokmen BB, et al., 2012). The aim of this research is to investigate the treatment effect of Vitamin U on a High-Fat Diet and nicotine-induced Non-Alcoholic Fatty Liver Disease in rats. In the pharmacological experiment, it is very much important to select a proper animal model. Here select the model to induced NAFLD is High-Fat Diet and nicotine. This model is very much mimic cause of NAFLD to human. The effects of treatment on various parameters like Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), cholesterol, triglyceride, total protein, etc. were studied. The study was further aimed to investigate the effect of the combination of Vitamin U along with Vitamin U in Non-Alcoholic Fatty Liver Disease (NAFLD). The status of pro-oxidant and the level of anti-oxidant enzymes will be estimated in the liver of rats in order to evaluate the treatment effect in NAFLD.
Material and Methods
Chemicals and reagents
The Vitamin-U (C6H14NO2S) powder chemically known as methyl methionine sulphonium chloride was purchased from Sigma-Aldrich is now Merck, India; Chemical Abstracts Service (CAS) no 3493-12-7, nicotine (C10H14N2) chemically known as 1-methyl-2-3-pyridyl-pirolidin is purchased from Sigma-Aldrich is now Merck, India; CAS no-54-11-5. The feed ingredients such as casein and cholesterol (both from Himedia laboratories, Mumbai, India), and yeast powder (Pet Care, Bangalore, India) were procured from commercial sources. Lard (Eli Lilly, Gurgaon, India). The glucose, triglyceride, Alkaline Phosphatase, cholesterol, SGPT, and SGOT assay kit was purchased from Robotnik. L-glutathione reduced standard (CAS no 70-18-8) and L-Epinephrine (CAS no-51-43-4) were supplied from Sigma-Aldrich now Mar. Sodium Carbonate, Sodium bicarbonate, Ethylenediamine Tetraacetic Acid (EDTA), Trichloroacetic Acid (TCA), Sodium Citrate, Sodium hydroxide, Potassium dihydrogen orthophosphate, 5,5-dithiobis 2-nitrobenzoic acid, and Thiobarbituric Acid (TBA) were provided from HiMedia (India).
Animals
The research protocol was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) of the Govt. of India was e followed and prior permission was granted from the Institutional Animal Ethics Committee for conducting the experimental studies. The Wister rats (200-250 g) obtained from the Bengal School of Technology, were used for this experiment. The animals were housed in standard cages (48 cm × 35 cm × 22 cm) at room temperature (22°C ± 2°C), with relative humidity (55%+5%) with a 12:12 hour light and dark cycle. All the animals were provided Normal Pellet Diet (NPD) and water ad libitum. For the induction of NAFLD, the required amount of cholesterol, lard, casein, yeast powder and sodium chloride were added with powder NPD.
HFD and nicotine induced Non-Alcoholic Fatty Liver Disease (NAFLD)
Female Wistar rats (n=6) that weighed 200-250 g were randomly segregated into six groups of nine animals in groups. The Group-I animals received a normal diet and the rest of the groups received HFD and nicotine (1.5 mg/kg, b.wt, I.P) and water ad libitum. After 28th days, blood was collected from the retro-orbital plexus under mild chloroform to ensure that all animals which received HFD and nicotine were hyperlipidemic or not. After confirmation of hyperlipidemia animals were divided into the following groups:
• Group-I (Negative control): Normal diet.
• Group-II (NAFLD control): HFD and nicotine (1.5 mg/kg, b.wt, I.P) (Sinha-Hikim AP, et al., 2017).
• Group-III: HFD and nicotine (1.5 mg/kg, b.wt, I.P) (Sinha-Hikim AP, et al., 2017)+Vitamin-U (100 mg/kg, b.wt) (Sokmen BB, et al., 2012).
• Group-IV: HFD and nicotine (1.5 mg/kg, b.wt, I.P) (Sinha-Hikim AP, et al., 2017)+Vitamin-U (150 mg/kg , b.wt) (Sokmen BB, et al., 2012).
• Group-V: HFD and nicotine (1.5 mg/kg, b.wt, I.P) (Sinha-Hikim AP, et al., 2017)+Vitamin-U (100 mg/kg, b.wt) (Sokmen BB, et al., 2012)+Vitamin E (200 mg/kg, b.wt, oral) (Oliveira CP, et al., 2003).
• Group-VI (Standard): Vitamin E (200 mg/kg, b.wt, oral) (Oliveira CP, et al., 2003).
Treatment has been done for 14 days (Srinivasan K, et al., 2005).
Analysis of serum biochemical parameter
All groups’ animals were treated orally for 14 days. Every day animals were clinically obverted. At the end of the intervention period, animals underwent 12-hour fasting. The blood was collected by retro-orbital plexus under mild chloroform. The blood sample was centrifuged at 2,000 g for 8-10 mins and serum was separated out and the following parameter was analyzed High Density Lipoproteins (HDL), Low Denisty Lipoproteins (LDL), Triglycerides (TG), glucose, SGOT (Aspartate Aminotransferase (AST)), SGPT (Alanine Transaminase (ALT)), Alkaline Phosphatase (all parameters was analyzed by ROBONIK semi-auto analyzer kit, using ROBONIK semi-auto analyzer). The serum LDH was analyzed by Reckon UV assay kit.
Liver homogenate preparation
After completing the intervention period (14 days) was sacrificed using overdose chloroform, livers of all groups’ animals were isolated and homogenized in Tris buffer pH 7.5 in homogenizer at a speed of 2000 g. The homogenate was centrifuged in the centrifuge machine (Remi-motors Ltd., Mumbai) and the supernatant was taken and to evaluate various oxidative parameters.
Analysis of Superoxide Dismutase (SOD)
SOD was analyzed spectrophotometric method by the supernatant of liver tissue (Otitoju O, 2005). In this method, SOD has the ability to inhibit auto-oxidation of epinephrine to adrenochrome at pH 10.2. This inhibition can be measured colorimeter at 480 nm. The 0.5 ml of distilled water was mixed with 0.5 ml of liver homogenate. All reagents should be in cold condition. Whole mixture was incubated with 0.15 ml chloroform and 0.25 ml. Then it was shaken and centrifuged at 2000 rpm for 10 min. The supernatant was separated. The Epinephrine was added just before taking SOD. The initial absorbance at zero minutes was noted and taken for 3 min with a 30-sec interval at 480 nm using a spectrophotometer. Its activity was expressed as U/mg of protein in liver tissue.
Analysis of reduced Glutathione (GSH)
Reduced Glutathione was analyzed spectrophotometric method by the supernatant of liver tissue (Moron MS, et al., 1979). DTNB (5,5’-dithio-bis- (2-nitrobenzoic acid)), a disulfide compound, was readily attacked, by the sulfhydryl group and forms a yellow-colored anion which was measured colorimeter at 412 nm. Tissue supernatant will be mixed with TCA and centrifuged. The supernatant will be mixed with DTNB and phosphate buffer and estimated at 412 nm. Its activity was expressed as μg of GSH/ mg of tissue.
Analysis of Lipid Peroxidation (LPO)
Lipid Peroxidation or level of Malondialdehyde (MDA) was determined spectrophotometrically. The method estimates Malondialdehyde, a product of the Lipid Peroxidation process. One molecule of MDA reacts with two molecules of TBA under the mildly acidic conditions to form a pink colored chromogen, whose intensity is measured colorimetrically at 535 nm. The testicular supernatant was incubated with 2 mL TCA. The mixture was cooled for 15 min and centrifuged separating the supernatant. The supernatant (2 mL) was then incubated with TBA by keeping it in boiling water for 10 min and cooled before measurements were done. The extent of Lipid Peroxidation was assessed by reading the absorbance of the test against blank at 535 nm using a spectrophotometer.
Statistical analysis
The biochemical result was evaluated by ANOVA followed by multiple comparison tests (Dunnett’stest) using the Graph Pad Prism 5 software. The Values are expressed as Mean ± SEM (Standard Error of Mean), n=6, aP<0.05, bP<0.01, cP<0.001 vs. Group-II.
Results
The present study entitled as the effect of Vitamin U on a High-Fat Diet and nicotine-induced Non-Alcoholic Fatty Liver Disease in rats. It involved the analysis of blood samples in serum and tissues belonging to different treatment groups. Each sample was analyzed for estimating the effect of Vitamin-U on a High-Fat Diet and nicotine induced Non-Alcoholic Fatty Liver Disease in rats.
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on SGOT
Table 1 and Figure 1shows that NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone, Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/ kg, b.wt, oral) serum SGOT level significantly (P<0.05, P<0.01, P<0.001) decreased compared with NAFLD control. However, Vitamin-E (200 mg/ kg, b.wt, and oral) alone did not produce significant change compared with NAFLD control.
| Ingredients diet | Diet (g/kg) |
|---|---|
| Powdered Normal Pellet Diet (NPD) | 365 |
| Lard | 310 |
| Casein | 250 |
| Cholesterol | 10 |
| Yeast powder | 1 |
| Sodium chloride | 1 |
Table 1: Composition of High Fat Diet (HFD) (Srinivasan K, et al., 2005)
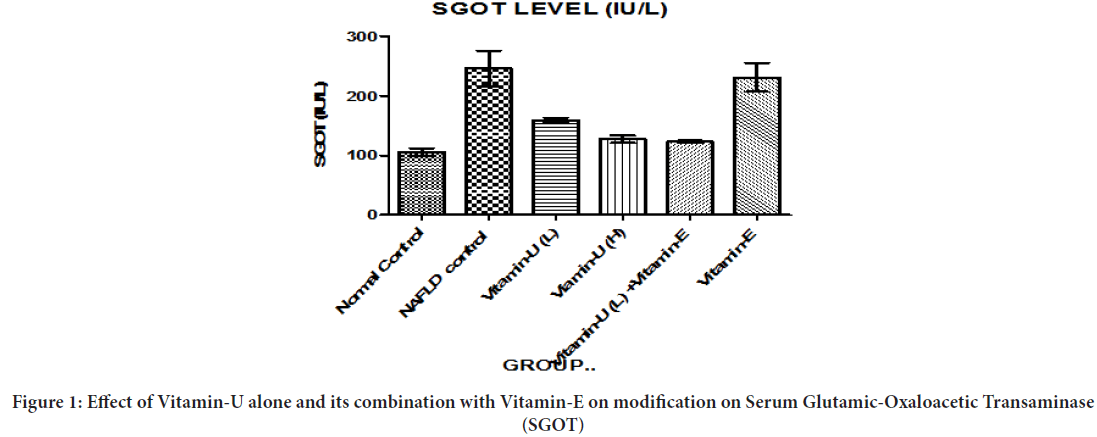
Figure 1: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Serum Glutamic-Oxaloacetic Transaminase (SGOT)
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on SGPT
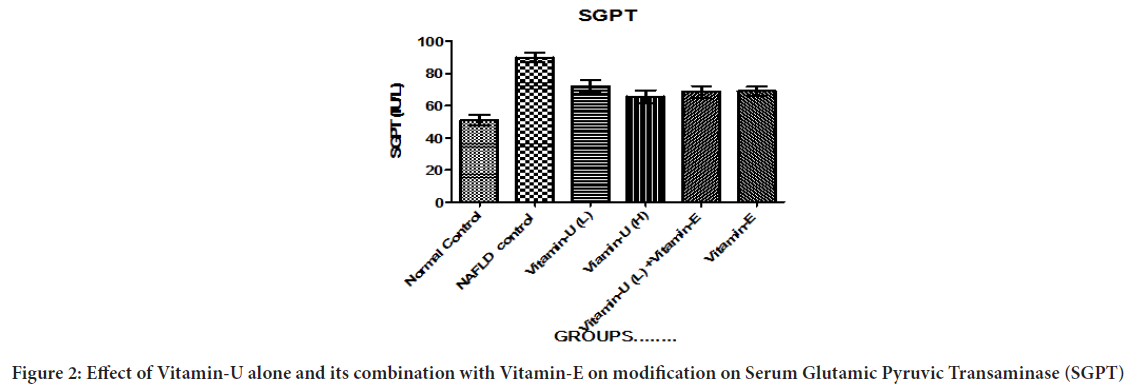
Table 1 and Figure 2 showed that NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone significantly (P<0.05, P<0.01) decreased serum SGPT level compared with NAFLD control rats. The NAFLD rats treatment with Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (30 mg/kg, b.wt oral) and Vitamin-E (200 mg/kg, b.wt, oral) alone more significantly (P<0.05, P<0.01, P<0.001) decreased serum SGPT level compared with NAFLD control rats.
Figure 2: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Serum Glutamic Pyruvic Transaminase (SGPT)
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Alkaline Phosphatase (ALP)
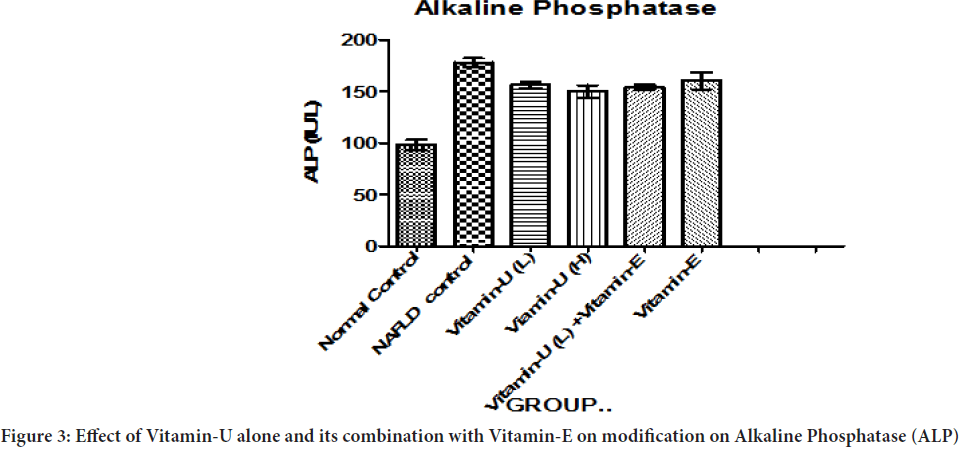
Table 2 and Figure 3shows that NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone and Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) serum ALP level significantly (P<0.05) decreased compared with NAFLD control. However, NAFLD rats treatment with Vitamin-U (150 mg/kg, b.wt, oral) alone significantly (P<0.05, P<0.01) decreased compared with NAFLD control. The NAFLD rat’s treatment with Vitamin-E (200 mg/kg, b.wt, and oral) alone did not produce significant change compared with NAFLD control.
| Groups | SGOT (IU/L) | SGPT (IU/L) | ALP (IU/L) | Cholesterol (mg/dl) | Triglyceride (mg/dl) |
|---|---|---|---|---|---|
| Group-I (Negative control): Normal diet | 106.0 ± 6.160 | 51.05 ± 3.342 | 98.24 ± 4.943 | 94.35± 2.917 | 119.9 ± 1.486 |
| Group-II (NAFLD control): HFD and nicotine (1.5 mg/kg, b.wt, I.P) | 245.9 ± 29.29 | 90.05 ± 3.12 | 178.0 ± 4.107 | 153.8 ± 4.799 | 178.2 ± 3.250 |
| Group-III : HFD and nicotine (1.5 mg/kg, b.wt, I.P) + Vitamin-U (100 mg/kg, b.wt, oral) (Low dose: L) | 158.8 ± 4.020a,b,c | 72.29 ± 3.821a,b | 156.5 ± 3.117a | 136.4 ± 4.334a,b | 156.5 ± 3.87a,b,c |
| Group-IV: HFD and nicotine (1.5 mg/kg, b.wt, I.P) + Vitamin-U (150 mg/kg , b.wt, oral) (High dose: H) | 127.5 ± 6.360a,b,c | 65.78 ± 3.88a,b,c | 150.0 ± 5.961a,b | 124.7 ± 2.753a,b,c | 127.1 ± 1.716a,b,c |
| Group V: HFD and nicotine (1.5 mg/kg, b.wt, I.P)+Vitamin-U (100 mg/kg, b.wt, oral)(L)+Vitamin E (200 mg/kg, b.wt, oral) | 123.0 ± 2.221a,b,c | 68.66 ± 3.608a,b,c | 154.0 ± 2.436a | 123.5 ± 2.012a,b.c | 124.5 ± 3.019a,b,c |
| Group-VI: (Standard): Vitamin E (200 mg/kg, b.wt, oral) | 230.7 ± 24.16a,b,c | 69.13 ± 2.764a,b,c | 160.3 ± 8.237ab | 137.8 ± 1.956a,b | 170.2 ± 2.522ab |
Note: The statistical significance of the difference between means was calculated by ANOVA followed by Dunnett's Multiple Comparison Test. N=6 Values are expressed as Mean ± SEM (Standard Error of Mean), n=6, aP<0.05, bP<0.01, cP<0.001 vs. Group-II. NAFLD: Non-Alcoholic Fatty Liver Disease; b.wt: body weight; I.P: Intraperitoneal
Table 2: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), Alkaline Phosphatase (ALP), Cholesterol and Triglyceride (TG)
Figure 3: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Alkaline Phosphatase (ALP)
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on cholesterol
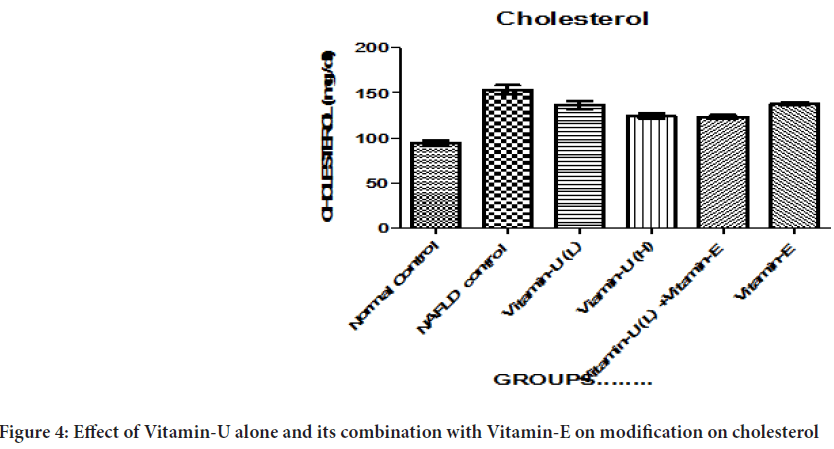
Table 2 and Figure 4 showed that NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone and Vitamin-E (200 mg/kg, b.wt, oral) alone significantly (P<0.05, P<0.01) decreased the serum cholesterol level compared with NAFLD control. The NAFLD rats treatment with Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) more significantly (P<0.05, P<0.01, P<0.001) decreased the cholesterol compared with NAFLD control.
Figure 4: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on cholesterol
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on triglyceride
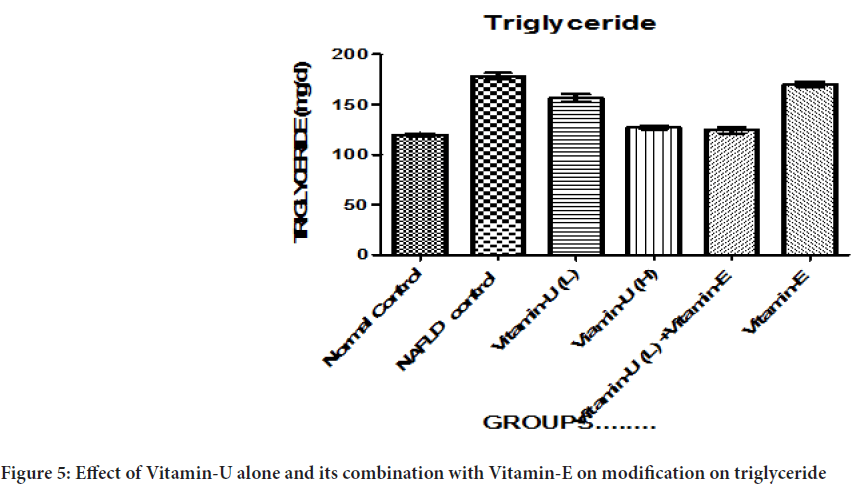
Table 2 and Figure 5 shows that NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone, Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) serum triglyceride level significantly (P<0.05, P<0.01, P<0.001) decreased compared with NAFLD control. However, Vitamin-E (200 mg/ kg, b.wt, oral) alone did not produce significant decreased compared with NAFLD control.
Figure 5: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on triglyceride
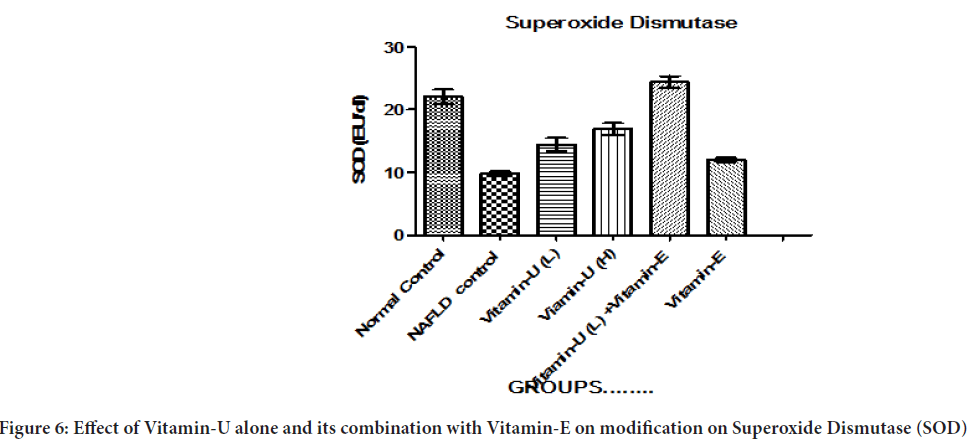
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on SOD
Table 3and Figure 6 shows that the NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone significantly (P<0.05, P<0.01) increased liver homogenate SOD level compared with NAFLD control. The NAFLD rats treatment with Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) more significantly (P<0.05, P<0.01, P<0.001) increased SOD level compared with NAFLD control. However NAFLD rat’s treatment with Vitamin-E (200 mg/kg, b.wt, oral) alone did not produce significant change compared with NAFLD control.
| Group | Superoxide Dismutase (EU/dl) | Reduced Glutathione (µg/ml) | Lipid Peroxidation (n mol/L) |
|---|---|---|---|
| Group-I (Negative control): Normal diet | 22.04 ± 1.141 | 36.00 ± 4.561 | 44.24 ± 1.540 |
| Group-II (NAFLD control): HFD and nicotine (1.5 mg/kg, b.wt, I.P) | 9.780 ± 0.3852 | 17.60 ± 0.9274 | 69.24 ± 1.165 |
| Group-III: HFD and nicotine (1.5 mg/kg, b.wt, I.P)+Vitamin-U (100 mg/kg, b.wt, oral) (Low dose: L) | 14.42 ± 1.045a,b | 25.85 ± 5.859 | 63.20 ± 1.744ab |
| Group-IV: HFD and nicotine (1.5 mg/kg, b.wt, I.P)+Vitamin-U (150 mg/kg , b.wt, oral) (High dose: H) | 16.90 ± 0.9274a,b,c | 25.60 ± 1.166 | 52.40 ± 3.326a,b |
| HFD and nicotine (1.5 mg/kg, b.wt, I.P)+Vitamin-U (100 mg/kg, b.wt, oral)(L)+Vitamin E (200 mg/kg, b.wt, oral) | 24.40 ± 0.9274a,b,c | 32.20 ± 1.068a | 41.00 ± 3.098a,b,c |
| Group-VI (Standard): Vitamin E (200 mg/kg, b.wt, oral) | 12.00 ± 0.3162ab | 19.08 ± 0.9547 | 45.20 ± 4.271a,b,c |
Note: The statistical significance of the difference between means was calculated by ANOVA followed by Dunnett's Multiple Comparison Test. N=6 Values are expressed as Mean ± SEM, n=6, aP<0.05, bP<0.01, cP<0.001 vs. Group-II
Table 3: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Superoxide Dismutase (SOD), Glutathione (GSH) and Lipid Peroxidation (LPO)
Figure 6: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Superoxide Dismutase (SOD)
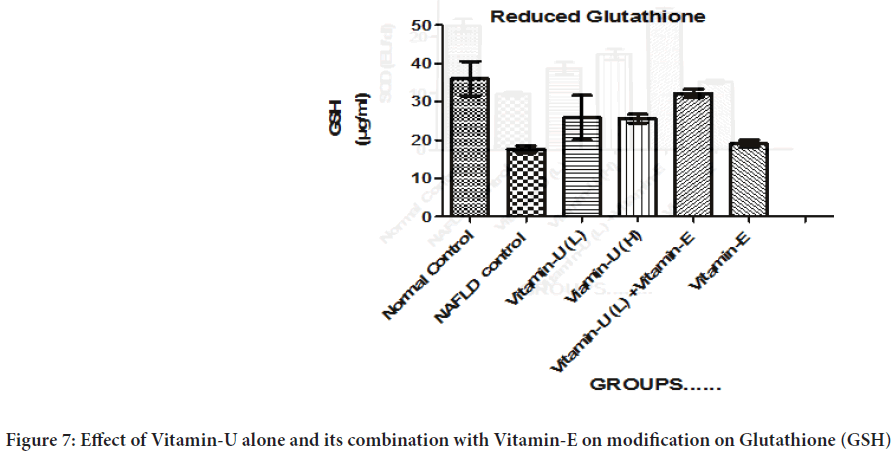
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on GSH
Table 3 and Figure 7shows that the NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) significantly (P<0.05) increased GSH level compared with the NAFLD control rats. However, NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) alone, Vitamin-U (150 mg/kg, b.wt, oral) alone and Vitamin-E (200 mg/kg, b.wt, oral) alone did not produce significant change compared with NAFLD control.
Figure 7: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Glutathione (GSH)
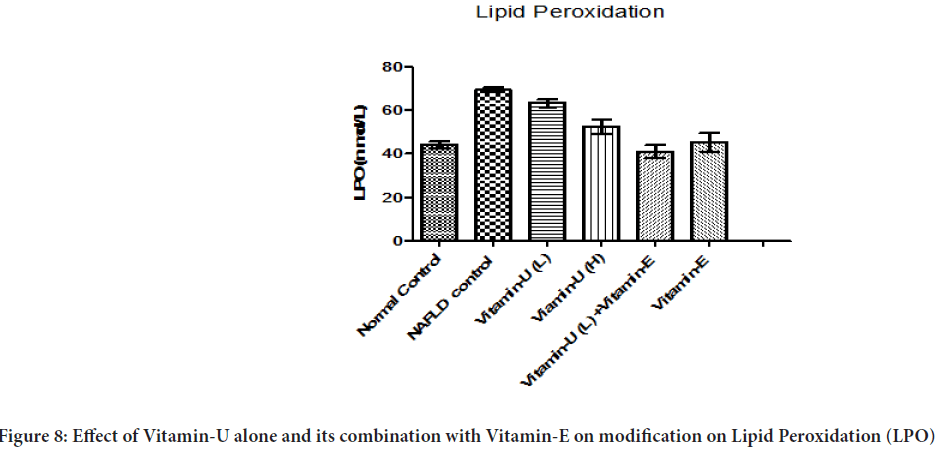
Effect of Vitamin-U alone and its combination with Vitamin-E on modification on LPO
Table 3 and Figure 8shows that the NAFLD rats treatment with Vitamin-U (150 mg/kg, b.wt, oral) alone significantly decreased liver homogenate LPO level compared with NAFLD control rats. However, NAFLD rats treatment with Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (30 mg/kg, b.wt oral) and Vitamin-E (200 mg/kg, b.wt, oral) alone more significantly (P<0.05, P<0.01, P<0.001) decreased liver homogenate LPO level compared with NAFLD control rats. The NAFLD rat’s treatment with Vitamin-U (100 mg/kg, b.wt, and oral) alone did not produce significant change compared with NAFLD control.
Figure 8: Effect of Vitamin-U alone and its combination with Vitamin-E on modification on Lipid Peroxidation (LPO)
Discussion
The liver is an important organ not only for the metabolism of biomolecules such as carbohydrate-protein and lipid but also detoxifies toxic substances. Nowadays NAFLD is a major problem in our society due to obesity.
In this study, HFD and nicotine were used for NAFLD induction. The HFD and nicotine increased adipose tissue lipolysis, play an important role in buildup lipid in the liver as a triglyceride. This combination of HFD and nicotine also leads to developing hepatic steatosis. Due to inhibition of Adenosine Monophosphate (AMP)-Activated Protein Kinase (AMPK) increase expression of Sterol Regulatory Element-Binding Protein 1c (SREBP-1c) (Buzzetti E, et al., 2016) that increase free Fatty Acid Synthase (FAS) and Acetyl-Coenzyme A Carboxylase (ACC), which is a rate-determining enzyme of fatty acid biosynthesis and decreased β-oxidation of fatty acid (Zhang BB, et al., 2009) resulting increase of blood TG and cholesterol and glucose level. Another important factor for hepatic lipogenesis Carbohydrate Response-Element Binding Protein (ChREBP) (Postic C and Girard J, 2008) which triggers peripheral insulin resistance and increased glucose production. In this study, HFD and nicotine increase cholesterol, TG. However, treatment with Vitamin U alone (100 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral), Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (30 mg/kg, b.wt, oral) and Vitamin-E (30 mg/kg, b.wt, oral)significantly cholesterol and TG (except Vitamin-E (30 mg/kg, b.wt, oral)) level decreased compare with the NAFLD control. This NAFLD HFD with nicotine model is very much similar to the cause of human NAFLD. The SGOT, SGPT, and ALP consider as biochemical markers for hepatocellular injury (Singh SN, et al., 2001; Koh PH, et al., 2011). A previous study showed that Vitamin-U able to lower SGOT, SGPT, and ALP levels in valproic acid-induced liver injury in rats (Sokmen BB, et al., 2012). In this study, HFD and nicotine increased SGOT, SGPT, and ALP levels. However, treatment with Vitamin U alone (100 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral), Vitamin-U (100 mg/kg, b.wt) combined with Vitamin-E (30 mg/kg, b.wt, oral) and Vitamin-E (200 mg/kg, b.wt, oral) alone SGOT, SGPT and ALP level significantly decreased compare with the NAFLD control. Inactivation of AMPK activates of c-Jun NH2-terminal Kinase (JNK)-mediated elevation of oxidative stress in the Endoplasmic Reticulum (ER), inflammation, and apoptosis (SinhaHikim AP, et al., 2017). Superoxide Dismutase (SOD) plays an important role to convert superoxide radicals into hydrogen peroxide (H2O2) which doses oxidation resulting in increased oxidative stress (Jena S, et al., 2012). In our study HFD and nicotine decrease SOD level in NAFLD control animals although treatment with Vitamin U alone (100 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral) alone, Vitamin-U (100 mg/ kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) and Vitamin-E (200 mg/kg, b.wt, oral) level significantly increase SOD. But Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (30 mg/kg, b.wt, oral) and Vitamin-U alone (150 mg/kg, b.wt, oral) alone more significant than Vitamin-E (200 mg/kg, b.wt, oral) and Vitamin-U (100 mg/kg, b.wt, oral) alone. Glutathione is another enzyme that is responsible for oxidative stress formation mainly by regulating thiol redox status in cell (Ballatori N, et al., 2009).The reduction of GSH (reduced Glutathione) level indicates that cellular damage condition. Previous study 15 reported Vitamin-U able to increased GSH level in valproic acid-induced liver injury in rats. In our study, GSH level was reduced in NAFLD control rats liver homogenate, so it was concluded that HFD and nicotine decreased GSH level in these NAFLD control groups. However, treatment with Vitamin-U alone (150 mg/kg, b.wt, oral), Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) significantly increased GSH level, whereas Vitamin U alone (100 mg/kg, b.wt, oral) alone and Vitamin-E (200 mg/ kg, b.wt, oral) alone not significant to lower GSH level. Lipid Peroxidation (LPO) level elevation indicates hepatotoxicity (Sathish P, et al., 2011). The HFD and nicotine increased lipogenesis which leads to Lipid Peroxidation. In our study, the LPO level was significantly increased in NAFLD control rats compared to normal control animals. During treatment with Vitamin U (100 mg/kg, b.wt, oral) alone, Vitamin-U (150 mg/kg, b.wt, oral) alone, Vitamin-U alone (150 mg/kg, b.wt, oral), Vitamin-U (100 mg/kg, b.wt, oral) combined with Vitamin-E (200 mg/kg, b.wt, oral) and Vitamin-E (200 mg/kg, b.wt, oral) alonelevel significantly decreased LPO level.
Conclusion
It was already explained NAFLD condition increased serum cholesterol, triglyceride, SGOT, SGPT, ALP level. In our study also HFD combined with nicotine increase these serum biomarker which has been mention previously. The HFD combined with nicotine also increased LPO level and deceased SOD and GSH level. During treatment it has been observed that Vitamin U combined with Vitamin-E more effective to overcome from NAFLD condition. However mechanism is not clear. At last it can be concluded that Vitamin-U combined with Vitamin-E showed positive to improved NAFLD condition.
Acknowledgements
This work was supported by Bengal School of Technology funds. The authors would like to thank, CM Hossain and Abhijit De for their immense support during the project work as supervisor.
References
- Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002; 87(7): 3023-3028.
[Crossref] [Google Scholar] [Pubmed]
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013; 10(6): 330-344.
[Crossref] [Google Scholar] [Pubmed]
- Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009; 35(3): 277-287.
[Crossref] [Google Scholar] [Pubmed]
- Duseja A. Nonalcoholic fatty liver disease in India-a lot done, yet more required!. Indian J Gastroenterol. 2010; 29(6): 217-225.
[Crossref] [Google Scholar] [Pubmed]
- Tunali SE, Kahraman S, Yanardag RE. Vitamin U, a novel free radical scavenger, prevents lens injury in rats administered with valproic acid. Hum Exp Toxicol. 2015; 34(9): 904-910.
[Crossref] [Google Scholar] [Pubmed]
- Sokmen BB, Tunali S, Yanardag R. Effects of vitamin U (S-methyl methionine sulphonium chloride) on valproic acid induced liver injury in rats. Food Chem Toxicol. 2012; 50(10): 3562-3566.
[Crossref] [Google Scholar] [Pubmed]
- Sinha-Hikim AP, Sinha-Hikim I, Friedman TC. Connection of nicotine to diet-induced obesity and non-alcoholic fatty liver disease: Cellular and mechanistic insights. Front Endocrinol. 2017; 8: 23.
[Crossref] [Google Scholar] [Pubmed]
- Oliveira CP, da Costa LCG, Tatai C, Nina BI, Lima ES, Abdalla DS, et al. Vitamin C and vitamin E in prevention of Nonalcoholic Fatty Liver Disease (NAFLD) in choline deficient diet fed rats. Nutr J. 2003; 2(1): 1-5.
[Crossref] [Google Scholar] [Pubmed]
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005; 52(4): 313-320.
[Crossref] [Google Scholar] [Pubmed]
- Otitoju O. Sub-chronic effect of Rambo insect powder contaminated diet on Superoxide Dismutase (SOD) activity in Wistar albino rats. Biokemistri. 2005; 17(2): 157-163.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979; 582(1): 67-78.
[Crossref] [Google Scholar] [Pubmed]
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism. 2016; 65(8): 1038-1048.
[Crossref] [Google Scholar] [Pubmed]
- Zhang BB, Zhou G, Li C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009; 9(5): 407-416.
[Crossref] [Google Scholar] [Pubmed]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J Clin Invest. 2008; 118(3): 829-838.
[Crossref] [Google Scholar] [Pubmed]
- Singh SN, Vats P, Suri S, Shyam R, Kumria MM, Ranganathan S, et al. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001; 76(3): 269-277.
[Crossref] [Google Scholar] [Pubmed]
- Koh PH, Mokhtar RA, Iqbal M. Andrographis paniculata ameliorates Carbon tetrachloride (CCl4)-dependent hepatic damage and toxicity: Diminution of oxidative stress. Redox Rep. 2011; 16(3): 134-143.
[Crossref] [Google Scholar] [Pubmed]
- Jena S, Anand C, Chainy GB, Dandapat J. Induction of oxidative stress and inhibition of superoxide dismutase expression in rat cerebral cortex and cerebellum by PTU-induced hypothyroidism and its reversal by curcumin. Neurol Sci. 2012; 33(4): 869-873.
[Crossref] [Google Scholar] [Pubmed]
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009; 390: 191-214.
[Crossref] [Google Scholar] [Pubmed]
- Sathish P, Paramasivan V, Palani V, Sivanesan K. N-acetylcysteine attenuates dimethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol. 2011; 654(2): 181-186.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Indrajit Bhattacharya1*, CM Hossain2 and Abhijit De32Department of Pharmaceutical Technology, Bengal School of Technology, West Bengal, India
3Department of Pharmaceutical Technology, Maulana Abul Kalam Azad University of Technology, West Bengal, India
Citation: Bhattacharya I: Effect of Vitamin U on High Fat Diet and Nicotine Induced Non-Alcoholic Fatty Liver Disease in Wistar Albino Rats
Received: 29-Aug-2022 Accepted: 23-Sep-2022 Published: 30-Sep-2022, DOI: 10.31858/0975-8453.13.9.610-617
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3