Research Article - (2022) Volume 13, Issue 6
Abstract
Objectives: Advanced Pancreatic Cancer (PC) causes nutritional deterioration and weight loss due to a decrease in digestive and absorption capacity associated with exocrine pancreatic insufficiency. Therefore, we conducted a prospective cohort study to evaluate the effectiveness of an elemental diet (ED).
Methods: We conducted two-centers, prospective consecutive observational study to assess the outcomes of patients with unresectable PC who underwent first-line chemotherapy. We enrolled patients receiving chemotherapy for unresectable PC from January 2017 to December 2019 and prescribed an oral ED (ED group). From December 2014 to December 2016, 43 patients before the introduction of ED therapy in our institutions were retrospectively studied as a historical cohort. Data on the nutritional markers at baseline and 3 months were extracted, and serial changes were compared between the 2 groups.
Results: A comparison of the background of the two groups revealed that the clinical stage was significantly worse and serum CA19-9 levels were significantly higher in the ED group. The serial changes at 3 months in body mass index, serum CEA, and serum CA19-9 were not statistically significant. The serum albumin level tended to show a much greater decrease in the historical cohort (P=0.15). Furthermore, the serum total protein level and prognostic nutrition index were significantly decreased in the historical cohort (P=0.02, <0.005, respectively).
Conclusion: This prospective study demonstrated that ED tended to improve the nutritional status during chemotherapy for unresectable PC.
Keywords
Elemental diet, Pancreatic cancer, Exocrine pancreatic insufficiency, Chemotherapy
Abbreviations
PC: Pancreatic Cancer; ED: Elemental Diet; CI: Confidence Interval; EPI: Exocrine Pancreatic Insufficiency; PS: Performance Status; BMI: Body Mass Index; PNI: Prognostic Nutrition Index; OS: Overall Survival
Introduction
Pancreatic Cancer (PC) is diagnosed at an advanced stage more frequently in comparison to other cancers, and the 5-year survival rate is the lowest among all types of cancer (Ilic M and Ilic I, 2016). In recent years, however, the prognosis of chemotherapy for PC has improved with the introduction of new drugs (Conroy T, et al., 2011; von Hoff DD, et al., 2013), and the long-term maintenance of the nutritional status and quality of life is more important than ever in the management of advanced PC. PC that obstructs the main pancreatic duct is also known to cause exocrine pancreatic insufficiency (EPI), especially when the tumor is located at the pancreatic head (Sikkens EC, et al., 2014). Since an elemental diet (ED) contains little fat (0.17 g/100 kcal) and the nitrogen source is decomposed into amino acids, PC patients lacking proteolytic enzymes can easily absorb nutrients. Therefore, the introduction of ED therapy is considered to contribute to the maintenance of the nutritional status and quality of life in PC patients and to be useful for continuing chemotherapy. The addition of an ED reduced postoperative weight loss in gastric cancer patients undergoing gastrectomy (Kimura Y, et al., 2019). However, there is little evidence on the effects of chemotherapy with an ED in PC (Yamazaki S, et al., 2016). Therefore, we conducted this prospective cohort study to evaluate the role of ED therapy in patients with unresectable PC who were receiving systemic chemotherapy.
Materials and Methods
Patients
This was a prospective consecutive observational study of ED in patients with unrespectable PC with a historical cohort at the Okayama University Hospital and the Japanese Red Cross Okayama Hospital. The study was approved by the local ethical committee at each hospital and written informed consent was obtained from all of the patients in the prospective cohort. The study was registered in the UMIN Clinical Trials Registry (UMIN-CTR: 000025967).
Consecutive patients receiving systemic chemotherapy for unresectable PC were prospectively enrolled between January 2017 and December 2019 (ED group). The inclusion criteria were unresectable PC with pathological confirmation and age ≥ 20 years. The exclusion criteria were a history of previous therapy for PC, including surgical resection, oral intake disability, history of inflammatory bowel disease, and ECOG (Eastern Cooperative Oncology Group) scale of Performance Status (PS) 3-4. Patients discontinuing chemotherapy due to disease progression or PS deterioration within 3 months after ED therapy was introduced were also excluded from the analysis to evaluate the effects of the ED at 3 months. Patients, who received first-line systemic chemotherapy for unresectable PC between December 2014 and December 2016, before ED was introduced at our institution, were retrospectively studied as a historical cohort. We applied the same inclusion and exclusion criteria as the prospective cohort, and patients whose detailed characteristics or clinical course were not available were excluded. None of the patients in either group received pancreatic enzyme replacement therapy.
All patients in the ED group received an oral ED (Elental®: EA Pharma Co., Tokyo, Japan) in addition to their regular diet. The ED was given at a dose of 300-600 mL/day (300-600 kcal/day) from the introduction of systemic chemotherapy. The dose of ED was set at the amount they were able to tolerate. Before introducing the ED, pharmacists explained the effectiveness of the ED and instructed the individual patients on how to take it.
Assessments
The primary endpoint was the evaluation of the impact of ED on the serum albumin level at 3 months after the introduction of chemotherapy. The secondary outcomes were the impact of ED on the prognosis of patients with PC. The body mass index (BMI), serum total protein, and Prognostic Nutrition Index (PNI) at baseline and at 3 months after the introduction of chemotherapy, were evaluated as surrogate markers of the nutritional status. The PNI was calculated as 10 × serum albumin (g/ dl)+0.005 × total lymphocyte count (per mm3) (Ikeya T, et al., 2015). In the ED group, we also evaluated adherence to the ED. During chemotherapy, computed tomographic scans were routinely performed at baseline and every 2-3 months.
Statistical analysis
Relevant factors were compared between the ED and historical cohort groups. The χ2 test was used for the comparison of categorical variables, and the Mann-Whitney U-test was used for the comparison of continuous variables. Overall Survival (OS) was calculated by the Kaplan-Meier method and compared by a log-rank test. P values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using the JMP software program (ver. 12.2.0, SAS, Cary, NC, USA).
Results
Patient characteristics
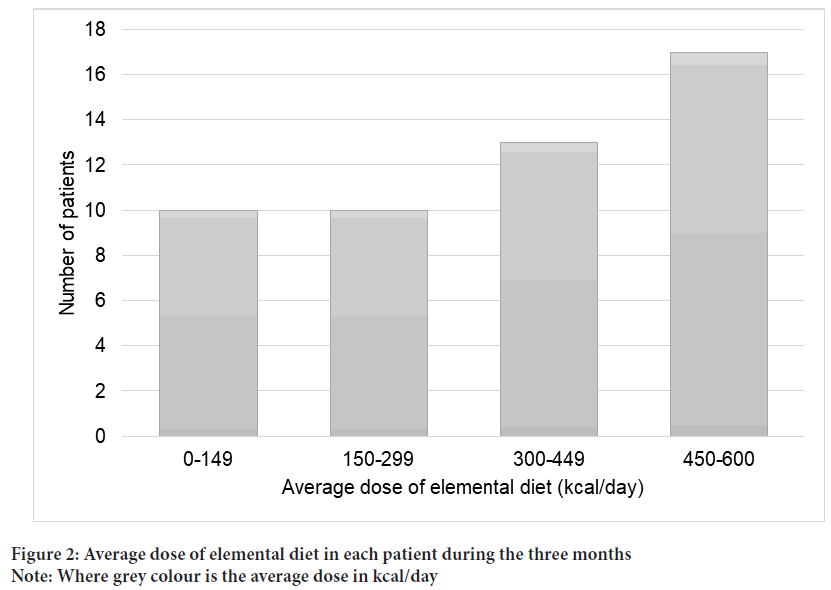
A total of 50 patients with unresectable PC were enrolled in the prospective cohort (ED group) between February 2017 and December 2019 (Figure 1). A total of 43 patients who started first-line chemotherapy for unresectable PC between December 2014 and December 2016 were eligible for inclusion in the historical cohort. The patient characteristics of the two of the two groups are shown in Table 1. In the ED group, clinical stage was worse and the serum CA19-9 levels were high. Otherwise, no significant differences were seen between the two groups, including the nutritional markers. The average dose of ED in each patient in the three months is shown in Figure 2. Thirty patients (60%) in the ED group took >300 kcal/day.
| Characteristics | ED group (n=50) |
Historical cohort (n=43) |
p-value |
|---|---|---|---|
| Age, years, median (range) | 69 (33-84) | 68 (48-78) | 0.23 |
| Male/female | 32/18 | 27/16 | 0.99 |
| Body mass index, kg/m2, median (range) | 21.4 (16.8-31.2) | 21.7 (14-27.5) | 0.81 |
| PS (0/1/2) | 12/34/4 | 7/36/0 | 0.09 |
| Tumor location, head | 16 | 17 | 0.52 |
| Biliary stent | 8 | 13 | 0.14 |
| Duodenal stent | 3 | 5 | 0.46 |
| Stage | |||
| Locally advanced | 8 | 19 | <0.01 |
| Metastatic | 42 | 24 | |
| Metastatic site | |||
| Liver | 25 | 8 | <0.01 |
| Lung | 15 | 2 | <0.01 |
| Peritoneum | 16 | 6 | 0.05 |
| Massive ascites | 4 | 1 | 0.37 |
| CEA, ng/mL, median (range) | 4.5(0.8-15000) | 3.5(0.25-30.9) | 0.2 |
| CA19-9, U/mL, median (range) | 709(2-12000) | 296(0.6-777) | <0.01 |
| Chemotherapy (first-line) | - | - | 0.22 |
| Folfirinox | 7 | 13 | |
| Gemcitabine+nab-Paclitaxel | 40 | 29 | |
| Gemcitabine | 2 | 1 | |
| S-1 | 1 | 0 | |
| Albumin, g/dL, median (range) | 3.9(1.7-4.6) | 3.9(3.2-4.4) | 0.32 |
| Total Protein, g/dL, median (range) | 6.8(5.6-7.8) | 6.8(4-8.2) | 0.32 |
| Diabetes | 20 | 24 | 0.13 |
| HbA1c,%, median (range) | 6.2(4.2-10.3) | 6.7(5.1-12) | 0.29 |
| PNI, median (range) | 45.1(22.1-56) | 46.5(37.5-53.5) | 0.24 |
Table 1: Baseline characteristics of the patients
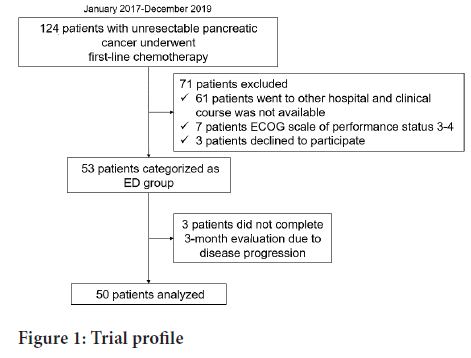
Figure 1: Trial profile
Figure 2: Average dose of elemental diet in each patient during the three months
Note: Where grey colour is the average dose in kcal/day
Serial changes of nutritional markers and tumor markers
The serial changes in nutritional markers and tumor markers are summarized in Table 2. The serial change in BMI, serum CEA, and serum CA19-9 was not statistically significant (P=0.51, 0.79 and 0.59, respectively). In the historical cohort, the serum albumin levels tended to show a much greater decrease (P=0.15). Furthermore, the serum total protein level and PNI were significantly decreased in the historical cohort (P=0.02 and P<0.005, respectively). While the serum HbA1c levels decreased in the historical cohort, it increased in the ED group, and the difference was statistically significant (P=0.02).
| Changes | ED group (n=50) | Historical cohort (n=43) |
p-value |
|---|---|---|---|
| Changes in albumin, g/dL, median (range) | -0.15(-1.4˜+1.3) | -0.2(-2.1˜+0.8) | 0.15 |
| Changes in total protein, g/dL, median (range) | -0.4(-1.7˜+1.1) | -0.6(-2.6˜+2.1) | 0.02 |
| Changes in body mass index, kg/m2, median (range) | -0.5(-6.41˜+4.93) | -0.7(-7.5˜+2.1) | 0.51 |
| Changes in PNI, median (range) | -1.6(-16.4˜+19.1) | -5(-27˜+6.5) | <0.005 |
| Changes in HbA1c,%, median (range) | 0.3(-3.6˜+4.8) | 0(-4.3˜+1.1) | 0.02 |
| Changes in CEA, ng/mL, median (range) | 0.4(-14868˜+233.6) | 0.36(-18.2˜+161.8) | 0.79 |
| Changes in CA19-9, U/mL, median (range) | -18.2(-11574˜+4602) | -113.6(-6762˜+66837) | 0.59 |
Table 2: Comparison of changes in nutritional markers from baseline to 3 months
Overall survival
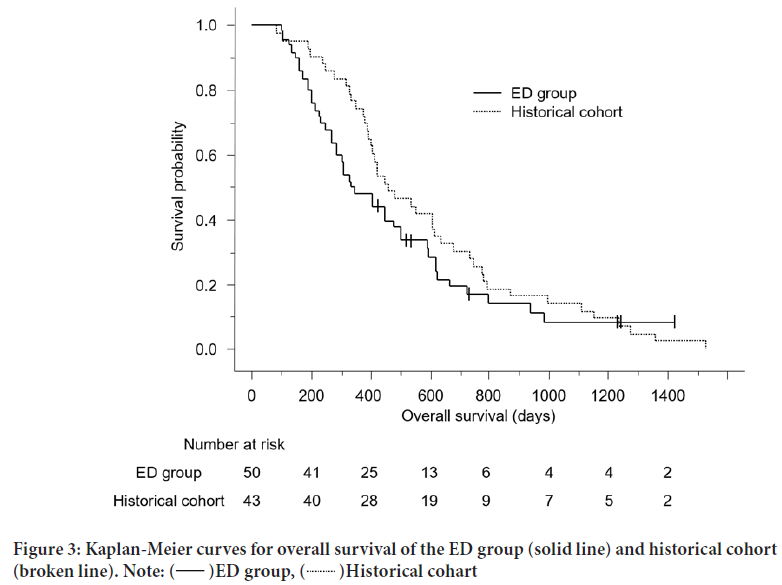
The Kaplan-Meier curves for OS are shown in Figure 3. The median OS was 341 days (95% confidence interval (CI), 270-500) in the ED group and 462 days (95% CI, 393-616) in the historical cohort (P=0.24).
Figure 3: Kaplan-Meier curves for overall survival of the ED group (solid line) and historical cohort
(broken line). 
Discussion
Our prospective cohort study of ED therapy in patients with unresectable PC suggested that the administration of an ED during systemic chemotherapy might improve the nutritional status in comparison to a historical cohort that did not receive an ED. The impact of the ED on the prognosis was unclear. Although cancer progression and the serum CA19-9 level in the ED group were worse in comparison to the historical cohort, the median survival time did not differ between the two groups. We believe that the role of ED in OS should be further investigated.
Patients with PC are known to be prone to EPI especially when the pancreatic duct is obstructed by the tumor (Sikkens EC, et al., 2014). Saito et al. showed that among patients with PC who underwent an NBT-PABA test, the results were lower than the normal threshold in 90% of the patients (Saito T, et al., 2017; Saito T, et al., 2018). The admission that the NBT-PABA (The N-benzoyl-L-tyrosyl-p-aminobenzoic acid) test is not an accurate test of the pancreatic exocrine function, the results, and tendency for patients with low NBT-PABA results to show low albumin and pre-albumin levels, suggested that EPI is quite prevalent in patients with PC, even in patients with PC located in the body or tail of the pancreas. The ED used in the present study (Elental®) is low in fat, is a good source of nitrogen and amino acids, and rarely requires a fully functional digestive system. Thus, we used this ED for patients with PC, without considering the cancer location. However, adherence to the ED was often low due to the odor and taste. In our study, pharmacists explained the efficacy of the ED and advised the patients how to take it individually using a leaflet. However, only thirty patients (60%) could take Elental® over 300 kcal/day. The present study was not sufficient to evaluate the effectiveness of the ED. It is essential to improve the flavor or the approach to counselling.
It has also been reported that Elental® does not seriously affect blood sugar control in diabetes patients, suggesting that its interference in perioperative blood sugar control is of minimal concern (Ohkura Y, et al., 2016). However, our study revealed that serum HbA1c levels were increased in the ED group. We hypothesize that the insulin levels of patients with PC decrease in comparison to patients with other cancers because the endocrine pancreas cells decline due to obstructive chronic pancreatitis. Moreover, 80% of Elental® consists of carbohydrate. Thus, blood sugar is a concern, and self-injected insulin must be introduced immediately for PC patients who receive ED therapy.
The nutritional status can potentially influence the clinical outcomes of chemotherapy for PC because serum albumin is a known prognostic factor for advanced PC (Zhang DX, et al., 2012). Thus, we decided to use the serum albumin level as the primary endpoint. According to our analysis, the serum albumin levels of patients in the ED group were slightly decreased at 3 months, whereas in the historical cohort, the serum albumin levels showed a much greater decrease. While there were no significant differences, these results suggested that an ED may maintain the nutritional status during chemotherapy for PC. In addition, the analysis of the serial changes in the serum totals protein level and PNI revealed a significant decrease in the historical cohort. Many studies found that the PNI is an independent prognostic indicator for various malignant tumors, including PC (Hua X, et al., 2019; Komura N, et al., 2019; Li S, et al., 2019). The preservation of the PNI may reflect that the ED maintained the nutritional status.
The present study was associated with some limitations. First, there was a selection bias in our study. To evaluate the impact of ED at 3 months after the introduction of chemotherapy, we excluded patients who developed early disease progression. Second, there were differences in the background characteristics. Cancer progression and the serum CA19-9 level in the ED group were substantially worse in comparison to the historical cohort. We consider that this occurred incidentally and because this was not a randomized controlled study.
Additionally, we evaluated BMI as one of the parameters of the nutritional status; however, the precise body composition was not evaluated in this study. It is possible that body weight might include the weight of ascites or edema; however, few patients in our study population had massive ascites (4 in the ED group and 1 in the historical cohort), and would have had little effect on our results.
Conclusion
In conclusion, we conducted this prospective cohort study to evaluate the role of ED therapy in patients with unresectable PC who were receiving systemic chemotherapy. This prospective study demonstrated that ED tended to improve the nutritional status during chemotherapy for unresectable PC. A prospective randomized study should be performed to evaluate the efficacy of ED therapy in patients with unresectable PC who receive systemic chemotherapy.
Acknowledgement
We express our deepest appreciation to Hirotaka Kanzaki (Department of Pharmacy, Okayama University Hospital) for statistical analysis.
Patient Consent
Informed consent was obtained from all individual participants included in the study.
Authors' Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hitomi Himei, Ryo Harada and Hironari Kato. The first draft of the manuscript was written by Hitomi Himei and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Japanese Red Cross Okayama Hospital (1/6/2017).
References
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016; 22: 9694-9705.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl JMed. 2011; 364: 1817-1825.
[Crossref] [Google scholar] [Pubmed]
- von Hoff DD, Ervin T, Arena FP, Chiorean GE, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl JMed. 2013; 369: 1691-1703.
[Crossref] [Google scholar] [Pubmed]
- Sikkens EC, Cahen DL, de Wit J, Casper L, van Casper E, Marco BJ, et al. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014; 48: e43-e46.
[Crossref] [Google scholar] [Pubmed]
- Kimura Y, Nishikawa K, Kishi K, Inoue K, Matsuyama J, Akamaru Y, et al. Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann Gastroenterol Surg. 2019; 3: 648-656.
- Yamazaki S, Takayama T, Higaki T, Moriguchi M, Yoshida N, Miyazaki T, et al. Pancrelipase with branched-chain amino acids for preventing nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Gastroenterol. 2016; 51: 55-62.
[Crossref] [Google scholar] [Pubmed]
- Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, et al. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol. 2015; 141:307-313.
[Crossref] [Google scholar] [Pubmed]
- Saito T, Hirano K, Isayama H, Nakai Y, Saito K, Umefune G, et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: A prospective cohort study. Pancreas. 2017; 46: 341-346.
[Crossref] [Google scholar] [Pubmed]
- Saito T, Nakai Y, Isayama H, Hirano K, Ishigaki K, Hakuta R, et al. A multicenter open-label randomized controlled trial of pancreatic enzyme replacement therapy in unresectable pancreatic cancer. Pancreas. 2018; 47: 800-806.
[Crossref] [Google scholar] [Pubmed]
- Ohkura Y, Haruta S, Tanaka T, Ueno M, Udagawa H. Effectiveness of postoperative elemental diet (Elental) in elderly patients after gastrectomy. World J Surg Oncol. 2016; 14:268.
[Crossref] [Google scholar] [Pubmed]
- Zhang DX, Dai YD, Yuan SX, Tao L. Prognostic factors in patients with pancreatic cancer. Exp Ther Med. 2012; 3: 423-432.
[Crossref] [Google scholar] [Pubmed]
- Hua X, Long ZQ, Huang X, Deng JP, He ZY, Guo L, et al. The value of Prognostic Nutritional Index (PNI) in predicting survival and guiding radiotherapy of patients with T1-2N1 breast cancer. Front Oncol. 2019; 9: 1562.
[Crossref] [Google scholar] [Pubmed]
- Komura N, Mabuchi S, Yokoi E, Shimura K, Kawano M, Matsumoto Y, et al. Prognostic significance of the pretreatment prognostic nutritional index in patients with epithelial ovarian cancer. Oncotarget. 2019; 10: 3605-3613.
- Li S, Tian G, Chen Z, Zhuang Y, Li G. Prognostic role of the prognostic nutritional index in pancreatic cancer: A meta-analysis. Nutr Cancer. 2019; 71: 207-213.
[Crossref] [Google scholar] [Pubmed]
Author Info
Hitomi Himei1, Hironari Kato1, Shigeru Horiguchi1, Hiroyuki Okada1, Ryo Harada2*, Ryosuke Sato2, Ayako Koike3, Hideki Mori3, Takashi Makita4 and Kumiko Ueda42Department of Gastroenterology and Hepatology, Japanese Red Cross Okayama Hospital, Okayama, Japan
3Department of Pharmacy, Japanese Red Cross Okayama Hospital, Okayama, Japan
4Department of Pharmacy, Okayama University Hospital, Okayama, Japan
Citation: Himei H: Effectiveness of an Oral Elemental Diet for Patients with Unresectable Pancreatic Cancer: A Prospective Cohort Study
Received: 02-May-2022 Accepted: 27-May-2022 Published: 03-Jun-2022, DOI: 10.31858/0975-8453.13.6.394-398
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3