Research Article - (2023) Volume 14, Issue 5
Abstract
Presently, evidence-based research studies on the efficacy and safety of mRNA COVID-19 vaccines are limited. The objective of this study is to conduct a systematic review and meta-analysis of Randomized Controlled Trials (RCTs) to learn about the efficacy of mRNA COVID-19 vaccines and the side effects associated with them. We used five databases to conduct an electronic search of material published between 2020 and June 2021. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) declaration was used to create and report on a procedure for a systematic review and meta-analysis. The systematic review comprised eleven RCTs, and nine RCTs were included for meta-analysis. To assess bias risk, the Cochrane collaboration tool was employed. In a total of 70604 cases, the overall effectiveness of the mRNA vaccines was determined to be 94.6% (95% CI 0.04-0.08). The administration of mRNA-based vaccine was associated with a greater number of side effects, such as, injection site pain, fever, swelling, fatigue, headache, chills, by yielding a summary OR of 4.35 (95% CI 2.05-9.24), 8.50 (95% CI 3.03-23.86), 3.66 (95% CI 1.12-12.01), 1.15 (95% CI 0.049-2.72), 1.49 (95% CI 0.90-2.46), 5.72 (95% CI 5.24-6.24) respectively. In all investigations, the mRNA-based COVID-19 vaccines caused mild to moderate local and systemic adverse effects following the first and second doses of immunization. mRNA vaccines were shown to have an overall effectiveness of 94.6%. The findings demonstrate the overall safety and effectiveness of all currently available mRNA-based COVID 19 vaccines, giving unambiguous data-driven evidence to support the continuing worldwide public health endeavor to immunize the whole population.
Keywords
mRNA COVID-19 vaccine, mRNA SARS- CoV-2 vaccine, COVID-19 vaccination trials, Vaccine safety, Vaccine efficacy
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 was first re- ported in a province of China called Wuhan. Also known as SARS-CoV-2, it is a single-stranded, unsegmented positive-sense ribonucleic acid coronavirus (Pormohammad A, et al., 2021). The infection rooted from SARS-CoV-2 leads to the COVID-19 (Pormohammad A, et al., 2021). It was on 11th March 2020 that the World Health Organization (WHO) declared the outbreak as a pandemic (Sahin U, et al., 2020). As of June 18, 2021 WHO reported 177,108,695 confirmed cases of COVID-19 includ- ing 3,840,223 deaths globally (WHO, 2021). India is also one of the worst affected countries which have left with a staggering 29,762,793 confirmed cases and 385137 deaths, as per the report of Ministry of health and family welfare on June 18, 2021 (WHO, 2021; MoHFW, 2021).
The Indian government has taken several steps to combat against the increasing cases, be it making it mandatory to wear masks and imposing fines on those who don’t, passing new guidelines for public transports and areas, imposing night or weekend curfews or even going under a full lockdown. But to control this pandemic, vaccines are a critical tool and getting vaccinated is one of the best ways to safeguard oneself and others from COVID-19 infection and spread. Vaccines instruct our immune system to recognize the targeted virus and develop antibodies against that virus to fight off the disease without getting disease itself (WHO, 2021).
SARS-CoV-2 chiefly has four principle structural proteins: S protein or spike protein, M protein or membrane protein, and Envelope (E) protein, these three are a part of the viral surface envelope and the N protein or nucleocapsid protein found in the ribonucleoprotein core. Between these surface proteins, the trimeric S-protein plays the role of primary target for vaccine development against SARS-CoV-2 due to its receptor recognition for the cell entry and cell membrane fusion process. S-protein contains two subunits: S1 and S2. S1 consists of RBD (Receptor-Binding Domain), whose role is identifying and binding to the host receptor: Angiotensin-Converting Enzyme 2 (ACE2), whereas S2 helps mediate the membrane fusion by creating a six-helical bundle. The spike protein and the Receptor Binding Domain act as capable targets for SARS-CoV-2 vaccines and the predominant antigenic target for developing a vaccine (Pormohammad A, et al., 2021).
S-protein plays the role of primary target for vaccine development against SARS-CoV-2 due to its receptor recognition for the cell entry and cell membrane fusion process. S-protein contains two subunits: S1 and S2. S1 consists of RBD (Receptor-Binding Domain), whose role is identifying and binding to the host receptor: Angiotensin-Converting Enzyme 2 (ACE2), whereas S2 helps mediate the membrane fusion by creating a six-helical bundle. The spike protein and the Receptor Binding Domain act as capable targets for SARS-CoV-2 vaccines and the predominant antigenic target for developing a vaccine (Pormohammad A, et al., 2021).
COVID-19 vaccines are not investigational, ongoing clinical trials have revealed COVID-19 vaccines as safe and effective way to build immunity against deadly virus by acting as a booster that strengthen the immune response. COVID-19 vaccines also protect from getting serious illness even if one gets COVID-19 after vaccination. Studies have shown that vaccinated people who get infected only have mild to moderate cases of COVID-19 as compared to those non-vaccinated. Thus, vaccine nearly eliminates the risk of hospitalization and death once immunized. Therefore, getting vaccination is safer means to protect population from any complications relating to COVID-19 (CDC, 2021).
Numerous methods have been acknowledged to build-up effective, safe and reliable vaccines against COVID-19. Different types of vaccines, as per Cochrane vaccine mapping tool, undergoing different phases of Clinical and pre-clinical development are as follows: RNA based vaccines, Non replicating viral vector, Protein subunit, Inactivated virus, DNA based vaccine, Replicating viral vector, Virus-like particle, Viral vector (Non-replicating)+APC (Antigen Presenting Cell), Live attenuated virus, RNA based vaccine+monoclonal antibodies, Inactivated virus+non COVID vaccine, Non replicating viral vector+RNA based vaccine (Cochrane, 2021).
Among all the above-mentioned approaches, mRNA-based method is one of the latest approaches to vaccine production. The mRNA vaccines contain an RNA molecule conveying a fragment of peptide or protein coding sequence from the virus that can be synthesized in the ribosomes resulting in generation of antigen which induce an immune reaction accompany with antibodies production (Pardi N, et al., 2018).
Recently, Pfizer and Moderna have developed mRNA vaccine by application of synthetic mRNA encoding in which the protein sequence of SAR CoV-2 spike protein (S-protein) is encapsulated within a lipid vesicle nanoparticle (Pormohammad A, et al., 2021).
For a vaccine to be used effectively in medical care and public health systems, they must meet the vital core competencies of vaccine which include efficacy and safety (adverse event, side effect profiles). There have been several studies conducted on the efficacy of COVID-19 vaccines but as per our knowledge there is a dearth in the comprehensive relative studies carried out on the safety and efficacy of mRNA COVID-19 vaccines.
The objective of undertaking this study is to know the efficacy of the mRNA COVID-19 vaccines and the varying side effects experienced because of them.
Materials and Methods
Search strategy
We searched all publications on clinical trials related to mRNA COVID19 Vaccines from the following databases: PubMed, Google Scholar, Science Direct, Cochrane and Web of Science and articles were selected using the MeSH terms “mRNA COVID-19 Vaccine”, “mRNA SARS-CoV-2 Vaccine” AND “COVID19 Vaccination trials” “clinical trial”, “phase trial”, “randomized control trials”, “control clinical trial” OR “Vaccine safety”, “Vaccine efficacy”. All articles published in the year 2020-2021 were searched only in English language by four independent reviewers. References and citations of eligible articles were also reviewed for any relevant literature. To carry out this analysis PRISMA instructions were strictly followed. Additional studies were identified by manually checking the reference list of included articles.
Selection process
Initially, articles were first reviewed by four independent reviewers (NC, SC, AS, RKS) based on title and abstract, all irrelevant and ineligible publications were eliminated and full texts articles of pertinent papers were assessed for eligibility and disagreements were resolved by discussion and for each publications consensus was reached.
Inclusion criteria
At the time of screening, all the studies on clinical trials were included in the systematic review, while in the meta-analysis we included randomized control trials studies in phase I, II, III of mRNA COVID 19 vaccines independent of applied dose, treatment or study duration, age, sex. Only full text articles in English were taken down.
Exclusion criteria
Studies excluded for the meta-analysis were: Non-randomized or pre-clinical studies, those studies with no control or placebo group, letters to the editors, studies with no significant extractable data, review articles and news reports. On the other hand, in systematic review non-randomized studies were included.
Data extraction
Four independent reviewers extracted data from the published studies that were chosen. The following data were obtained from each article:
1. First authors
2. Published year
3. Title of study
4. Name of vaccine
5. Company
6. Trial initiation date
7. Trial phase
8. Study design
9. Inclusion and Exclusion criteria
10. Age group
11. Trial Country
12. Storage temperature
13. Dosage, route of administration, Dosage interval
14. Experimental group
15. Control group
16. Side effects
17. Efficacy-related data
Four of the authors extracted data independently and reviewed extracted data randomly. Any discrepancies or inconsistencies were fixed through discussion.
Quality assessment
Four independent reviewers assessed the quality of the included articles using the Cochrane risk tool/Cochrane assessment tool. The Cochrane assessment tool included the seven quality parameters of random sequence generation, allocation concealment, blinding of patients and personnel, binding outcome assessment, incomplete data outcome addressed, selective reporting and other bias. Two questions were asked for all the parameters in form of Yes or No. In seven studies treatment allocation was concealed. Patients were blinded in six studies. The outcome assessor was blinded in four studies.
Data analysis
Starting with data cleaning, the data was then prepared for analysis which was done in Microsoft Office 365 and analysis was finally performed with the help of Meta-Essential Software Version 1.5. The point estimates of the effect size, odds ratios, and 95% confidence intervals were calculated to make an estimate about the vaccine efficacy and side effects. Random effects model was used to estimate the pooled effects. Moreover, to know the heterogeneity between studies, the I2 statistic was used. The presence and effect of publication bias were examined.
Results
Characteristics of included studies
In total 52,566 publications were screened for COVID-19 vaccines’ side effects and efficacies. Out of these studies, 11 met the systematic review’s inclusion criteria (non-randomized and randomized), while 9 randomized studies were included in the meta-analysis (Figure 1). The characteristics of selected articles have been summarized in Table 1. Studies with different vaccine phase reports, number of doses, injection concentration, different case, and control group numbers are considered as same dataset for the meta-analysis. Only studies in English were included. Out of 9 randomized studies, 6 were double-blinded. In seven studies treatment allocation was concealed. Patients were blinded in six studies. The outcome assessor was blinded in four studies (Table 2).
| S. No. | Author/Year | Journal/Database | Company | Trial initiation date | Trial Phase | Sample size(N1=Experimental group; N2=Control/Placebo group) |
|---|---|---|---|---|---|---|
| 1 | Polack FP, 2020 | The New England Journal of Medicine/ PubMed | Pfizer- BioNtech | July 27, 2020 | II/III | N1=18,860; N2=18,846 |
| 2 | Baden LR, 2020 | Moderna | July 27 and October 23, 2020 | III | N1=15,210; N2=15,210 | |
| 3 | Walsh EE, 2020 | Pfizer-BioNtech | 4 May and 22 June, 2020 | I | N1= 156; N2=39 | |
| 4 | Kremsner P, 2020 | The Central European Journal of Medicine/ Google Scholar | CureVac | June, 2020 | I | N1=216; N2=32 |
| 5 | Mulligan MJ, 2020 | Nature/ PubMed | Pfizer-BioNtech | 4 May and 19 June, 2020 | I/II | N1=36; N2=9 |
| 6 | Anderson EJ, 2020 | The New England Journal of Medicine/ PubMed | Moderna | 16 April and 12 May, 2020 | I | N=40 |
| 7 | Chu L, 2021 | Vaccine/PubMed | Moderna | 29 May and 8 July, 2020 | II | N1=400; N2=200 |
| 8 | Sahin U, 2020 | Nature/ PubMed | Pfizer- BioNtech | 23 April and 22 May, 2020 | I | N1=72; N2=18 |
| 9 | Jackson LA, 2020 | The New England Journal of Medicine/ PubMed | Moderna | March 16 and April 14, 2020 | I | 45 (3 Cohorts of 15(25 mcg)+15(100 mcg)+15(250 mcg) participants) |
| 10 | Frenck RW, 2021 | The New England Journal of Medicine/ PubMed | Pfizer- BioNtech | October 15 and January 12, 2021 | II/III | N1=1131; N2= 1129 |
| 11 | Li J, 2021 | Nature Medicine/ PubMed | 18 July and 14 August, 2020 | I | N1=96 ; N2= 48 |
Table1: Characteristics of included studies
| S. No | Author/Year | Random sequence generation | Allocation concealment | Blinding of patient and personnel | Blinding outcome assessment | Incomplete outcome data addressed | Selective reporting | Other Bias |
|---|---|---|---|---|---|---|---|---|
| 1 | Polack FP, 2020 | Yes | Yes | Yes | No | Yes | No | No |
| 2 | Baden LR, 2020 | Yes | No | No | No | Yes | Yes | No |
| 3 | Walsh EE, 2020 | Yes | Yes | Yes | Yes | No | No | No |
| 4 | Kremsner P, 2020 | No | Unclear | No | Unclear | Yes | Yes | No |
| 5 | Mulligan MJ, 2020 | Yes | Yes | Yes | No | Yes | No | No |
| 6 | Anderson EJ, 2020 | Yes | No | No | No | Yes | No | No |
| 7 | Chu L, 2021 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 8 | Sahin U, 2020 | Yes | No | No | Unclear | Yes | No | No |
| 9 | Jackson LA, 2020 | No | Yes | Yes | Yes | Yes | Yes | No |
| 10 | Frenck RW, 2021 | Yes | Yes | Yes | Yes | Yes | No | No |
| 11 | Li J, 2021 | Yes | Yes | No | No | Yes | Yes | No |
Table 2: Quality assessment of selected studies
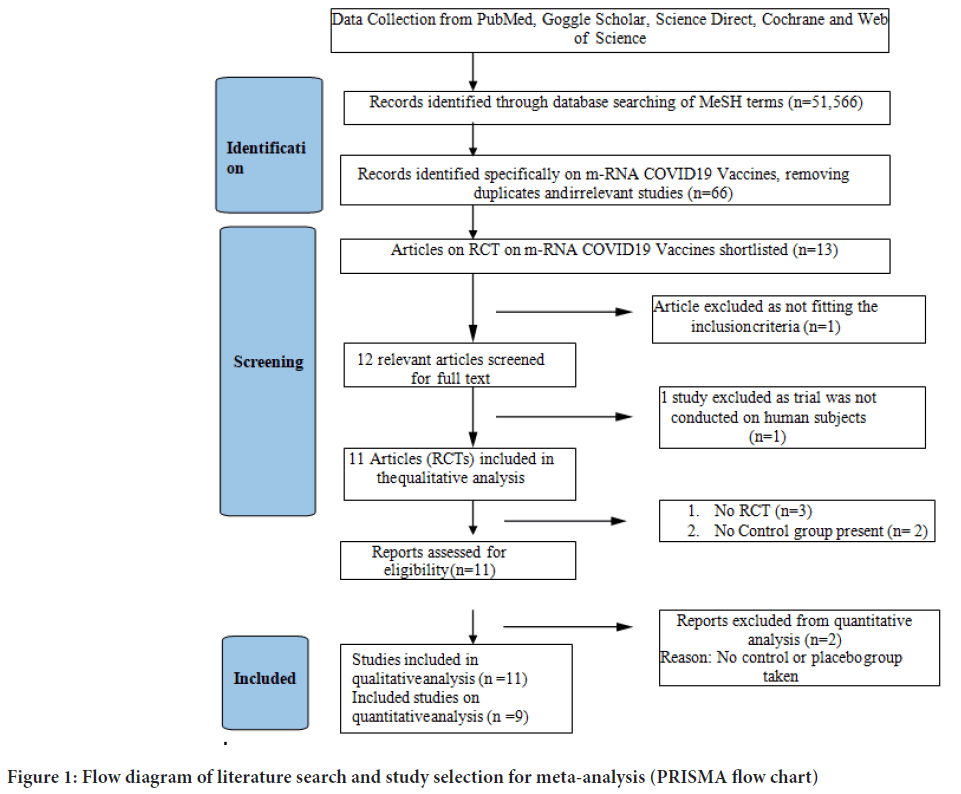
Figure 1: Flow diagram of literature search and study selection for meta-analysis (PRISMA flow chart)
Characteristics of participants
In total 71704 cases were included in the study. Out of these, 36173 received the COVID-19 vaccine and 35531 controls who received placebo were included in this study. All vaccines and placebos were Intramuscularly (IM) injected. The participants were from different age group varying from 16 years of age to over 65 years of age. And they were injected with different concentrations of the vaccine given in two phases: I and II.
Efficacy of mRNA COVID-19 vaccines
The overall efficacy of the mRNA vaccines was found out to be 94.6% (95% CI 0.04-0.08) efficacy in a total of 70604 cases (Figure 2).
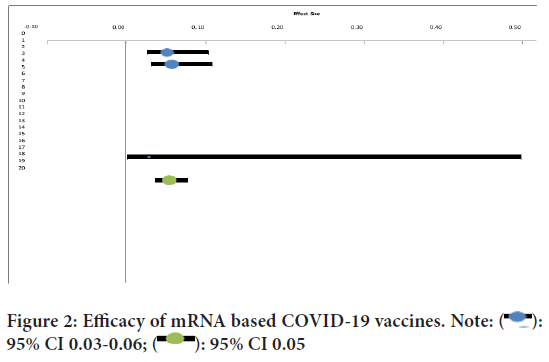
Figure 2: Efficacy of mRNA based COVID-19 vaccines. Note:  :
95% CI 0.03-0.06;
:
95% CI 0.03-0.06;  : 95% CI 0.05
: 95% CI 0.05
Side effects of mRNA COVID-19 vaccines
The administration of mRNA-based vaccine was associated with a greater number of side effects, such as, injection site pain, fever, swelling, fatigue, headache, chills, by yielding a summary OR of 4.35 (95% CI 2.05-9.24), 8.50 (95% CI 3.03-23.86), 3.66 (95% CI 1.12-12.01), 1.15 (95% CI 0.049- 2.72), 1.49 (95% CI 0.90-2.46), 5.72 (95% CI 5.24-6.24) respectively.
Serious adverse side effects of mRNA COVID-19 vaccines
It is expected with any vaccine that there would be some temporary side effects due to activation of body’s immune response and also due injection site trauma. There can be both, perceived (in case of placebo groups) and real adverse side effects, which can be short-term and long-term. This study indicates that RNA-based vaccines too have higher incidences of side effects, including injection site pain, swelling, fever, headache, fatigue, and chills. Additionally, it was also seen that rate of serious adverse side effects like anaphylactic shock, was not very high. A few participants had severe adverse events; four serious adverse events were seen with BNT162b2 recipients. Apart from this there were two deaths of BNT162b2 recipients, one died from arteriosclerosis and one from a cardiac arrest. In the placebo group participants, one participant died from hemorrhagic stroke, one from myocardial infarction and two died from unidentified causes. Bell’s palsy occurred in 3 participants who received the vaccine, and one from the placebo group. Some cases of hypersensitivity were also observed.
Discussion
The motive of vaccination is to shield individuals from infection and transmission. Albeit the exigency use allowance for some of the COVID-19 vaccines has been permitted by the Food and Drug Administration in the US and the Department of Health and Human Services of each country, the vaccines’ efficacy and side effects have not yet been extensively discussed, although popular media and politicians have made many uncorroborated claims. Consequently, in the prevailing meta-analysis, we provide systematic and comprehensive data regarding the vaccines’ safety and efficacy against SARS-CoV-2. Here, we mainly focused on available mRNA focused RCTs publications on the safety and efficacy of COVID-19 vaccines. The present study was carefully surveyed for the general and specific target antigen efficacy of each mRNA vaccine group. Our analysis showed that variation in the efficacy of vaccines after the first doses are noteworthy in comparison with the efficacies after the second doses. Therefore, matriculation of the second dose should produce a more reliable outcome and efficacy compared to a single dose. In total, mRNA-based COVID-19 vaccines had 94.6% efficacy. The mRNA-based vaccine evoked high levels of neutralizing antibodies after one month of the first (70%) and second (99.5%) doses. Safety against variants has been shown with the mRNA- based vaccine against the United Kingdom (B.1.1.7, also called 20I/501Y. V1) variant (Pormohammad A, et al., 2021; Muik A, et al., 2021; Shen X, et al., 2021; Bernal JL, et al., 2021), however they may be less effective against the variant first detected in South Africa (B.1.351, known as 20H/501Y. V2) (Pormohammad A, et al., 2021; Shen X, et al., 2021; Mascola JR, et al., 2021). A week after the second dose of mRNA-based vaccine, induction of neutralizing antibody titers in the serum sample was 6-fold lower for participants bearing B.1.351 variant compared to original Wuhan-Hu-1 spike protein (Pormohammad A, et al., 2021; Mascola JR, et al., 2021; Bian L, et al., 2021).
Conclusion
The mRNA based COVID-19 Vaccines has shown mild to moderate local and systemic side effects after first and second doses of vaccination in all studies. The overall efficacy of mRNA vaccines was found out to be 94.6%. Only a small number of people who receive the vaccine have experienced serious adverse events. All RCTs based studies followed up the experimental and control (placebo) groups after one month after both prime and booster doses, therefore, all reports are related to short term impacts. The long-term assessment of mRNA COVID 19 Vaccine was not carried out. This meta-analysis allows us to assimilate pertinent new evidence for summarizing and analyzing the clinical manifestations of all available mRNA COVID 19 Vaccines in randomized controlled trials phase I, II, and III. The outcome supports the overall safety and efficacy of all current mRNA based COVID 19 vaccines, providing clear data driven evidence to support the ongoing global public health effort to immunize the whole population.
Limitations
• The study focuses only on mRNA vaccines, does not focus on all the other kind of vaccines currently available for use.
• The overall side effects and efficacy have been reported, not taking into count the first and second doses separately.
• Some studies included had considerable bias.
• The study does not focus on the age diversity and different dose concentrations of vaccines.
• There is a lack of data on specific categories like kids and pregnant ladies, only healthy adults were included in the experimental and control groups.
• Data on long term safety and duration of efficacy was not focused on. Only short-term impacts of the mRNA vaccine were targeted.
• Our systematic review and meta-analysis only considered studies published in English language.
• The subgroup analysis of the dose concentrations and age groups was not conducted.
References
- Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. Efficacy and safety of COVID-19 vaccines: A systematic review and meta-analysis of randomized clinical trials. Vaccines. 2021; 9(5): 467.
[Crossref] [Google Scholar] [Pubmed]
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020; 586(7830): 594-599.
[Crossref] [Google Scholar] [Pubmed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. World Health Organization. 2021.
- MoHFW. India COVID-19 vaccine tracker. Ministry of Health and Family Welfare. 2021.
- WHO. Getting the COVID-19 vaccine. World Health Organization. 2021.
- CDC. Benefits of getting a COVID-19 vaccine. Centers for Disease Control and Prevention. 2021.
- Cochrane. The COVID-19 NMA initiative. Cochrane. 2021.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. 2018; 17(4): 261-279.
[Crossref] [Google Scholar] [Pubmed]
- Muik A, Wallisch AK, Sänger B, Swanson KA, Mühl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B. 1.1. 7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021; 371(6534): 1152-1153.
[Crossref] [Google Scholar] [Pubmed]
- Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, et al. SARS-CoV-2 variant B. 1.1. 7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021; 29(4): 529-539.
[Crossref] [Google Scholar] [Pubmed]
- Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021; 385(7): 585-594.
[Crossref] [Google Scholar] [Pubmed]
- Shen X, Tang H, Pajon R, Smith G, Glenn GM, Shi W, et al. Neutralization of SARS-CoV-2 variants B. 1.429 and B. 1.351. N Engl J Med. 2021; 384(24): 2352-2354.
[Crossref] [Google Scholar] [Pubmed]
- Mascola JR, Graham BS, Fauci AS. SARS-CoV-2 viral variants-tackling a moving target. JAMA. 2021; 325(13): 1261-1262.
[Crossref] [Google Scholar] [Pubmed]
- Bian L, Gao F, Zhang J, He Q, Mao Q, Xu M, et al. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021; 20(4): 365-373.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Neeru Chaudhary*, Rakhi Ahuja, Amena Sherwani and Surbhi ChauhanCitation: Chaudhary N: Efficacy and Safety of mRNA based COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Received: 24-Apr-2023 Accepted: 18-May-2023 Published: 25-May-2023, DOI: 10.31858/0975-8453.14.5.301-305
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






