Research Article - (2022) Volume 13, Issue 10
Abstract
The ever-increasing spectrum of microbial disease and emergence of life-threatening antibiotic-resistant pathogens necessitates more intensive search for microbial antagonistic agents from diverse symbionts and habitats. Based on these hypotheses the current study was initiated to investigate in vitro antibacterial and antifungal activities of both crude and pure compounds isolated from potential Actinomycetes isolates recovered from soda lakes of Ethiopia. Two soda lakes, Arenguade/Hora Hadho/Chitu were included in this study. A total of fifteen 12 water samples were collected and Actinomycetes cultures were isolated by serial dilution plating technique on various media. Morphological and biochemical characterization of selected isolates was done as described in the International Streptomyces Project Guidelines. Minimum inhibitory concentrations of the crude extract was determined for fungus using the protocol described in National Committee for Clinical Laboratory Standards and for the bacteria disc diffusion assay method as described in the Clinical and Laboratory Standards Institution. Ethanol extracts obtained from Solid State Fermentation a total of 36 isolates of Acti nomycetes were subjected to primary screening and tested for activity against the bacterial and fungal reference strain. Accordingly, the extracts from 10 isolates that were most active in the primary screen were 6 (16.66%) from Lake Chitu that were inhibited the growth of Gram-positive bacterial and fungal strain; 4 (11.11%) from Lake Arenguade that were inhibited Gram-negative bacteria and fungal. From evaluated the antibacterial and antifungal potential of two soda lakes water-derived Actinomycete; the best ones in terms of activity spectra were Actinomycetes species isolated from Lake Chitu.
Antibacterial and antifungal metabolite production was detected from isolates of Lake Chitu with maximum zone of inhibition within 5-6 days of incubation against Candida albicans ATCC 62376, Aspergillus niger, C. neoformans, F. oxysporum f. sp. melonis (CECT 20474), and Shigella boydii (clinical). All test microorganisms, except Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and S. aureus (clinical isolate) were sensitive to the culture filtrate starting from 8-12 days of incubation. In terms of the effectiveness of the antibacterial activity, the antibacterial metabolite produced from different carbon sources were more effective on S. aureus (clinical isolate), A. niger, C. neoformans, S. aureus ATCC25923, Fusarium oxysporum f. sp. melonis (CECT 20474), Shigella boydii (clinical isolate) respectively. However, the most resistant test isolates with least activity was detected against Candida albicans ATCC 62376 and E. coli ATCC 25922.
Keywords
Actinomycetes, Antibiotics, Microbial diversity, Soda lakes, Streptomyces
Introduction
The emergence of multi-drug resistant pathogens necessitates continuous search for novel antimicrobial agents. There is the continuous need for novel antibiotics to overcome the serious problem of evolving pathogens, naturally resistant bacteria and fungi, and multidrug resistance among common bacterial pathogens. Also, searching for novel strains from pristine environments is an important approach for obtaining novel bioactive molecules. Ironically, despite the extensive research done on antibiotic-producing Streptomycetes, interactions of Actinomycetes with other bacteria, the ecological rationale and physiological property of the Streptomycetes the extent to which produce antibiotics is the most striking and poorly understood (Barka EA, et al., 2016). Among the microbes, the secondary metabolites recovered from the soda lakes and untouched forest soil Actinomycetes were more added values (Kumar MS and Das AP, 2016; Katz L and Baltz RH, 2016; van der Meij A, et al., 2017; Avinash TS and Ravishankar RV, 2017; Ganesan P, et al., 2017). New resistant strains emerge more quickly while the rate of discovery of new antibiotics is slowing down. Today, superbugs are estimated to cause 700,000 deaths every year and are expected to rise to 10 million deaths each year by 2050 (BBC, 2018). This necessitates screening for new and novel drugs producing microorganisms, including Actinomycetes (Singh LS, et al., 2009; Rajivgandhi G, et al., 2019).
In this regard, Ethiopian soda lakes and untouched soil habitats from various climatic zones possessing remarkable biodiversity may harbor new Actinomycetes strains that have the potential for new antibiotic discovery. Previous studies by a few investigators confirmed the existence of potential antibiotic-producing Actinomycetes from different ecosystems of Ethiopia. Accordingly, alkaliphilic Actinomycetes capable of producing antimicrobial metabolites active against bacteria and fungi were isolated from Ethiopian soda lakes (Biniam W, 2018; Mehabie D, 2011; Gebreyohannes G, et al., 2013) rhizosphere of different plants, farmlands and garden soils (Atsede M and Fassil A, 2018; Bizuye A, et al., 2013). Most microorganisms are still untapped in terms of their capacity to produce secondary metabolites since only a small fraction can be cultured in the laboratory. Thus, improving cultivation techniques to extend the range of secondary metabolite producers accessible under laboratory conditions is an important first step in prospecting underexplored sources for the isolation of novel antibiotics. For industrial application, further, purification, structural elucidation, characterization, and optimization of the culture condition are required (Kibret M, et al., 2018).
Materials and Methods
Description of the study area
Two soda lakes, namely, Arenguade/Hora Hadho/Chitu were included respectively in this study. As a result of their sensitivity to ecological disturbances, soda lakes are among the ecological systems for which continuous scientific investigations on their microbial diversity should be given high priority (Kebede E, 1997).
Lake Chitu is located at a distance of about 180 Km South of Addis Ababa at an altitude of 1600 m above sea level (Saha MR, et al., 2010). Likewise, Lake Arenguade is located about 50 Km Southeast of Addis Ababa. For this study, Lake Chitu and Lake Arenguade were thus chosen for their peculiar and interesting features to understand the microbial ecology of the soda lakes in the region. These lakes are similar in that both are crater in origin, have closed basins, have very small catchment areas (limited external material input), and are highly productive. On the other hand, they have several differences. Lake Chitu is shallow (max. depth 17 m), located inside the rift valley in semiarid lowland area (1600 m.a.s.l.) and is highly alkaline (>600 meq/L) and saline (>6%), while Lake Arenguade is deeper (max. depth 32 m), located outside the rift valley in the highland area around Bishoftu (2000 m.a.s.l) and is less saline (<0.3%) and alkaline (about 50 meq/L). These features are good bases for a comparative microbial community study across sharp environmental gradients (Saha MR, et al., 2010).
Water sample collection
From each lake, a total of 12 water samples were collected in 500 mL sterile screw-capped bottles and sufficient space was provided for aeration and thorough mixing as described in Duckworth AW, et al., 1998. All samples were labeled and coded as LCHAACC13 (Lake Chitu Alkaliphilic Actinomycetes Culture Collection), LAAACC15 (Lake Arenguade/Hora Hado/ Alkaliphilic Actinomycetes Culture Collection), followed by a specific number to indicate isolate number. Samples were transported to Adama Science and Technology University, Applied Microbiology Laboratory and stored in the refrigerator at 4°C until analyzed.
Isolation and screening of alkaliphilic Actinomycetes
Media for the cultivation of Actinomycetes: For the cultivation of Actinomycete, both nutrient limiting and nutrient-rich media were used. The isolation media for the two soda lakes were chosen based on the culturability of general actinobacterial and the geochemical properties of the soda lakes (NCCLS, 2006). Actinomycetes cultures were isolated by serial dilution plating technique on various media including Starch Casein Agar (SCA) composed of (g/l) soluble starch, 10; Casein, 0.3; KNO3, 2; NaCl, 2; K2HPO4, 2; MgSO4.7H2O, 0.05; CaCO3,0.02; FeSO4.7H2O, 0.01; agar, 18; and Na2CO3, 10; and distilled water, 1,000 ml. starch nutrient agar, and Humic Acid agar composed of (g/l) humic acid, 1; Na2HPO4,0.5; KCl, 1.71; MgSO4.7H2O, 0.05; FeSO4.7H2O, 0.01; CaCO3, 0.02; yeast extract, 1 and; agar, 18. Na2CO3 was sterilized (at 121°C, 15 psi) separately as a 25% solution and added to the rest of the medium after cooling. To inhibit Gram-negative bacterial contamination 1 ml nalidixic acid was added to the medium from a stock solution prepared by dissolving 0.01 g of nalidixic acid in 10 ml of 100% methanol by swirling and mixing in a 37°C water bath (NCCLS, 2006).
Morphological and biochemical characterization of selected isolates: Identification of Actinomycetes to the genus level was done by a polyphasic approach, including cultural, morphological, physiological, and biochemical characteristics as described in the International Streptomyces Project Guidelines. The isolation media for the five sites were chosen based on the culturability of general actinobacterial and the geochemical properties of the soda lakes and forest soils. The four media Starch Casein Agar, Starch Casein Broth, Actinomycetes Isolation Agar, and Glycerol Asparagine Agar media were used in combination with temperature (28°C-55°C), salt (1%-10% w/v) and pH (7.0-13) ranges to simulate the sampling environment. Glycerol Asparagine Agar media was designed for the isolation of Actinomycetes from Chitu and Arenguade soda lakes, whilst the rests are general actinobacterial media.
Bacterial and fungal reference strain: Shigella boydii(clinical isolate), and Fusarium oxysporumf. sp. melonis(CECT 20474), Aspergillus niger, Can- dida albicansATCC 62376 and C. neoformans(clinical isolates) were used as bacterial and fungal reference (test) strains. The test bacterial and fungal strains were obtained from Ethiopian Health and Nutrition Research Institute (EHNRI) and Biomedical Laboratory, Faculty of Life Sciences, AAU.
Media for test microorganisms
Nutrient agar and nutrient broth: For the antimicrobial susceptibility test and screening, those test bacteria were seeded on nutrient agar media. Twenty-eight grams of Nutrient Agar (NA) (based on manufacturer’s recipe) was dissolved in 1 L of distilled water and sterilized at 121°C, 15 psi. Then about 25 ml of sterile NA media was poured on sterile Petri-dishes and allowed to cool overnight before use. Similarly, 13 g Nutrient Broth dissolved in 1 L distilled water and sterilized. About 5 ml of sterile nutrient broth was added on sterile screw-capped test tubes then inoculated with test microorganisms and incubated overnight at 37°C. The broth culture was adjusted to 0.5 McFarland standards before it was used (NCCLS, 2006).
Muller Hinton agar: For the antibacterial metabolite susceptibility test, thirty-eight grams of Muller Hinton agar based on the manufacturer’s recipe were dissolved in 1 L of distilled water and sterilized at 121°C, 15 psi. About 25 ml was poured into sterile Petri dishes to grow the test microorganisms (bacteria).
Standardization of inoculum
Standardization of the inoculum density of isolates for susceptibility test was done by the method described in Bharti A, et al., 2010. To determine the active phase of test organisms, each isolate was grown in 100 mL of nutrient broth for bacteria and Potato Dextrose Agar for fungi in 250 mL Erlenmeyer flask on a rotary shaker at 120 r/min and 37°C. Samples were taken every 2 h and the density of the turbidity standard was determined using a spectrophotometer (JENWAY, London) at 660 nm. The optical density values were extra plotted against time to determine the different phases of the growth curve. Samples from the exponential phase were taken to adjust the inoculum density with 0.5 McFarland turbidity standard prepared by adding about 0.05 ml of 1% BaCl2 and 9.95 ml of 1% H2SO4 were mixed to give 0.5 McFarland standards by which approximately 1.5 × 108 bacterial cell suspensions are expected to be present per ml of culture as cited in Singh LS, et al., 2009.
Primary screening of Actinomycetes for antimicrobial activity
Antimicrobial activity was first screened using the conventional agar cylinders method on Potato Dextrose Agar (PDA) and Muller Hint Agar (MHA) plates for fungi and bacteria respectively (Benhadj M, et al., 2019; Wayne PA, 2002). Mycelium plugs (7 mm diameter) of active isolates incubated at a different time (3, 7, 10, and 14 days) were inoculated onto PDA and MHA plates previously inoculated with target pathogens. Secondly, a double layer method was used for confirmation. The active strain was inoculated as a spot in the center of ISP2 plates at 30°C for 7 days. After incubation, the plates were then covered by 10 ml of PDA and MHA previously inoculated with target fungi and bacteria respectively (Benhadj M, et al., 2019). The inhibition zones around each spot were measured (mm) after 24 h at 37°C for bacteria, 48 h and 7 days at 30°C for yeast and molds respectively. Two replicates were prepared for each test and plates with indicator strain were used as control. The isolates were then selected based on a wide spectrum activity against tested microorganisms for further studies (Bharti A, et al., 2010).
Secondary screening
The bioactivity testing of the ethanol crude extracts obtained from the selected active isolates was done for antifungal and antibacterial susceptibility based on primary screening results. Minimum inhibitory concentrations of the crude extract was determined for fungus using the protocol described in National Committee for Clinical Laboratory Standards (NC- CLS) (Benhadj M, et al., 2020) and for bacteria as described in Clinical Laboratory Standards (CLS) (Bharti A, et al., 2010). The entire secondary bioactivity test with crude extracts was conducted in triplicates and inhibition zone diameters were measured.
Data management and analysis
The collected and recorded data were analyzed using Microsoft Excel. The data on temperature and pH tolerance was analyzed by comparing the mean growth in the log colony forming-unit through Analysis of Variances (ANOVAs). All statistical results with P<0.05 were considered to be statistically significant.
Results
Metabolite production and optimization of selected isolates
The preliminary antimicrobial activities of the isolates against selected pathogens by the cross streak method showed that they possess antimicrobial properties, as observed from their inhibitory activities against the selected bacterial and fungal strain. From ethanol extracts obtained from Solid State Fermentation (SSF) (Primary screening), a total of 36 isolates of Actinomycetes were subjected to primary screening and tested for activity against bacterial and fungal reference strain. Accordingly, the extracts from 10 isolates that were most active in the primary screen were 6 (16.66%) from LCHAACC13 (Lake Chitu Alkaliphilic Actinomycetes Culture Collection) that were inhibited the growth of Gram-positive bacterial and fungal strain; 4 (11.11%) from LAAACC15 (Lake Arenguade Alkaliphilic Actinomycetes Culture Collection) that were inhibited Gram-negative bacteria and fungal. From the 10 selected isolates, the best ones in terms of activity spectra were Actinomycetes spp. isolated from LCHAACC13. Extracts from 10 isolates were most active in this primary screen, namely that designated Lc-13, Lc-15, Lc-17, La-13, La-15, Lc-19, Lc-21, La-17, La-19, and Lc-23, were selected for further characterization. Isolates those were coded with Lc-13-23 and La-13, 15, 17, and 19 were obtained from Lake Chitu (Lc) and Arenguade (La) respectively.
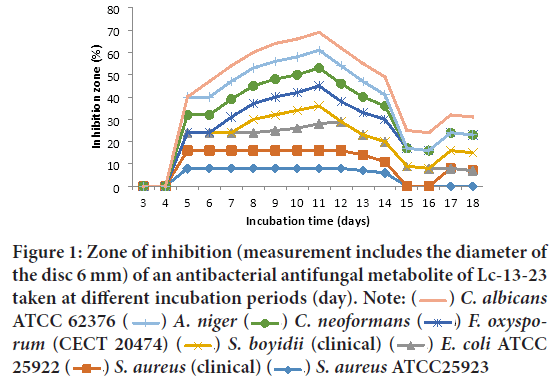
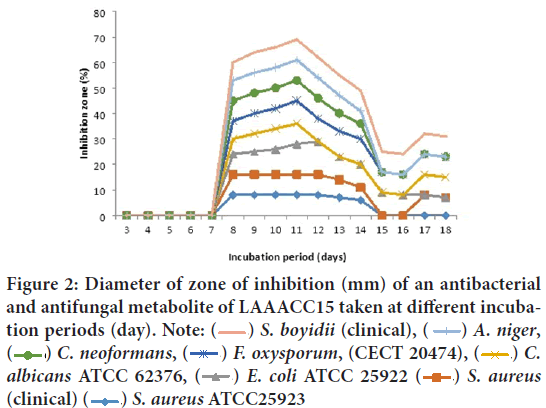
Dates of incubation period on the production of antibacterial and antifungal metabolite by isolates of two soda lakes: From evaluated the antibacterial and antifungal potential of two soda lakes water-derived Actino-mycetes; no antibacterial metabolite production was observed up to 4 and 7 days of incubation for LCHAACC13 (Lake Chitu Alkaliphilic Actinomycetes Culture Collection) and LAAACC15 (Lake Arenguade/Hora Hadho/ Alkaliphilic Actinomycetes Culture Collection) respectively as shown in Figures 1 and 2 below. However, antibacterial and antifungal metabolite production was detected from isolates of LCHAACC13 with maximum zone of inhibition within 5-6 days of incubation against Candida albicans ATCC 62376, Aspergillus niger, C. neoformans, Fusarium oxysporum f. sp. melonis (CECT 20474), and Shigella boydii (clinical) (Figure 1). The most resistant test isolates as increasing the time of incubation were Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and S. aureus (clinical isolate). All test microorganisms, except Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and S. aureus (clinical isolate) were sensitive to the culture filtrate starting from 8-12 days of incubation and in terms of multiple inhibition and diameter of zone of inhibition, the highest level of antibacterial and antifungal production was obtained at 10th, 11th, and 12th day of incubation by LCHAACC13 and LAAACC15 respectively (Figures 1 and 2). Some decrement in activity starting from 13 days of incubation and least activity were observed from 16 days and above.
Figure 1: Zone of inhibition (measurement includes the diameter of
the disc 6 mm) of an antibacterial antifungal metabolite of Lc-13-23
taken at different incubation periods (day). Note: 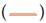 C. albicans ATCC 62376
C. albicans ATCC 62376  A. niger
A. niger  C. neoformans
C. neoformans  F. oxysporum (CECT 20474)
F. oxysporum (CECT 20474)  S. boyidii (clinical)
S. boyidii (clinical)  E. coli ATCC 25922
E. coli ATCC 25922  S. aureus (clinical)
S. aureus (clinical)  S. aureus ATCC25923
S. aureus ATCC25923
Figure 2: Diameter of zone of inhibition (mm) of an antibacterial
and antifungal metabolite of LAAACC15 taken at different incubation periods (day). Note: 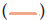 S. boyidii (clinical),
S. boyidii (clinical),  A. niger,
A. niger,  C. neoformans,
C. neoformans,  F. oxysporum, (CECT 20474),
F. oxysporum, (CECT 20474),  C.
albicans ATCC 62376,
C.
albicans ATCC 62376,  E. coli ATCC 25922
E. coli ATCC 25922  S. aureus (clinical)
S. aureus (clinical)  S. aureus ATCC25923
S. aureus ATCC25923
On the other hand, no strong antibacterial and antifungal metabolite production and activities were observed from LAACC15 (Lake Arengude/Hora Hado/Alkaliphilic Culture Collection) up to 7 days of incubation (Figure 2). However, antimicrobial activity of the culture filtrate of LAACC15 was detected on all test microorganisms except E. coli ATCC 25922, S. aureus ATCC25923, and S. aureus (clinical isolate) starting from the 8th, 10th, 11th, and 12th days of incubation with maximum and decrement activity at 14th and 16 days onwards respectively (Figure 2). The best antibacterial activity was found against Shigella boydii (12 mm) clinical isolate, followed by A. niger (10 mm), C. neoformans (clinical isolate), F. oxysporum f. sp. melonis (CECT 20474), and C. albicans ATCC 62376 (9 mm) (Figure 2).
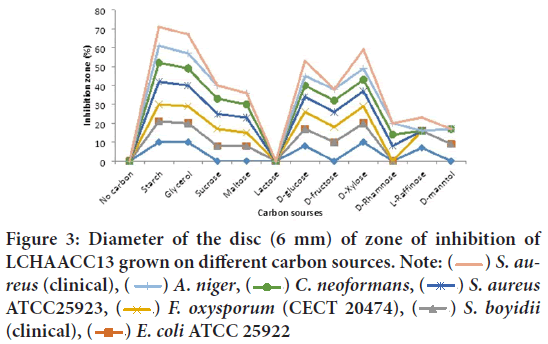
Carbon sources on growth and production of antibacterial and antifungal metabolite by two soda lakes: Among the carbon sources for the production of antibacterial metabolite by LCHAACC13, high antibacterial and antifungal metabolite production was obtained media containing starch, followed by glycerol, D-xylose, D-glucose, D-fructose, sucrose, and maltose respectively only carbon sources in the submerged fermentation (Figure 3). Interestingly, both strains of soda lakes were unable to use lactose as a sole carbon source. In terms of the effectiveness of the antibacterial activity, the antibacterial metabolite produced from different carbon sources were more effective on S. aureus (clinical isolate), A. niger, C. neoformans, S. aureus ATCC25923, Fusarium oxysporum f. sp. melonis (CECT 20474), Shigella boydii (clinical isolate) (12 mm) respectively. However, the most resistant test isolate with least activity was detected against Candida albicans ATCC 62376 and E. coli ATCC 25922.
Figure 3: Diameter of the disc (6 mm) of zone of inhibition of
LCHAACC13 grown on different carbon sources. Note:  S. aureus (clinical),
S. aureus (clinical),  A. niger,
A. niger,  C. neoformans,
C. neoformans,  S. aureus ATCC25923,
S. aureus ATCC25923,  F. oxysporum (CECT 20474),
F. oxysporum (CECT 20474),  S. boyidii (clinical),
S. boyidii (clinical),  E. coli ATCC 25922
E. coli ATCC 25922
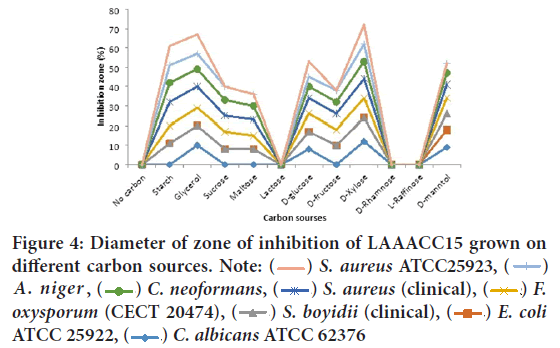
For LAAACC15, high antibacterial and antifungal metabolite production were recorded by the presence of Glycerol, followed by starch, D-xylose respectively (Figure 4). Among tested bacterial reference strains, S. aureus ATCC25923, A. niger, C. neoformans and S. aureus (clinical isolate), Fusarium oxysporum f. sp. melonis (CECT 20474), Shigella boydii (clinical isolate) (12 mm) were more sensitive. Similarly, like the isolate of Lake Chitu, for Lake Arenguade the most resistant test isolate with the least activity was detected against Candida albicansATCC 62376 and E. coliATCC 25922.
Figure 4: Diameter of zone of inhibition of LAAACC15 grown on
different carbon sources. Note:  S. aureus ATCC25923,
S. aureus ATCC25923,  A. niger ,
A. niger , C. neoformans,
C. neoformans,  S. aureus (clinical),
S. aureus (clinical),  F.
oxysporum (CECT 20474),
F.
oxysporum (CECT 20474),  S. boyidii (clinical),
S. boyidii (clinical),  E. coli ATCC 25922,
E. coli ATCC 25922,  C. albicans ATCC 62376
C. albicans ATCC 62376
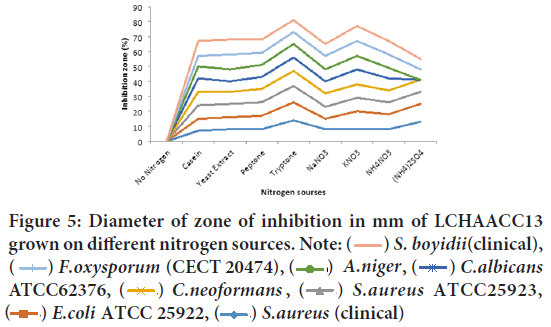
Nitrogen concentration on growth and production of antibacterial and antifungal metabolite by two soda lakes: Primary screening of antibacterial metabolite of LCHACC13 from different Nitrogen sources showed that maximum zone of inhibition against Shigella boydii (clinical), Fusar- ium oxysporumf. sp. melonis(CECT 20474), Aspergillus niger, C. neofor mans(clinical isolates), and S. aureus(clinical) in the presence of tryptone, NaNO3, Casein, and KNO3 were used as nitrogen sources respectively, while yeast extract and (NH4)2SO4 were least effective in terms of multiple inhibition of the test microorganisms (Figure 5). The most resistant test isolates were Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and Candida albicans ATCC 62376.
Figure 5: Diameter of zone of inhibition in mm of LCHAACC13
grown on different nitrogen sources. Note:  S. boyidii(clinical),
S. boyidii(clinical),  F.oxysporum (CECT 20474),
F.oxysporum (CECT 20474), A.niger,
A.niger,  C.albicans ATCC62376,
C.albicans ATCC62376,  C.neoformans ,
C.neoformans ,  S.aureus ATCC25923,
S.aureus ATCC25923,  E.coli ATCC 25922,
E.coli ATCC 25922,  S.aureus (clinical)
S.aureus (clinical)
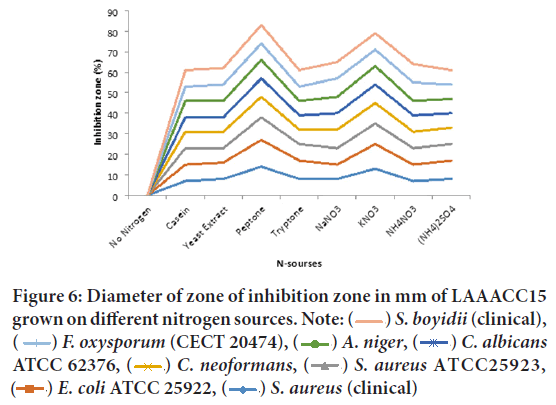
The culture filtrate of LAAACC15 grown in different nitrogen sources, however showed very strong antibacterial and antifungal activity against Shigella boydii (clinical), Fusarium oxysporum f. sp. melonis (CECT 20474), Aspergillus niger, Candida albicans ATCC 62376 and C. neoformans (clinical isolates) when peptone and KNO3 were used as nitrogen sources and very weak antibacterial and antifungal activity when (NH4)2SO4 and NH4NO3 as nitrogen sources (Figure 6). The most resistant test iso lates were Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and S. aureus (clinical).
Figure 6: Diameter of zone of inhibition zone in mm of LAAACC15
grown on different nitrogen sources. Note:  S. boyidii (clinical),
S. boyidii (clinical),  F. oxysporum (CECT 20474),
F. oxysporum (CECT 20474),  A. niger,
A. niger,  C. albicans ATCC 62376,
C. albicans ATCC 62376,  C. neoformans,
C. neoformans,  S. aureus
S. aureus  E. coli ATCC 25922,
E. coli ATCC 25922,  S. aureus (clinical)
S. aureus (clinical)
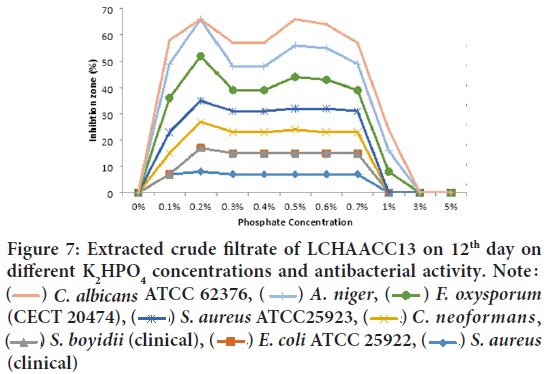
Phosphate concentration on growth and production of antibacterial and antifungal metabolite by two soda lakes: From 12 days culture filtrate of LCHAACC13 grown at different phosphate concentration showed very strong antibacterial and antifungal activity was observed against Can- dida albicans ATCC 62376, Aspergillus niger, Fusarium oxysporum f. sp. melonis(CECT 20474), Staphylococcus aureusATCC25923, C. neoformans(clinical isolates), and Shigella boydii(clinical), at the phosphate concentration range between 0.1%-0.7% and maximum activity at 0.2%, 0.5%, and 0.6%, whereas when the phosphate concentration was increased to 1% and above no antibacterial and antifungal metabolite production and activities were reduced (Figure 7). The most resistant test isolates at all phosphate concentration were E. coliATCC 25922, and S. aureus(clinical).
Figure 7: Extracted crude filtrate of LCHAACC13 on 12th day on different K2HPO4 concentrations and antibacterial activity. Note :  C. albicans ATCC 62376,
C. albicans ATCC 62376,  A. niger,
A. niger,  F. oxysporum (CECT 20474),
F. oxysporum (CECT 20474),  S. aureus ATCC25923,
S. aureus ATCC25923,  C. neoformans,
C. neoformans,  S. boyidii (clinical),
S. boyidii (clinical),  E. coli ATCC 25922,
E. coli ATCC 25922,  S. aureus (clinical)
S. aureus (clinical)
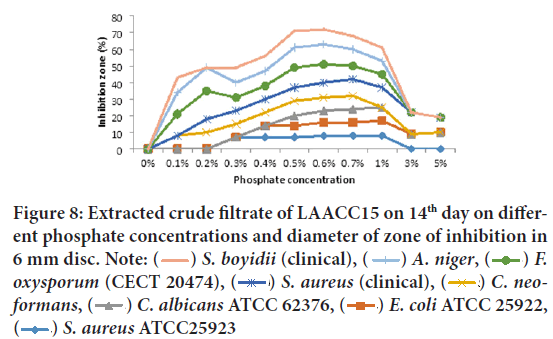
Antibacterial and antifungal metabolite productions and activities tested from 14 days of culture filtrate of LAACC15 showed very strong antibacterial and antifungal activity was observed against Shigella boydii (clinical), Aspergillus niger, Fusarium oxysporumf. sp. melonis(CECT 20474), S. aureus (clinical), C. neoformans (clinical isolates), and Shigella boydii (clinical), at the phosphate concentration range 0.1%-1% and maximum activity at 0.5% and 0.6%, whereas when the phosphate concentration was increased to 3% and above antibacterial and antifungal metabolite production and activities were reduced (Figure 8). The most resistant test isolates at all phosphate concentration were E. coliATCC 25922, Staphylococcus aureusATCC25923, and Candida albicansATCC 62376.
Figure 8: Extracted crude filtrate of LAACC15 on 14th day on different phosphate concentrations and diameter of zone of inhibition in
6 mm disc. Note:  S. boyidii (clinical),
S. boyidii (clinical),  A. niger,
A. niger,  F.
oxysporum (CECT 20474),
F.
oxysporum (CECT 20474),  S. aureus (clinical),
S. aureus (clinical), C. neoformans,
C. neoformans,  C. albicans ATCC 62376,
C. albicans ATCC 62376,  E. coli ATCC 25922,
E. coli ATCC 25922,  S. aureus ATCC25923
S. aureus ATCC25923
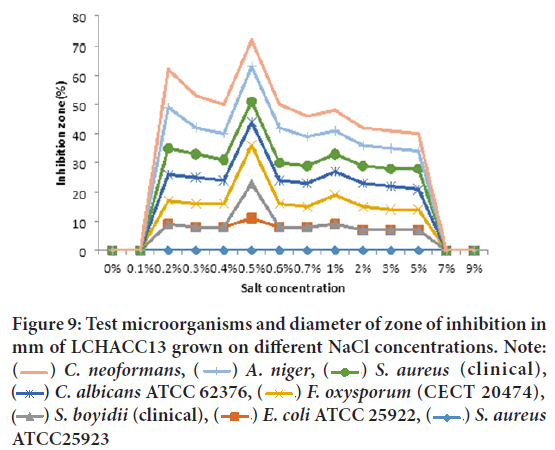
Salt concentration on growth and production of antibacterial and antifungal metabolite by two soda lakes: Extracted CF of LCHAACC13 showed very strong antibacterial and antifungal activity was observed against C. neoformans (clinical isolates), Aspergillus niger, S. aureus (clinical), Candida albicans ATCC 62376, and Fusarium oxysporum f. sp. melonis (CECT 20474), at the salt concentration range 0.1%-5% and maximum activity at 0.5%. On the other hand, weak antibacterial and antifungal activity was detected from the crude filtrate of isolates grown on 3%, 5%, and 7% NaCl concentration and no antibacterial metabolite from the isolates grown without NaCl (Figure 9). The most resistant test isolates at all salt concentration were E. coli ATCC 25922, Staphylococcus aureus ATCC25923, and Shigella boydii (clinical).
Figure 9: Test microorganisms and diameter of zone of inhibition in
mm of LCHACC13 grown on different NaCl concentrations. Note:  C. neoformans,
C. neoformans,  A. niger,
A. niger, S. aureus (clinical),
S. aureus (clinical),  C. albicans ATCC 62376,
C. albicans ATCC 62376,  F. oxysporum (CECT 20474),
F. oxysporum (CECT 20474),  S. boyidii (clinical),
S. boyidii (clinical),  E. coli ATCC 25922,
E. coli ATCC 25922,  S. aureus ATCC25923
S. aureus ATCC25923
ATCC25923
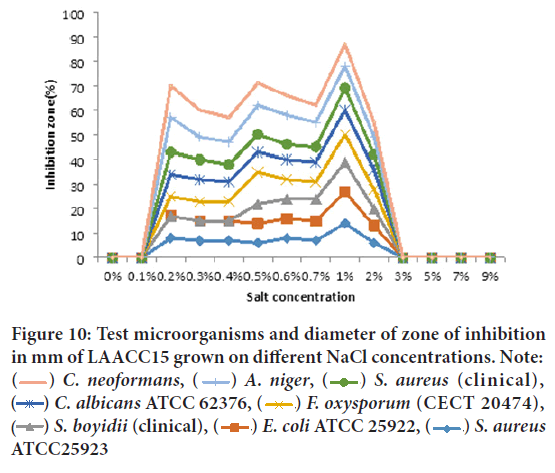
On the other hand, extracted crude filtrate of LAACC15 showed very strong antibacterial and antifungal activity against C. neoformans (clinical isolates), Aspergillus niger, S. aureus (clinical), Candida albicans ATCC 62376, and Fusarium oxysporum f. sp. melonis (CECT 20474), at the salt concentration range 0.1%-3% and maximum activity at 1%. Unlike the crude filtrate of Lake Chitu, no antibacterial and antifungal activity was detected from the crude filtrate of the isolates grown on 3%, 5%, and 7% NaCl concentration and no antibacterial metabolite from isolates grown without NaCl (Figure 10). The most resistant test isolates at all salt concentration were E. coli ATCC 25922, Staphylococcus aureus ATCC25923, and Shigella boydii (clinical).
Figure 10: Test microorganisms and diameter of zone of inhibition in mm of LAACC15 grown on different NaCl concentrations. Note:  C. neoformans,
C. neoformans,  A. niger,
A. niger,  S. aureus (clinical),
S. aureus (clinical),  C. albicans ATCC 62376,
C. albicans ATCC 62376,  F. oxysporum (CECT 20474),
F. oxysporum (CECT 20474),  S. boyidii (clinical),
S. boyidii (clinical),  E. coli ATCC 25922,
E. coli ATCC 25922,  S. aureus ATCC25923
S. aureus ATCC25923
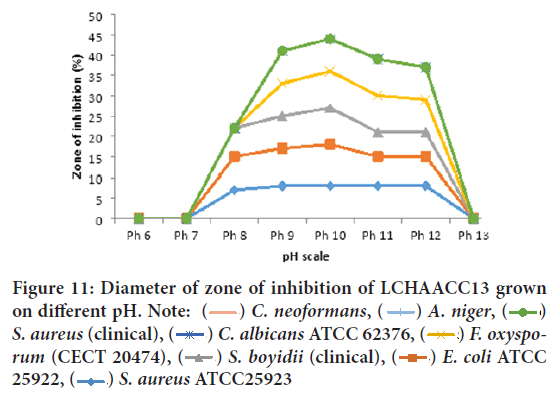
Effect of pH on growth and antibiotic production by LCHAACC 13 and LAAACC 15: The final pH measured for both isolates grown on different cultivation media indicated that the pH remained alkaline regardless of the medium constituents and for both isolates antibacterial and antifungal metabolite production was more favored when the pH of the cultivation media adjusted between 9-11, with maximum activity at 10 against C. neoformans (clinical isolates), Aspergillus niger, S. aureus (clinical), Candida albicans ATCC 62376, and Fusarium oxysporum f. sp. melonis (CECT 20474). The most resistant test isolates at all salt concentration was E. coli ATCC 25922, Staphylococcus aureus ATCC25923, and Shigella boydii (clinical). As shown in Figure 11 for both isolates production of antibacterial metabolite was favored when the culture condition was alkaline and both culture filtrate of LCHAACC13 and LAACC15 was stable at alkaline pH but weak stability at pH 12 and unstable at pH 13 values.
Figure 11: Diameter of zone of inhibition of LCHAACC13 grown
on different pH. Note:  C. neoformans,
C. neoformans,  A. niger,
A. niger,  S. aureus (clinical),
S. aureus (clinical),  C. albicans ATCC 62376,
C. albicans ATCC 62376,  F. oxysporum (CECT 20474),
F. oxysporum (CECT 20474),  S. boyidii (clinical),
S. boyidii (clinical),  E. coli ATCC 25922,
E. coli ATCC 25922,  S. aureus ATCC25923
S. aureus ATCC25923
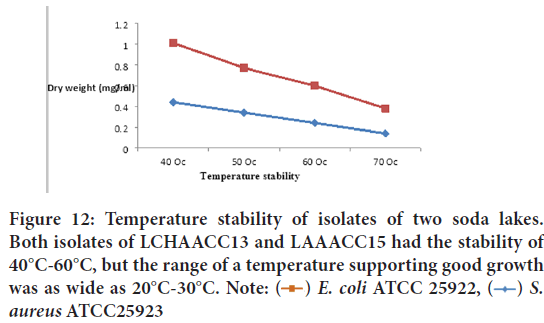
Effect of cultural temperature and growth period: It was observed that the crude filtrate of LCHAACC13 and LAAACC13 were stable from 40°C-60°C with maximum activity at 40°C against E. coli and S. aureus ATCC25923.
Discussion
From the finding, we observed that growth, antibacterial, and antifungal metabolite production and activities by isolates were positively affected by seven independent variables, namely incubation period, carbon (starch), nitrogen (NaNO3, Casein, and KNO3) sources, phosphate concentrations, pH, salt concentration, and temperature stability. We evaluated in vitro antibacterial and antifungal potential of two soda lakes water-derived Actinomycetes and we observed that no strong antibacterial and antifungal metabolite production up to 4 and 7 days of incubation for Lake Chitu alkaliphilic Actinomycetes culture collection and Lake Arenguade/Hora Hadho/alkaliphilic Actinomycetes culture collection) respectively. However, from isolates of LCHAACC13, we observed that with a maximum zone of inhibition within 5-6 days of incubation against Candida albicans ATCC 62376, Aspergillus niger, C. neoformans, Fusarium oxysporum f. sp. melonis (CECT 20474), and Shigella boydii (clinical) (Figure 12). The most resistant test isolates as increasing the time of incubation were Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and S. aureus (clinical isolate). All test microorganisms, except Staphylococcus aureus ATCC25923, E. coli ATCC 25922, and S. aureus (clinical isolate) were sensitive to the culture filtrate starting from 8-12 days of incubation and in terms of multiple inhibition and diameter of zone of inhibition, the highest level of antibacterial and antifungal production was obtained at 10th, 11th, and 12th day of incubation by LCHAACC13 and LAAACC15 respectively.
Figure 12: Temperature stability of isolates of two soda lakes.
Both isolates of LCHAACC13 and LAAACC15 had the stability of
40°C-60°C, but the range of a temperature supporting good growth
was as wide as 20°C-30°C. Note:  E. coli ATCC 25922,
E. coli ATCC 25922,  S.
aureus ATCC25923
S.
aureus ATCC25923
Some decrement in activity starting from 13 days of incubation and least activity were observed from 16 days and above. Our result is in agreement with Wang X, et al., 2018, they evaluated the antimicrobial activity of the strain ActiF450 against selected S. aureusand observed that antimicrobial activity started after 2 days of incubation and reached a maximum after 7 days. They observed a potent and broad-spectrum activity against a range of human fungal pathogens including moulds and yeasts, such as Arthroderma vanbreuseghemii, A. fumigatus, A. niger, C. albicans, C. glabarta, C. krusei, C. parapsilosis, F. oxysporum, F. solani, Microsporum canis, Rhodotorula mucilaginosa and Scodapulariopsis candida. In addition, high antibacterial activity was recorded against pathogenic staphylococci. Yu J, et al., 2008 screened 180 actinobacteria for their novel biocontrol agents against plant pathogens for anti-fungal activity both in vitro and in vivo and found that three isolates exhibited potent anti-fungal activity against Botrytis cinerea and F. oxysporum f. sp. cucumerinum evaluated antifungal activity of Streptomyces rimosus MY02 (Arumugam T, et al., 2017) and observed the diameter of inhibition zone of the culture supernatant from S. rimosus MY02 against Fusarium oxysporium f sp. cucumerinum was 33.19 mm.
The results showed that among the carbon sources antibacterial and antifungal activity was higher when the isolate was grown in a medium having Starch Casein Agar for Lake Chitu isolates enhanced the production of secondary metabolites and the zone of inhibition against reference bacterial and fungal strain which is in agreement with that starch casein medium (Vilches C, et al., 1990; Pandey A, et al., 2005) found to be good base for antibacterial and antifungal metabolite production and D-xylose for Lake Arenguade in agreement with dextrose prove to be the best carbon source for antibiotic production by S. kanamyceticus M27 with a maximum zone of inhibition (22 mm) and 19 mm respectively (Atta HM, 2015). Our result is also supported (Grant WD, 2006; Calvo AM, et al., 2002; Ripa EA, et al., 2009; Balachandar R, et al., 2018) that they concluded that the rate at which carbon source is metabolized can influence the formation of biomass or production of primary or secondary metabolites and fast growth due to high concentrations of rapidly metabolized sugars is often associated with low productivity of secondary metabolites.
Other researchers namely, (Singh LS, et al., 2009) soybean meal proved to be a suitable carbon source for antibiotic production by S. tanashiensis A2D; (Hassan MA, et al., 2001) no secondary metabolite production with supplemented medium with galactose, lactose, raffinose, and maltose as a sole carbon source; (Cruz R, et al., 1999) readily metabolized carbon sources, such as glucose, can suppress antibiotic production by preventing the synthesis of a key enzyme in the biosynthesis pathway referred to as “catabolic repression”; (Atta HM, 2015; Cruz R, et al., 1999; Drew SW and Demain AL, 1997) S. griseocarneus, Streptomyces violatus, and S. sannanensis strain RJT-1, glucose found to be suitable carbon source for the antibiotic production; while dextrose at a concentration of 2 g/100 ml gave maximal antibiotic titers and a higher dose decreased the yield by S. kanamyceticus (Atta HM, 2015; Hassan MA, et al., 2001) high concentration of glucose as a repressor of secondary metabolisms and maximum cell growth rates can inhibit antimicrobial agent production (Balachandar R, et al., 2018). An example of catabolic repression of secondary metabolism in Actinomycetes is that of actinomycin synthesis by S. antibioticus after more glucose is added to the media (Yang SS and Ling MY, 1989) and in S. aureofaciens, the production of tetracycline was critically influenced when 10% soluble starch was enriched with sweet potato residues (Mariadhas VA, et al., 2014).
Among the nitrogen sources used for the isolate of LCHAACC13 (Figure 6), yeast extract was suitable in higher production of antibacterial metabolites that were active against E. coli and S. aureus followed by NaNO3, casein, and KNO3 that were effective against most of the test bacteria (Tables 1 and 2). The culture filtrate of LAAACC15 grown in different nitrogen sources was showed that very weak antibacterial activity against Shigella boydii as compared to the antibacterial activity obtained from the usual cultivation media Starch Casein Agar (SCA) which contains a combination of Casein and KNO3 as nitrogen sources (Figure 6). This might because yeast extract is rich in vitamins, minerals, amino acids, digested nucleic acids, and other growth factors which could induce antibacterial metabolite synthesis. The results are comparable with (Drew SW and Demain AL, 1997) as they observed yeast extract gave the highest antimicrobial metabolite production followed by NaNO3, peptone, and KNO3 by new Streptomyces species; (Atta HM, 2015) in which they tested different nitrogen source contained the basal mineral salts plus 2% dextrose and amino acids and inorganic nitrogen compounds employed at a concentration equivalent to 84 mg of nitrogen per 100 ml. They obtained the highest antibiotic in a synthetic medium containing (NH4) H2PO4 as the nitrogen sources and the optimal concentration for antibiotic production at a concentration of 0.68%.
| Pathogens | Active isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lc-13 | Lc-15 | Lc-17 | La-13 | La-15 | Lc-19 | Lc-21 | La-17 | La-19 | Lc-23 | |
| S. aureus ATCC25923 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| S. aureus (clinical) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| E. coli ATCC 25922 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| S. boydii (clinical) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| F. oxysporum (CECT 20474) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Aspergillus niger | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| C. albicans ATCC 62376 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| C. neoformans (clinical) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
Note: +++: Good activity; ++: Moderate activity; +: Weak activity; –: No activity; Lc: Lake Chitu; La: Lake Arenguade
Table 1: Preliminary antimicrobial screening of 10 active isolates against different microbial pathogens
| Pathogens | C | Active isolates (Zone of inhibition in mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lc-13 | Lc-15 | Lc-17 | La-13 | La-15 | Lc-19 | Lc-21 | La-17 | La-19 | Lc-23 | ||
| S. aureus ATCC25923 | 14 | 10 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| S. aureus (clinical) | 14 | 12 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| E. coli ATCC 25922 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| S. boydii (clinical) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| F. oxysporum (CECT 20474) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Aspergillus niger | 14 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| C. albicans ATCC 62376 | 13 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| C. neoformans (clinical) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
Note: C: Control (Streptomycin for bacteria; ketoconazole for fungi); +++: Good activity; ++: Moderate activity; +: Weak activity; –: No activity
Table 2: Secondary antimicrobial screening of 10 active isolates against different microbial pathogens
Additionally, Duckworth AW, et al., 1998 in the production of polyene antibiotics, soybean meal has been considered as a good nitrogen source because of the balance of nutrients, low phosphorous content, and slow hydrolysis and concluded that the presence of nitrogen regulation revealed on the enzymatic level in the fermentation of cephamycin and patulin (Liangzhi LI, et al., 2007) observed that higher growth and antibacterial activity in ammonium nitrate as nitrogen source followed by sodium nitrate and potassium nitrate exhibited maximum growth and antibacterial activity (El‐Enshasy HA, et al., 2000) investigated the effects of nitrogen sources on streptolydigin production and distribution of secondary metabolites from Streptomyces lydicus. They found that when soybean meal was used as the source of nitrogen, three analogs of streptolydigin were detected, and among the nitrogen sources glutamic acid was most favorable for the formation of streptolydigin.
Antibacterial and antifungal metabolite production were tested from 12 days culture filtrate of LCHAACC13 grown at different phosphate concentration and the result showed that detectable activity was observed at the phosphate concentration range between 0.1%-0.7% and maximum activity at 0.2%, whereas when the phosphate concentration was increased to 1% and above no antibacterial metabolite production (Figures 8 and 9). It has been reported that phosphate concentrations above 0.005% suppress production of natamycin by Streptomyces natalensis (Martín JF, 1977) that KH2PO4 is not favorable for the production of antibiotic by S. violatus, however, KH2PO4 at a concentration of 1 g/l yields an inhibition zone of 22 mm, equivalent to an antibiotic concentration of 128 µg/ml. Elucidation of the precise interaction between phosphate metabolism and antibiotic biosynthesis has been complicated by the fact that phosphate is involved in a host of related and non-related metabolic pathways (Fenta L, 2017).
In addition, Kim YJ, et al., 2007 also found that when adding a large amount of inorganic phosphate, consumption of carbon and nitrogen sources and respiration are accelerated resulting in good growth, but the production of antibiotics is usually reduced. However, it is presumed that when the concentration of inorganic phosphate in the culture is high, the intracellular concentration of ATP is increased and the primary metabolism is accelerated. When the amount of inorganic phosphate is lowered, the ATP concentration decreases; this decrease depresses metabolic conversions which are required for the production of antibiotics (Fenta L, 2017). In general, three separate regulatory roles, viz. independently inhibition of enzyme action, regulation of protein expression, and limit on the cell growth have been postulated for phosphate (Fenta L, 2017).
For both isolates, antibacterial and antifungal metabolite production was more favored when the pH of the cultivation media adjusted between 9-11, with maximum activity at 11 for Lake Chitu and 10 Arenguade respectively. The results are comparable with alkaliphilic Actinomycetes Nocardiopsis alkaliphila sp. nov. (Hozzein WN, et al., 2004; Schippers A, et al., 2002) grow optimally at pH 9.5-10. N. metallicus (Groth I, et al., 1997) exhibited growth in the pH range 7-10.5, whereas in Bogoriella caseilytica (Arasu MV, et al., 2009), a new alkaliphilic Actinomycete, optimum growth occurred at pH values 9-10. The results are comparable with some Streptomyces species recorded to produce antibiotics against bacteria, fungi, and yeast at alkaline pH (Singh LS, et al., 2009). The results are in contrast to the result reported using Streptomyces sp. for antimicrobial production (Yilmaz EI, et al., 2008). Our finding supports the fact that generally, alkaline environment is more suitable for the growth of Streptomyces and thus production of antimicrobial compound (Noaman NH, et al., 2004; Vasavada SH, et al., 2006) reported that when the pH was maintained at 5, production of geldanamycin was increased from 414 to 768 mg/L. Vasavada SH, et al., 2006 studied the effects of the temperature, pH, incubation period, for the production of antimicrobial metabolite production and they found temperature of 35°C and pH 8 were the best for growth and antimicrobial agent production and 14 to 15 days of incubation was found to be the best for maximum growth and antimicrobial activity, respectively.
An increase in the concentration of sodium chloride in the medium influenced the growth and antimicrobial compound production. Extracted CF of LCHAACC13 showed that maximum antibacterial metabolite production at 1% salt concentration against Salmonella typhii and Streptococcus Pneumonia, whereas, Escherichia coli and Pseudomonas aeruginosa as shown in Figure 13 below. On the other hand, weak antibacterial and antifungal activity was detected from the crude filtrate of both isolates grown on 5%, 7%, and 9% NaCl concentration and no antibacterial metabolite from both isolates grown without NaCl. Duckworth AW, et al., 1998 reported the antimicrobial potential of Actinomycetes to isolate that grew in the presence of 20% (w/v) NaCl, while antibiotic production being maximum with 5% (w/v) NaCl in the production medium.
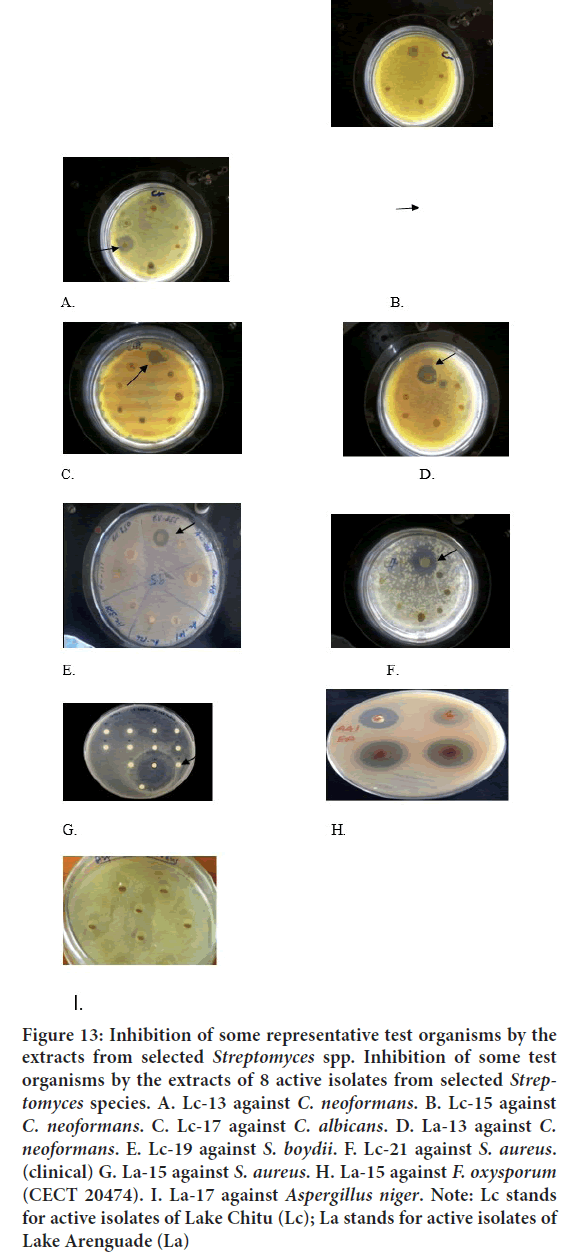
Figure 13: Inhibition of some representative test organisms by the extracts from selected Streptomyces spp. Inhibition of some test organisms by the extracts of 8 active isolates from selected Streptomyces species. A. Lc-13 against C. neoformans. B. Lc-15 against C. neoformans. C. Lc-17 against C. albicans. D. La-13 against C. neoformans. E. Lc-19 against S. boydii. F. Lc-21 against S. aureus. (clinical) G. La-15 against S. aureus. H. La-15 against F. oxysporum (CECT 20474). I. La-17 against Aspergillus niger. Note: Lc stands for active isolates of Lake Chitu (Lc); La stands for active isolates of Lake Arenguade (La)
Vastrad BM and Neelagund SE, 2014 reported that good growth as well as an antimicrobial activity when salt concentrations of 12 g/L and a decline in growth and antimicrobial activity after 20 g/L. In Streptomyces fradiaeNCIM 2418 (Yang SS and Swei WJ, 1996), neomycin production was enhanced by sodium nitrate and ammonium chloride, copper sulphate, and zinc sulphate positively influenced on neomycin production. In Streptomyces rimosus, supplementation of 1% (w/w) CaCO3, enhance the production of tracycline in Solid State Fermentation when corncob was used as the solid medium.
Usually, cultivation for antibiotic production is performed under one constant temperature from the beginning to the end, but the temperature adequate for growth is not always the same as that for production. It is known that thermophilic Actinomycetes such as thermo Actinomycetes produce new antibiotics at a temperature higher than 40°C, but Streptomyces usually produces antibiotics at temperatures near 27°C. Generally, the range of a temperature supporting good growth is as wide as 25°C, but the temperature range adequate for good production of secondary metabolites is narrow and 5°C~25°C (Cruz R, et al., 1999). S. violates showed a narrow range of incubation temperature for relatively good growth and antibiotic production. The increase of the incubation temperature from 20°C-30°C increased the growth of the cells and the production of the antibiotic by 3.45-fold and 4.39-fold, respectively. Maximum antibiotic production was obtained at 30°C. A higher incubation temperature (35°C) harmed growth and antibiotic production. The size of inoculum affected the ability of S. violates to produce the antibiotic in the tested cultures. An increase of the inoculum size from 0.5 ml to 2 ml/50 ml medium enhanced the production of the antibiotic by approximately 4.6-fold. Thus temperature must be considered separately growth and for production. It would be of interest in antibiotic screening to use temperature shifts.
Conclusion
The present study provides evidence that Ethiopian Soda lakes are a rich and valuable resource of potentially active Actinomycetes. Concisely, this study highlighted for the first time the antimicrobial potentials of soda lakes associated actinobacteria with the ability to suppress major human fungal and bacterial pathogens in vitro. Further studies are needed to enhance the isolation and selection of Actinomycetes from unexplored ecosystems for antibiotic discovery. Hopefully, these new agents will meet the challenges as we attempt to manage serious underlying infectious diseases. In addition, knowledge of the actinobacteria gene clusters may provide important answers toward understanding the metabolites biosynthetic pathway.
References
- Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, et al. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev. 2016; 80(1): 1-43.
[Crossref] [Google Scholar] [Pubmed]
- Kumar MS, Das AP. Molecular identification of multi drug resistant bacteria from urinary tract infected urine samples. Microb Pathog. 2016; 98: 37-44.
[Crossref] [Google Scholar] [Pubmed]
- Katz L, Baltz RH. Natural product discovery: Past, present, and future. J Ind Microbiol Biotechnol. 2016; 43(2-3): 155-176.
[Crossref] [Google Scholar] [Pubmed]
- van der Meij A, Worsley SF, Hutchings MI, van Wezel GP. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol Rev. 2017; 41(3): 392-416.
[Crossref] [Google Scholar] [Pubmed]
- Avinash TS, Ravishankar RV. Biocontrol of Fusarium wilt disease of cucumber (Cucumis sativus L.) in greenhouse and field. Int J Agric Technol. 2017; 13(4): 531-543.
- Ganesan P, Reegan AD, David RH, Gandhi MR, Paulraj MG, Al-Dhabi NA, et al. Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu, India. Alexandria J Med. 2017; 53(2): 101-110.
- BBC. The lists of WHO a danger that pharmaceutical companies will develop only treatments that are easier and more profitable to make. BBC. 2018.
- Singh LS, Mazumder S, Bora TC. Optimisation of process parameters for growth and bioactive metabolite produced by a salt-tolerant and alkaliphilic actinomycete, Streptomyces tanashiensis strain A2D. J Mycol Med. 2009; 19(4): 225-233.
- Rajivgandhi G, Ramachandran G, Maruthupandy M, Vaseeharan B, Manoharan N. Molecular identification and structural characterization of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT 235641) and its antibacterial efficacy against isolated ESBL producing bacteria. Microb Pathog. 2019; 126: 138-148.
- Biniam W. Bioactive compounds from alkaliphilic actinomycetes. Addis Ababa University. 2018.
- Mehabie D. Antibiotic production and optimization of culture condition of Actinomycetes from some soda lakes of Ethiopia. Addis Ababa University. 2011.
- Gebreyohannes G, Moges F, Sahile S, Raja N. Isolation and characterization of potential antibiotic producing actinomycetes from water and sediments of Lake Tana, Ethiopia. Asian Pac J Trop Biomed. 2013; 3(6): 426-435.
[Crossref] [Google Scholar] [Pubmed]
- Atsede M, Fassil A. Isolation and screening of antibiotic producing actinomycetes from rhizosphere and agricultural soils. Afr J Biotechnol. 2018; 17(22): 700-715.
- Bizuye A, Moges F, Andualem B. Isolation and screening of antibiotic producing actinomycetes from soils in Gondar town, North West Ethiopia. Asian Pac J Trop Dis. 2013; 3(5): 375-381.
- Kibret M, Guerrero-Garzón JF, Urban E, Zehl M, Wronski VK, Rückert C, et al. Streptomyces spp. from Ethiopia producing antimicrobial compounds: Characterization via bioassays, genome analyses, and mass spectrometry. Front Microbiol. 2018; 9: 1270.
[Crossref] [Google Scholar] [Pubmed]
- Kebede E. Response of Spirulina platensis (=Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. J Appl Psychol. 1997; 9(6): 551-558.
- Saha MR, Ripa FA, Islam MZ, Khondkar P. Optimization of conditions and in vitro antibacterial activity of secondary metabolite isolated from Streptomyces sp. MNK7. J Appl Sci Res. 2010; 6(5): 453-459.
- Duckworth AW, Grant S, Grant WD, Jones BE, Meijer D. Dietzia natronolimnaios sp. nov., a new member of the genus Dietzia isolated from an East African soda lake. Extremophiles. 1998; 2(3): 359-366.
[Crossref] [Google Scholar] [Pubmed]
- NCCLS (US). Performance standards for antimicrobial disk susceptibility tests: approved standards. National Committee for Clinical Laboratory Standards. 2006.
- Bharti A, Kumar V, Gusain O, Bisht GS. Antifungal activity of actinomycetes isolated from Garhwal region. J Sci Engg Tech Mgt. 2010; 2(2).
- Benhadj M, Gacemi-Kirane D, Menasria T, Guebla K, Ahmane Z. Screening of rare actinomycetes isolated from natural wetland ecosystem (Fetzara Lake, northeastern Algeria) for hydrolytic enzymes and antimicrobial activities. J King Saud Univ Sci. 2019; 31(4): 706-712.
- Wayne PA. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. NCCLS document M27-A2. 2002.
- Benhadj M, Metrouh R, Menasria T, Gacemi-Kirane D, Slim FZ, Ranque S. Broad-spectrum antimicrobial activity of wetland-derived Streptomyces sp. ActiF450. EXCLI J. 2020; 19: 360.
[Crossref] [Google Scholar] [Pubmed]
- Wang X, Zhang M, Gao J, Pu T, Bilal M, Wang Y, et al. Antifungal activity screening of soil Actinobacteria isolated from Inner Mongolia, China. Biol Control. 2018; 127: 78-84.
- Yu J, Liu Q, Liu Q, Liu X, Sun Q, Yan J, et al. Effect of liquid culture requirements on antifungal antibiotic production by Streptomyces rimosus MY02. Bioresour Technol. 2008; 99(6): 2087-2091.
[Crossref] [Google Scholar] [Pubmed]
- Arumugam T, Kumar PS, Kameshwar R, Prapanchana K. Screening of novel Actinobacteria and characterization of the potential isolates from mangrove sediment of south coastal India. Microb Pathog. 2017; 107: 225-233.
[Crossref] [Google Scholar] [Pubmed]
- Vilches C, Méndez C, Hardisson C, Salas JA. Biosynthesis of oleandomycin by Streptomyces antibioticus: Influence of nutritional conditions and development of resistance. J Gen Microbiol. 1990; 136(8): 1447-1454.
[Crossref] [Google Scholar] [Pubmed]
- Pandey A, Shukla AN, Majumdar SK. Utilization of carbon and nitrogen sources by Streptomyces kanamyceticus M 27 for the production of an anti-bacterial antibiotic. Afr J Biotechnol. 2005; 4(9). [Crossref]
- Atta HM. Biochemical studies on antibiotic production from Streptomyces sp.: Taxonomy, fermentation, isolation and biological properties. J Saudi Chem Soc. 2015; 19(1): 12-22.
- Grant WD. Alkaline environments and biodiversity, in extremophilies. Encyclopaedia of Life Support Systems (EOLSS). 2006.
- Calvo AM, Wilson RA, Bok JW, Keller NP. Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev. 2002; 66(3): 447-459.
[Crossref] [Google Scholar] [Pubmed]
- Ripa EA, Nikkon K, Zaman S, Khondkar P. Optimal conditions for antimicrobial metabolites production from a new Streptomycessp. RUPA-08PR isolated from Bangladeshi soil. Mycobiology. 2009; 37(3): 211-214.
[Crossref] [Google Scholar] [Pubmed]
- Balachandar R, Karmegam N, Saravanan M, Subbaiya R, Gurumoorthy P. Synthesis of bioactive compounds from vermicast isolated actinomycetes species and its antimicrobial activity against human pathogenic bacteria. Microb Pathog. 2018; 121: 155-165.
[Crossref] [Google Scholar] [Pubmed]
- Hassan MA, El-Naggar MY, Said WY. Physiological factors affecting the production of an antimicrobial substance by Streptomyces violatus in batch cultures. Egypt J Biol. 2001; 3(1): 1-10.
- Cruz R, Arias ME, Soliveri J. Nutritional requirements for the production of pyrazoloisoquinolinone antibiotics by Streptomyces griseocarneus NCIMB 40447. Appl Microbiol Biotechnol. 1999; 53(1): 115-119.
[Crossref] [Google Scholar] [Pubmed]
- Drew SW, Demain AL. Effect of primary metabolites on secondary metabolism. Annu Rev Microbiol. 1977; 31(1): 343-356.
[Crossref] [Google Scholar] [Pubmed]
- Yang SS, Ling MY. Tetracycline production with sweet potato residue by solid state fermentation. Biotechnol Bioeng. 1989; 33(8): 1021-1028.
[Crossref] [Google Scholar] [Pubmed]
- Mariadhas VA, Thankappan SR, Naif AA, Veeramuthu D, Savarimuthu I, Paul A, et al. Nutritional requirements for the production of antimicrobial metabolites from Streptomyces. Afr J Microbiol Res. 2014; 8(8): 750-758.
- Liangzhi LI, Bin QI, Yingjin YU. Nitrogen sources affect streptolydigin production and related secondary metabolites distribution of Streptomyces lydicus AS 4.2501. Chin J Chem Eng. 2007; 15(3): 403-410.
- El‐Enshasy HA, Farid MA, El‐Sayed ES. Influence of inoculum type and cultivation conditions on natamycin production by Streptomyces natalensis. J Basic Microbiol. 2000; 40(5‐6): 333-342.
[Crossref] [Google Scholar] [Pubmed]
- Martín JF. Control of antibiotic synthesis by phosphate. Adv Biochem Engin. 1977; 6: 105-127.
- Fenta L. Isolation and characterization of phosphate solubilizing bacteria from tomato (Solanum l.) rhizosphere and their effect on growth and phosphorus uptake of the host plant under greenhouse experiment. Int J Adv Res. 2017.
- Kim YJ, Song JY, Moon MH, Smith CP, Hong SK, Chang YK. pH shock induces overexpression of regulatory and biosynthetic genes for actinorhodin productionin Streptomyces coelicolor A3 (2). Appl Microbiol Biotechnol. 2007; 76(5): 1119-1130.
[Crossref] [Google Scholar] [Pubmed]
- Hozzein WN, Li WJ, Ali MI, Hammouda O, Mousa AS, Xu LH, et al. Nocardiopsis alkaliphila sp. nov., a novel alkaliphilic actinomycete isolated from desert soil in Egypt. Int J Syst Evol Microbiol. 2004; 54(1): 247-252.
[Crossref] [Google Scholar] [Pubmed]
- Schippers A, Bosecker K, Willscher S, Spröer C, Schumann P, Kroppenstedt RM. Nocardiopsis metallicus sp. nov., a metal-leaching actinomycete isolated from an alkaline slag dump. Int J Syst Evol Microbiol. 2002; 52(6): 2291-2295.
[Crossref] [Google Scholar] [Pubmed]
- Groth I, Schumann P, Rainey FA, Martin K, Schuetze B, Augsten K. Bogoriella caseilytica gen. nov., sp. nov., a new alkaliphilic actinomycete from a soda lake in Africa. Int J Syst Evol Microbiol. 1997; 47(3): 788-794.
[Crossref] [Google Scholar] [Pubmed]
- Arasu MV, Duraipandiyan V, Agastian P, Ignacimuthu S. In vitro antimicrobial activity of Streptomyces spp. ERI-3 isolated from Western Ghats rock soil (India). J Mycol Méd. 2009; 19(1): 22-28.
- Yilmaz EI, Yavuz M, Kizil M. Molecular characterization of rhizospheric soil streptomycetesisolated from indigenous Turkish plants and their antimicrobial activity. World J Microbiol Biotechnol. 2008; 24(8): 1461-1470.
- Noaman NH, Fattah A, Khaleafa M, Zaky SH. Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol Res. 2004; 159(4): 395-402.
[Crossref] [Google Scholar] [Pubmed]
- Vasavada SH, Thumar JT, Singh SP. Secretion of a potent antibiotic by salt-tolerant and alkaliphilic actinomycete Streptomyces sannanensis strain RJT-1. Curr Sci. 2006; 91(10): 1393-1397.
- Vastrad BM, Neelagund SE. Optimization of medium composition for the production of neomycin by Streptomyces fradiae NCIM 2418 in solid state fermentation. Biotechnol Res Int. 2014: 1-11.
[Crossref] [Google Scholar] [Pubmed]
- Yang SS, Swei WJ. Oxytetracycline production by Streptomyces rimosus in solid-state fermentation of corncob. World J Microbiol Biotechnol. 1996; 12(1): 43-46.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Kenesa Chali1*, Zerihun Belay1 and Ketema Bacha22Department of Biology, Jimma University, Jimma, Ethiopia
Citation: Chali K: In Vitro Bioassay of Antibacterial and Antifungal Activity Studies of Actinomycetes from Soda Lakes of Ethiopia
Received: 15-Sep-2022 Accepted: 10-Oct-2022 Published: 17-Oct-2022, DOI: 10.31858/0975-8453.13.10.701-710
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3