Research Article - (2022) Volume 13, Issue 12
Abstract
Patients with Autoimmune Diseases (AD) are at increased risk of complications, which adversely affect the quality of life and prognosis. Recently, a large amount of literature suggested reduced oxidative stress by hydrogen supplements in reducing inflammation and improving prognosis. In this study, clinical trial NCT05196295, we randomly assigned different dosages (low, medium, and high) of hydrogen-rich coral calcium (HRCC) to patients with autoimmune diseases, including Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE) patients in MingSheng Hospital in Taiwan (age 54 years, 80% females). Participants were measured with their baseline health biomarkers including inflammatory indicators, haematologic, urinary biomarkers, and health status prior to the trial. Clinical and follow-up assessments were collected after 1 month of the treatment. We performed analyses to compare the baseline with the changes after the HRCC treatment. Together with the groups treated with different dosages, the treatment demonstrated no adverse effects on participants’ responses to the HRCC. While the primary endpoint of the trial was met, the potential therapeutic effects of HRCC are addressed for future studies. This clinical trial of HRCC in safety response is the first-in-human study.
Keywords
Rheumatoid arthritis (RA), Poor quality of life (QOL), Oxidative stress, molecular hydrogen, DAS- 28, Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Complete Blood Count (CBC)
Abbreviations
AD: Autoimmune Diseases; HRCC: Hydrogen-Rich Coral Calcium; RA: Rheumatoid Arthritis; SLE: Systemic Lupus Erythematosus; QOL: Quality of Life; CRP: C-Reactive Protein; DAS-28: Disease Activity Score (the number 28 refers to the 28 joints that are examined in this assessment); 8-OHdG 8-hydroxy-2’-deoxyguanosine, measurement of oxidative stress; IL- 6: Interleukin-6; BFI-T: Brief Fatigue Inventory-Taiwan; WBC: White Blood Cells; RBC: Red Blood Cells; MCH: Mean Corpuscular Hemoglobin; MCV: Mean Corpuscular Volume; MCHC: Mean Corpuscular Hemoglobin Concentration; HbA1C: Hemoglobin A1c; TG: Triglycerides; CHOL: Cholesterol; HDL-C: High Density Lipoproteins; LDL-C: Low Density Lipoproteins; hs-CRP: High-Sensitivity CRP; ESR: Erythrocyte Sedimentation Rate; eGFR: Estimated Glomerular Filtration Rate; PLT: Platelets
Introduction
Autoimmune diseases (AD) are inflammatory diseases that cause the immune system to attack joints, bones, muscles, and organs (van der Heijde D, et al., 2018; Kumar BS, et al., 2017) affecting approximately 5%-8% of the world population (Wang L, et al., 2015). Common symptoms include joint pain, fatigue, skin problems, digestive issues, recurring fever, and inflammation-swelling, redness, and warmth in a joint or affected area. AD can cause damage to vital organs, including the lungs, heart, nervous system, kidneys, skin, and eyes, thereby affecting the quality of life and survival time.
Patients with Rheumatoid Arthritis (RA) have poor Quality of Life (QOL) resulting from the overproduction of pro-inflammatory cytokines such as TNF-α and IL6, and often have an elevated Erythrocyte Sedimentation Rate (ESR, also known as sedimentation rate), or C-Reactive Protein (CRP) level, which indicate the presence of an inflammatory process in the body (Greenmyer JR, et al., 2020). Patients with RA report a high level of disease activities, measured by the DAS-28 (Kumar BS, et al., 2017; Greenmyer JR, et al., 2020), including measurement of patient feedback, joint swelling and tenderness, CRP, and ESR, which is considered the gold standard of RA remission (Sheehy C, et al., 2014; Greenmyer JR, et al., 2020).
Current treatments of RA are based on biological response modifiers (biologicals) such as inhibitors of TNF-alpha and certain interleukins to overcome the overproduction of pro-inflammatory cytokines (Tank ND, et al., 2017), and medi cations that slow disease and prevent joint deformity, called Disease-Modifying Antirheumatic Drugs (DMARDs) (Tank ND, et al., 2017). Despite all the options of treatments, RA patients experience sleep and skin complications, movement difficulties, joint pains, and stroke (Table 1).
| Number | Patient | Sex | Age | Dosages | Qualitative response before treatment | Qualitative response after treatment |
|---|---|---|---|---|---|---|
| 1 | HR013 | F | 48 | 6 Capsules/day | N/A-patient did not report | Sleep improved, dry skin |
| 2 | HR014 | F | 52 | 6 Capsules/day | RA hand symptoms | Easier bowel movement, pains at joints maintained |
| 3 | HR015 | F | 58 | 6 Capsules/day | Sleep condition very bad; bad eye sight; anxiety | Sleep improved, ear itchiness, dry hand and eye improved |
| 4 | HR016 | M | 84 | 6 Capsules/day | Hear and eye sight problems | Don't think HRCC helps |
| 5 | HR017 | F | 55 | 6 Capsules/day | Numb hands, easily anxious | Knees became more comfortable but conditions rebounded |
| 6 | HR006 | F | 68 | 3 Capsules/day | Patients with lupus erythematosus, pancreatic cancer | Joints pains |
| 7 | HR008 | F | 56 | 3 Capsules/day | Willing to try HRCC | Energy improved |
| 8 | HR010 | F | 57 | 3 Capsules/day | Hypertension treatment intake | Sleep improved |
| 9 | HR011 | F | 38 | 3 Capsules/day | Walk habit, weight loss difficulty | Ankles condition improved |
| 10 | HR012 | F | 50 | 3 Capsules/day | Can't hear well | Skin itchiness improved |
| 11 | HR001 | M | 70 | 1 Capsule/day | No specific changes | Swelling wrist, breathing difficulty, hip pain maintained |
| 12 | HR002 | F | 32 | 1 Capsule/day | N/A-patient did not report | No specific changes |
| 13 | HR003 | F | 40 | 1 Capsule/day | Dry hands, lupus erythematosus, sinusitis | N/A-patient did not report |
| 14 | HR004 | M | 68 | 1 Capsule/day | N/A-patient did not report | Sleep difficulties |
| 15 | HR005 | F | 54 | 1 Capsule/day | N/A-patient did not report | Brain fog improved, energy and sleep conditions improved, measles and eczema improved. |
Table 1: Qualitative responses of the 15 patients
While there is no cure for RA, patients can be effectively regulated with supplements and self-management interventions (Ohta S, 2014). Recently, a large body of scientific research has suggested active hydrogen molecules as a therapeutic agent in preventing or treating inflammation and oxidative-stress-related human or animal disease models (Ara J, et al., 2018; Barancik M, et al., 2020; Ishibashi T, et al., 2012; Javorac D, et al., 2019; Meng J, et al., 2016; Nakayama M, et al., 2017; Ohsawa I, et al., 2007; Ono H, et al., 2017; Tamaki N, et al., 2016; Terasaki Y, et al., 2019). There are many ways to use hydrogen such as inhalation of hydrogen, drinking Hydrogen-Rich Water (HRW), and molecular hydrogen as treatments (Yang F, et al., 2020). Hydrogen element in the clinical trial has been used in the non-gaseous form, for example, HRW to improve patients with Parkinson’s disease. Hydrogen-rich saline containing 1 ppm hydrogen had started to demonstrate effects in reducing the active phase of rheumatoid arthritis; hydrogen-rich tablets in treating soft-tissue injuries in male occupational athletes; oral hydrogen-rich capsules, a blend of the hydrogen-generating minerals was also shown to improve insulin resistance in obese patients (Yang F, et al., 2020).
It has been discovered with therapeutic antioxidants and anti-inflammatory effects of molecular hydrogen in oxidative-stress-related diseases. Molecular hydrogen selectively reduces cytotoxic oxygen radicals in brain injuries (Ohsawa I, et al., 2007); it is a unique biological capacity to act as an antioxidative and anti-inflammatory substance against organ damage during ischemia and inflammation therapeutic antioxidant for the improvement of cerebral infarction in neuroprotection (Ono H, et al., 2017), an anti-inflammatory agent in chronic-dialysis patients (Nakayama M, et al., 2017), a diminisher of neurologic injury following experimental circulatory arrest in swine, an accelerator in the rat models of oral palatal wound healing (Tamaki N, et al., 2016), and a regulator of reactive oxygen species and oxidative stress in the cardiovascular and central nervous systems (Barancik M, et al., 2020), an eliminator of the fine particles from the lungs and blood by enhancing phagocytic activity (Choi J, et al., 2017).
Additional health benefits of hydrogen include antifatigue and positive metabolic effects. Forced swimming mice drinking hydrogen water (Ara J, et al., 2018) showed a reduction in blood glucose, lactate, and BUN in serum after the treatment. Athletes showed boosting in running performance, and torso strength in healthy adults given hydrogen inhalation (Javorac D, et al., 2019). Further coral hydrate (Wu HT, et al., 2022), the molecular hydrogen has shown effects in reducing total sleep time and sleep falling time to improve alcohol intoxication in mice (Wu HT, et al., 2022).
Molecular hydrogen might protect against Rheumatoid Arthritis-Associated Interstitial Lung Disease (RA-ILD) by decreasing oxidative stress in mice (Terasaki Y, et al., 2019). In addition, Meng Y, et al., 2016 discovered decreased 8-hydroxy-2’-deoxyguanosine (8-OHdG), reduced oxidative stress in fibrous synovial cells (RA-FLSs), and reduced abnormal proliferation in RA mice (Meng Y, et al., 2016). In clinical trials, reduced oxidative stress and disease activity has been demonstrated in patients with rheumatoid arthritis treated with a high concentration of molecular hydrogen (Ishibashi T, et al., 2012), reduced serum biomarkers for RA, Tumor Necrosis Factor-α (TNF α), Interleukin-6 (IL-6), Matrix Metalloproteinase-3 (MMP-3), and urinary 8-hydroxydeoxyguanosine (8-OHdG) were reported in the trial received infused molecular hydrogen in saline (Ishibashi T, et al., 2014). The role of molecular hydrogen as a free radical, or antioxidant in autoimmune oxidative stress, and the effects of molecular hydrogen in inflammation reduction play a role in several preventive and therapeutic applications. The latest international research shows that supplementation of molecular hydrogen as an aid, adjuvant (Si Y, et al., 2021; Yang F, et al., 2020) can speed up the course of AD disease.
It is interesting to explore how molecular hydrogen may have the ability to speed up recovery the of AD disease. The purpose of this study is to determine the safety and possible efficacy of Hydrogen-Rich Coral Calcium (HRCC), the molecular hydrogen in different dose exposures as the first clinical study in AD patients.
Materials and Methods
Safety measurements assessed
Our primary outcome measures are any adverse effects/symptoms (where time frame is up to 28 days). Any adverse effects will be codified according to Common Terminology Criteria for Adverse Events (NCI CTCAE v5.0). Secondary outcome measures include-
• Changes in physiological parameter (Blood routine) (Time frame: Change from baseline blood routine at day 28).
• Changes in physiological parameter (Urine routine) (Time frame: Change from baseline urine routine at day 28) and
• Changes in Brief Fatigue Inventory-Taiwan (BFI-T) (6 questions) (Time frame: Change from baseline BFI-T at day 28).
The BFI-T is a 9-item, 11-point scale developed to assess subjective fatigue. The first three questions measure fatigue severity from 0, indicating “no fatigue,” to 10. Full range from 0 to 100. The specific safety variables to be assessed and laboratory tests to be conducted included urinary biomarkers of sugar, protein, bilirubin, ketones, occult blood, specific gravity, PH, urobilinogen, nitrite, leukocyte, and microscopic examination, Serum biomarkers of Hemoglobin (HGB), WBC count, platelet, RBC count, hematocrit, MCH, MCV, MCHC, Seg. neutrophils, lymphocyte, eosinophilic, basophils, monocyte, SGPT, CRE, UA, AC sugar, HbA1C, TG, CHOL, HDL-C, LDL-C, CRP, hs-CRP, ESR, and eGFR.
Study design
15 rheumatologic and autoimmune patients (14 RA patients and 1 Systemic Lupus Erythematosus [SLE] patient) were recruited from the Min-Sheng General Hospital for this study. Participants were screened by doctors for their eligibility and underwent a series of tests (questionnaires and examinations). Consenting participants will then be allocated into 3 groups by different dosages (Low, n=5; Medium, n=5; High, n=5). Patients receive a different dosage, 1 (Low), 3 (Medium), or 6 (High) capsules of HRCC with their conventional treatment every day for one month. Investigators examined for any changes in haematologic, urine analysis, and health status during and following the exposure period.
Materials
Hydrogen capsules (Pure hydrogen) were purchased from HoHo Biotech Co., Ltd. (Taipei, Taiwan). Each hydrogen capsule contains 170 mg Hydrogen-Rich Coral Calcium (HRCC) with 1.7 × 1021 molecules of hydrogen (around 24 cups of 1,200 ppb/0.6 mM, 200 ml of Hydrogen Water). Therefore, the low dose contains 170 mg, the medium dose, 510 mg, and the high dose contains 1,020 mg HRCC.
Timeline
Participants were instructed on the experimental procedure and obtained their consent on week 0. Participants had their blood drawn and urine tested at 10 ml, measured body weights, blood pressure, and blood oxygen, received 7 days of capsule consumption, and completed the pre-test BFI-T surveys during week 1. Participants had their body weights, blood pressure, and blood oxygen measured, reported any adverse effects, and received 21 days of capsule consumption and the instructions for intake on week 2. Participants had their blood drawn and urine tested at 10 ml and completed post-test BFI-T surveys on week 5. Research assistants provided weekly care and reminded the time for a return.
Statistics
In this report, 14 RA patients are used for the analyses for DAS-28 and BFI-T (RA-specific indicators). 15 (RA+SLE) patients are used for CRP, ESR, and urinary and plasma indicators. Data are expressed as mean and standard deviation (STD). Statics, p<0.05 indicates a significant difference. Analyses were conducted using paired sample T-test. Statistical software Prism is used for statistical analyses.
Results
Demographics
All the data are from the 15 patients (14 RA+1 SLE) (3 males, 12 females; mean age: 54 years [range, 44-96]). The 14 RA patients are described below (Table 2). No patients administered HRCC complained of any adverse effects or changes in general conditions such as hyper-or hypotension, paleness, dizziness, diarrhea, pain, and general discomfort (Table 1). On the contrary, 2 out of the 15 treated patients reported improved energy, 2 out of 15 patients improved sleep conditions, and 1 out of 15 relieved defecating after the treatment (Table 1).
| Number of patients | Total | Male | Female |
|---|---|---|---|
| 14 | 3 | 11 | |
| Age, mean ± SD days | 66 | 74 | 49 |
| BFI-T | 59.28 | 73.33 | 55.45 |
| DAS-28 | 5.18 | 6.39 | 4.85 |
Table 2: The demographics and baseline of RA
DAS-28
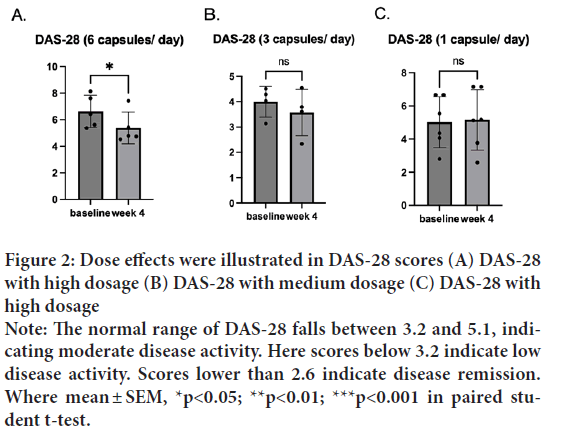
Rheumatoid arthritis activity is measured by Disease Activity Score 28 (DAS-28) at a given moment in time. DAS-28 is calculated based on several different factors, including lab results, patient feedback, and joint swelling and tenderness. Before the treatment, the average DAS score was above the higher bound of the normal range of 5.1 (M=5.18, SD=1.59). After the treatment, one sample t-test indicated that DAS-28 after the treatment (M=4.64, SD=1.44) was significantly lower than DAS-28 before the treatment where t(14)=2.64, p=0.02, d=13) (Figure 1). Interestingly, the dose effects were pronounced in the DAS-28; the group treated with a high dosage (6 capsules/day) significantly reduced the score and t(5)=4.4, p=0.01, d=4) (Figures 2A-2C), while the medium (3 capsules/day) and low (1 capsule/ day) dosages did not show a difference.
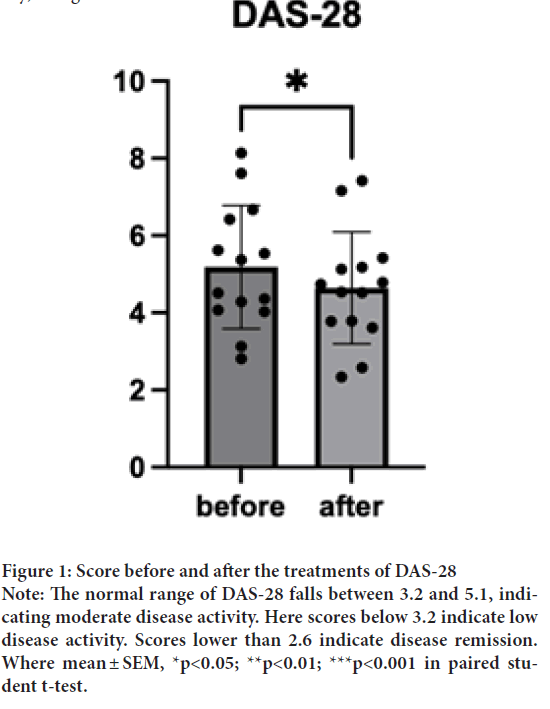
Figure 1: Score before and after the treatments of DAS-28
Note: The normal range of DAS-28 falls between 3.2 and 5.1, indicating moderate disease activity. Here scores below 3.2 indicate low
disease activity. Scores lower than 2.6 indicate disease remission.
Where mean ± SEM, *p<0.05; **p<0.01; ***p<0.001 in paired student t-test.
Figure 2: Dose effects were illustrated in DAS-28 scores (A) DAS-28
with high dosage (B) DAS-28 with medium dosage (C) DAS-28 with
high dosage
Note: The normal range of DAS-28 falls between 3.2 and 5.1, indicating moderate disease activity. Here scores below 3.2 indicate low
disease activity. Scores lower than 2.6 indicate disease remission.
Where mean ± SEM, *p<0.05; **p<0.01; ***p<0.001 in paired student t-test.
Brief Fatigue Inventory-Taiwan (BFI-T)
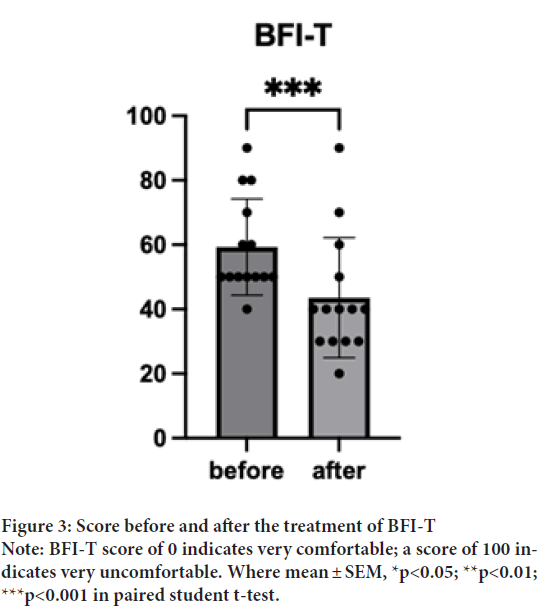
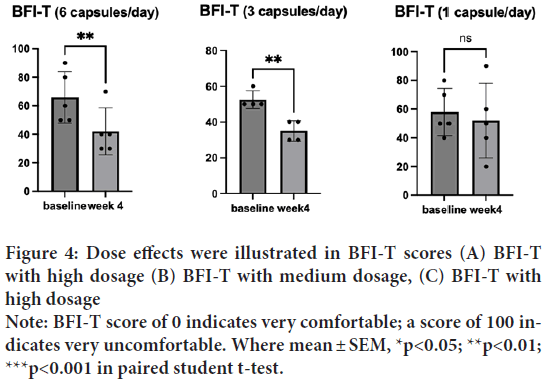
BFI-T before the treatment was 59.29 (SD=14.92). BFI-T after the treatment was 43.57 (SD=18.65). One sample t-test indicated significantly lower BFI-T after the treatment t(14)=5.078, p=0.0002, d=13) (Figure 3). This result indicated that HRCC treatments may enhance patients’ general health conditions. Interestingly, the dose effects were pronounced in BFI-T; the group treated with a high dosage (6 capsules/day) significantly reduced the score t(5)=6, p=0.004, d=4). So does the medium dosage (3 capsules/day) t(4)=7, p=0.006, d=3) (Figures 4A-4C).
Figure 3: Score before and after the treatment of BFI-T
Note: BFI-T score of 0 indicates very comfortable; a score of 100 indicates very uncomfortable. Where mean ± SEM, *p<0.05; **p<0.01;
***p<0.001 in paired student t-test.
Figure 4: Dose effects were illustrated in BFI-T scores (A) BFI-T
with high dosage (B) BFI-T with medium dosage, (C) BFI-T with
high dosage
Note: BFI-T score of 0 indicates very comfortable; a score of 100 indicates very uncomfortable. Where mean ± SEM, *p<0.05; **p<0.01;
***p<0.001 in paired student t-test.
ESR and CRP
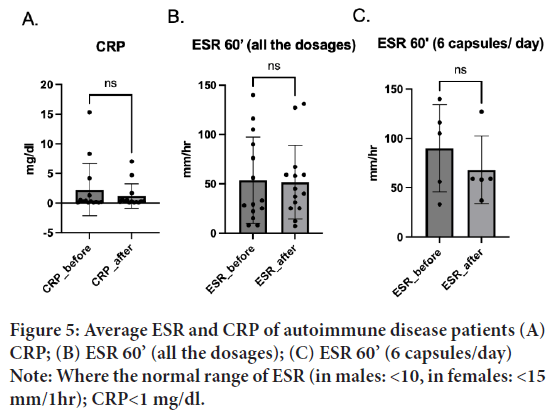
Erythrocyte sedimentation rate (ESR), known as sedimentation rate, and the C-Reactive Protein (CRP), the protein liver produces in response to inflammation are the indicators of the inflammatory process in the body. People with rheumatoid arthritis often have elevated rates in both indicators. CRP baseline was 2.8 mg/dl (SD=4.86), which decreased to 1.3 mg/dl (SD=2.22) after the treatment (N=14). Interestingly, most of the low dosage treated patients showed a trend of decrease in C.R.P. Despite no significant difference being observed after the treatment; t(14)=1.47, p=0.17, d=13) (Figure 5A), a trend of decrease in CRP is observed after the treatment.
Figure 5: Average ESR and CRP of autoimmune disease patients (A)
CRP; (B) ESR 60’ (all the dosages); (C) ESR 60’ (6 capsules/day)
Note: Where the normal range of ESR (in males: <10, in females: <15
mm/1hr); CRP<1 mg/dl.
ESR 60’
Before the treatment, the baseline was 53.27 (SD=42.27) and decreased to 51.40 (SD=36.03) after the treatment (N=15). No significant difference was found before and after the treatment, t(15)=0.29, p=0.77, d=14 (Figure 5B). Interestingly, most high dosage (6 capsules) treated patients showed a trend of decrease in ESR60’; t(5)=1.73, p=0.16, d=4) (Figure 5C). Larger groups of samples are interested in future studies.
Complete Blood Count (CBC)
Complete Blood Count (CBC) provides basic information about health, which can be used to detect or monitor many different health conditions. CBC is often considered along with other factors in determining the health condition (Ahmed MM, et al., 2020). In this report, we examined HRCC effects on WBC, including monocyte and neutrophil, RBC, HGB, and platelets, the indicators associated with an inflammation response in the patients.
WBC
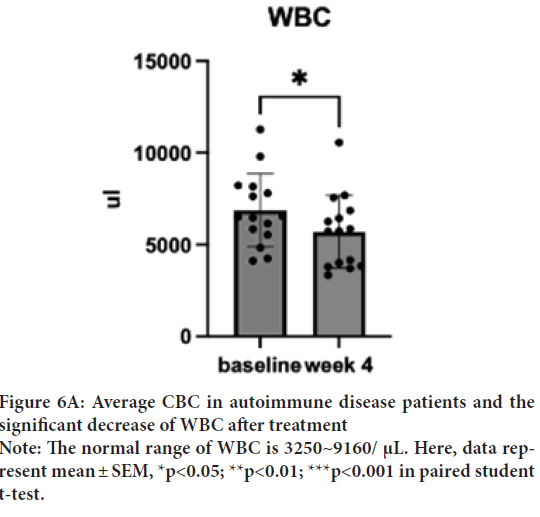
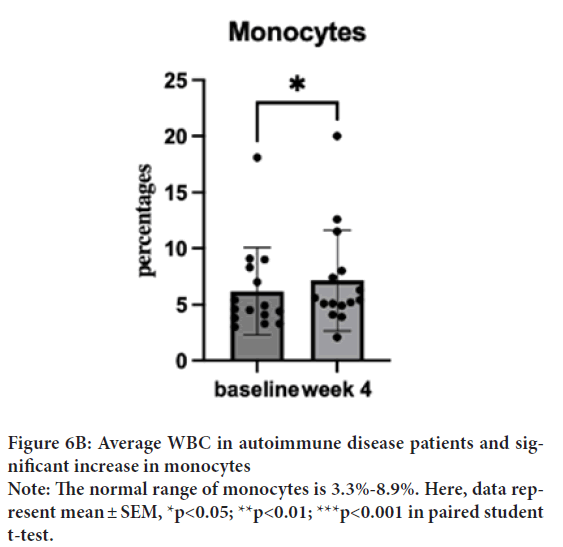
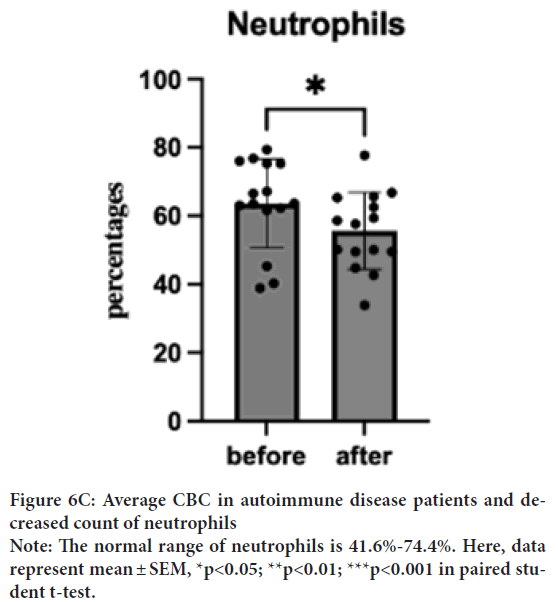
WBC baseline was 6891 μL (SD=2051.5), which decreased to 5331 μL (SD=1625.5) in a week and to 5331 μL (SD=2207.1) after the treatment. Despite a normal baseline, one sample T-test was performed to investigate the degree of changes. One sample T-test suggested a significant decrease of WBC after the treatment; t(15)=2.27, p=0.04, d=14) (Figure 6A). Interestingly, most of the dosages treated patients show a trend of increased monocyte and decreased neutrophils. One sample t-test showed a significant increase in monocytes from 6.22% (SD=5.17) to 7.47% (SD=5.24) (Figure 6B); t(15)=2.55, p=0.02, d=14 and decrease in neutrophils from 67.76% (SD=12.95) to 55.72% (SD=13.74); t(15)=2.56, p=0.02, d=14 (Figure 6C). The WBC of low dosage (1 capsule/day) demonstrated a dose effect. Similar effects are shown in the neutrophil group.
Figure 6A: Average CBC in autoimmune disease patients and the
significant decrease of WBC after treatment
Note: The normal range of WBC is 3250~9160/ μL. Here, data represent mean ± SEM, *p<0.05; **p<0.01; ***p<0.001 in paired student
t-test.
Figure 6B: Average WBC in autoimmune disease patients and significant increase in monocytes
Note: The normal range of monocytes is 3.3%-8.9%. Here, data represent mean ± SEM, *p<0.05; **p<0.01; ***p<0.001 in paired student
t-test.
Figure 6C: Average CBC in autoimmune disease patients and decreased count of neutrophils
Note: The normal range of neutrophils is 41.6%-74.4%. Here, data
represent mean ± SEM, *p<0.05; **p<0.01; ***p<0.001 in paired student t-test.
HGB
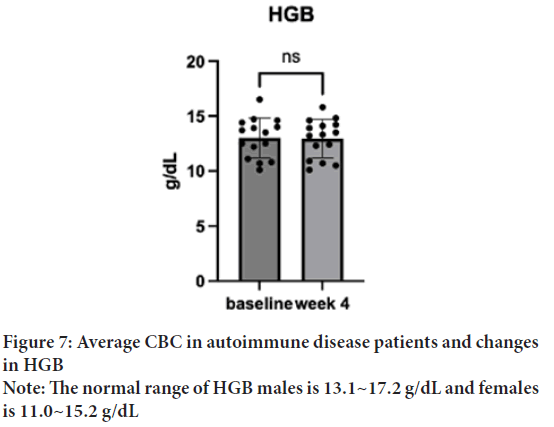
In both sex groups, the HGB baseline was 12.125 g/dL (SD=1.67), which increased to 12.32 g/dL (SD=1.47) in a week and 12.75 g/dL (SD=1.98) after the treatment. One sample t-test suggested no significant difference in the changes found after the treatment; t(15)=0.5, p=0.62, d=14) (Figure 7). The males showed a baseline of 10.4 g/dL, which increased to 11.1 g/dL in a week and 10.97 g/dL after the treatment. The females showed a baseline of 12.7 g/dL, which increased to 12.84 g/dL in a week, and to 13.43 g/dL after the treatment. The overall values are increased in both groups, while 10.97 g/dL after the treatment is still out of the normal range for the males. 12.84 g/dL of females after the treatment is within the normal range.
Figure 7: Average CBC in autoimmune disease patients and changes
in HGB
Note: The normal range of HGB males is 13.1~17.2 g/dL and females
is 11.0~15.2 g/dL
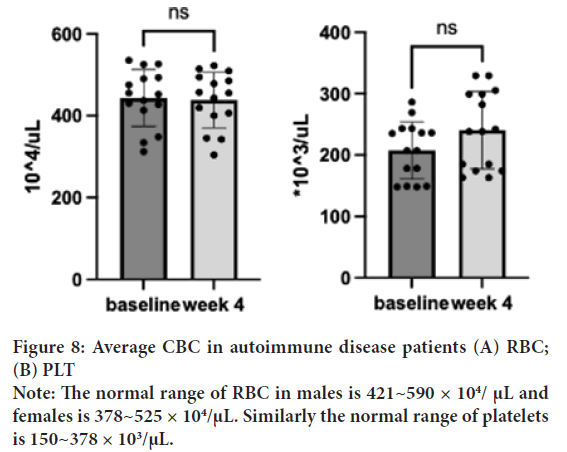
RBC
RBC baseline was 435.13 μL (SD=79.0) in the normal range decreased to 430 μl (SD=76.6) in a week and to 436.73 μl (SD=76.1) after the treatment. One sample t-test suggested no significant difference in the changes found after the treatment; t(15)=0.31, p=0.21, d=14) (Figure 8A).
Figure 8: Average CBC in autoimmune disease patients (A) RBC;
(B) PLT
Note: The normal range of RBC in males is 421~590 × 104/ μL and
females is 378~525 × 104/μL. Similarly the normal range of platelets
is 150~378 × 103/μL.
PLT
PLT baseline was 219 μL (SD=31.9) in the normal range, 239 μL (SD=91.3) in a week, and 245 (SD=64.4) μL after the treatment. Although the baseline was normal, one sample t-test was performed to investigate the degree of changes resulting; t(15)=2.14, p=0.05, d=14) (Figure 8B). A trend of increase in PLT is found after the treatment.
Discussion
This study protocol was approved for the IRB where the primary endpoint, safety is established. This study illustrates that HRCC in the capsules with different dosages, used for a month pronounced no toxicity, nor adversity effects in ranges of the DAS-28, CRP, ESR, and plasma or urinary biomarkers, nor unexpected circumstances in the patients. According to patients’ qualitative responses, the use of hydrogen supplement HRCC did not result in adverse acute side effects. Given the first safety, the endpoint is established, and the efficacy endpoint can be pursued in future trials.
Results of DAS-28 and BFI-T scores showed a significant decrease and a trend of decreased ESR and CRP under the effect of treatments. This result indicated that HRCC treatments might enhance patients’ general health conditions and reduce the DAS-28 score. Nevertheless, larger groups of longitudinal samples are interested in future studies to understand the effects of ESR and GRP. Given the fact that DAS-28 and BFI-T measure the disease activities of the joints and the overall comfort levels, it is reasonable the patients demonstrated an overall improvement in physical health in a short timeframe. Serum and urinary biomarkers measure physiology; therefore, it may take time to take effect. Future studies are recommended to last for at least 3 months upon patients’ first visit in consideration of increased experimental length.
In our study, a significant decrease in the WBCs and neutrophils and an increase in the monocytes were demonstrated after the treatment. It is worth exploring the role and mechanisms of HRCC in interacting with the plasma and urinary biomarkers. For example, the role of HRCC in oxygen transportation, the vital role of RBC. We also found a trend of increase in estimated glomerular filtration rate (eGFR), the measure of renal function in two of the AD patients. eGFR baseline was 137.35 ml/min/1.73 m2, in the normal range, increased to 154.05 ml/min/1.73 m2 after the treatment. It is interesting to understand how HRCC plays a role in the inflammation and metabolic pathways, and how it interacts with the eGFR signaling pathway.
Furthermore, patients reported improved energy, improved sleep condition, and defecating relief in the IRB qualitative responses which illustrate the potential of hydrogen as an adjuvant used with medicines for RA and other autoimmune diseases. Similar effects such as antifatigue effects were seen in the chronic forced swimming mice drinking hydrogen water (Ara J, et al., 2018) and boosting running performance, and torso strength in healthy adults given hydrogen inhalation (Javorac D, et al., 2019). As a few patients reported increased energy levels, it is worthwhile to explore the effects of HRCC on the cell energy level, and the mechanisms regarding the role of HRCC on the metabolic pathways. The limitations of the study include its sample size, duration, and dosage, so safety signals that arise from rare susceptibilities, longer use, or higher dosage may not be detected. Also, as HRCC contains calcium, we may have to include more biomarkers in addition to those for general and RA patients to better assess the potential effects of calcium supplementation in the study. Future experiments will include historical control and the RA treatment groups as references.
Considering the results of safety, longitudinal and control studies can be proceeded to answer additional questions such as the role of HRCC in regulating RA-related biomarkers and the effects of HRCC in RA treatments. Future studies will also look into the individual response of HRCC in SLE patients versus in RA patients. In this report, the SLR (HR006) patient demonstrated increased PLT from the abnormal 148 × 103/μL to 163 × 103/μL, whereas 150~378 × 103/μL is the normal range. This particular patient also demonstrated decreased cholesterol (L.D.) from 137 to 81 (mg/ dL), while other patients do not illustrate such degrees of change. HRCC effects in responses to different autoimmune diseases are worth to be investigated. Current side effects of RA treatments include serious infections, hepatitis B infection in carriers of the virus, allergic reactions, nervous system problems, blood problems, heart failure, Immune reactions including lupus-like syndrome, liver problems, and psoriasis (Scheinfeld N, 2005). While how the HRCC capsules act on the target or aid in regulating the RA-related biomarkers is unknown, it is important to minimize patient discomfort, reduce clinic visits, and reduce disability.
Conclusion
To date, the results of this study as the first human clinical trial suggested that HRCC capsules are safe and do not result in adverse effects by dose. Initial results of changes in biomarkers including alleviation in DAS-28, BFI-T, and WBC as a result of HRCC demonstrate the potential of HRCC as an adjuvant to conventional RA and AD medicines. Future studies will be conducted to validate the efficacy of HRCC in an effort to reduce the side effects of current medicines and enhance the quality of life.
Ethical Approval
The research was conducted in accordance with the principles embodied in the Institutional review board and Good Clinical Practice-ICH GCP. This research protocol has been approved by the Institution Review Board (IRB) from Min-Sheng General Hospital (MSIRB). The approved MSIRB number is 2021007-A.
Acknowledgment
This article was supported by HoHo Biotech (ho202102040). We are grateful to Min-Sheng General Hospital, Taoyuan, Taiwan for its assistance in the conduct of the clinical trial.
References
- van der Heijde D, Daikh DI, Betteridge N, Burmester GR, Hassett AL, Matteson EL, et al. Common language description of the term rheumatic and musculoskeletal diseases (RMDs) for use in communication with the lay public, healthcare providers and other stakeholders endorsed by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR). Ann Rheum Dis. 2018; 77(6): 829-832.
[Crossref] [Google scholar] [Pubmed]
- Kumar BS, Suneetha P, Mohan A, Kumar DP, Sarma KV. Comparison of Disease Activity Score in 28 joints with ESR (DAS28), Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire Disability Index (HAQ-DI) & Routine Assessment of Patient Index Data with 3 measures (RAPID3) for assessing disease activity in patients with rheumatoid arthritis at initial presentation. Indian J Med Res. 2017; 146(Suppl 2): S57.
[Crossref] [Google scholar] [Pubmed]
- Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: A comprehensive update. J Intern Med. 2015; 278(4): 369-395.
[Crossref] [Google scholar] [Pubmed]
- Greenmyer JR, Stacy JM, Sahmoun AE, Beal JR, Diri E. DAS28-CRP cutoffs for high disease activity and remission are lower than DAS28-ESR in rheumatoid arthritis. ACR Open Rheumatol. 2020; 2(9): 507-511.
[Crossref] [Google scholar] [Pubmed]
- Sheehy C, Evans V, Hasthorpe H, Mukhtyar C. Revising DAS28 scores for remission in rheumatoid arthritis. Clin Rheumatol. 2014; 33(2): 269-272.
[Crossref] [Google scholar] [Pubmed]
- Tank ND, Karelia BN, Vegada BN. Biological response modifiers in rheumatoid arthritis: Systematic review and meta-analysis of safety. J Pharmacol Pharmacother. 2017; 8(3): 92-105.
[Crossref] [Google scholar] [Pubmed]
- Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014; 144(1): 1-1.
[Crossref] [Google scholar] [Pubmed]
- Ara J, Fadriquela A, Ahmed MF, Bajgai J, Sajo ME, Lee SP, et al. Hydrogen water drinking exerts antifatigue effects in chronic forced swimming mice via antioxidative and anti-inflammatory activities. BioMed Res Int. 2018.
[Crossref] [Google scholar] [Pubmed]
- Barancik M, Kura B, LeBaron TW, Bolli R, Buday J, Slezak J. Molecular and cellular mechanisms associated with effects of molecular hydrogen in cardiovascular and central nervous systems. Antioxidants. 2020; 9(12): 1281.
[Crossref] [Google scholar] [Pubmed]
- Ishibashi T, Sato B, Rikitake M, Seo T, Kurokawa R, Hara Y, et al. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med gas res. 2012; 2(1): 1-8.
[Crossref] [Google scholar] [Pubmed]
- Javorac D, Stajer V, Ratgeber L, Betlehem J, Ostojic S. Short-term H2 inhalation improves running performance and torso strength in healthy adults. Biol Sport. 2019; 36(4): 333-339.
[Crossref] [Google scholar] [Pubmed]
- Meng J, Yu P, Jiang H, Yuan T, Liu N, Tong J, et al. Molecular hydrogen decelerates rheumatoid arthritis progression through inhibition of oxidative stress. Am J Transl Res. 2016; 8(10): 4472.
[Google scholar] [Pubmed]
- Ishibashi T, Sato B, Shibata S, Sakai T, Hara Y, Naritomi Y, et al. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind, placebo-controlled pilot study. Int Immunopharmacol. 2014; 21(2): 468-473.
[Crossref] [Google scholar] [Pubmed]
- Nakayama M, Itami N, Suzuki H, Hamada H, Osaka N, Yamamoto R, et al. Possible clinical effects of molecular hydrogen (H2) delivery during hemodialysis in chronic dialysis patients: Interim analysis in a 12 month observation. PloS one. 2017; 12(9): e0184535.
[Crossref] [Google scholar] [Pubmed]
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat med. 2007; 13(6): 688-694.
[Crossref] [Google scholar] [Pubmed]
- Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. 2017; 26(11): 2587-2594.
[Crossref] [Google scholar] [Pubmed]
- Tamaki N, Orihuela-Campos RC, Fukui M, Ito HO. Hydrogen-rich water intake accelerates oral palatal wound healing via activation of the Nrf2/antioxidant defense pathways in a rat model. Oxid Med Cell Longev. 2016.
[Crossref] [Google scholar] [Pubmed]
- Terasaki Y, Terasaki M, Kanazawa S, Kokuho N, Urushiyama H, Kajimoto Y, et al. Effect of H2 treatment in a mouse model of rheumatoid arthritis-associated interstitial lung disease. J Cell Mol Med. 2019; 23(10): 7043-7053.
[Crossref] [Google scholar] [Pubmed]
- Choi J, An ES, Ban YH, Seo DW, Kim TS, Lee SP, et al. Hydrogen-enriched water eliminates fine particles from the lungs and blood by enhancing phagocytic activity. J Biomed Res. 2017; 31(6): 503.
[Crossref] [Google scholar] [Pubmed]
- Wu HT, Chao TH, Ou HY, Tsai LM. Coral hydrate, a novel antioxidant, improves alcohol intoxication in mice. Antioxidants. 2022; 11(7): 1290.
[Crossref] [Google scholar] [Pubmed]
- Si Y, Tian H, Dong B, Zhang Y, Wen Y, Jia X, et al. Effects of hydrogen as adjuvant treatment for unstable angina. Exp Biol Med. 2021; 246(18): 1981-1989.
[Crossref] [Google scholar] [Pubmed]
- Yang F, Yue R, Luo X, Liu R, Huang X. Hydrogen: A potential new adjuvant therapy for COVID-19 patients. Front Pharmacol. 2020; 11: 543718.
[Crossref] [Google scholar] [Pubmed]
- Ahmed MM, Ghauri SK, Javaeed A, Rafique N, Hussain W, Khan N. Trends of utilization of complete blood count parameters for patient management among doctors in Azad Kashmir. Pak J Med Sci. 2020; 36(5): 999.
[Crossref] [Google scholar] [Pubmed]
- Scheinfeld N. Adalimumab: A review of side effects. Expert Opin Drug Saf. 2005; 4(4): 637-641.
[Crossref] [Google scholar] [Pubmed]
- Moorkens E, Godman B, Huys I, Hoxha I, Malaj A, Keuerleber S, et al. The expiry of Humira® market exclusivity and the entry of adalimumab biosimilars in Europe: An overview of pricing and national policy measures. Front Pharmacol. 2021: 1993.
[Crossref] [Google scholar] [Pubmed]
Author Info
Min-Chung Shen1*, Jia-Ru Chung2, Kuang-Yih Wang2, Chao-Fang Chu3, Wen-Hsin Tsou2, Hsia-Yun Chou2, Tian-Yu Wang2, Tzu- Hao Chang3, Wen-Wen Chen4, Feng-Cheng Liu5, Frank L Douglas2 and Rong-Hong Hsieh12HOHO Biotech Co., LTD, Taipei, Taiwan
3Department of Biomedical Informatics, Taipei Medical University, Taipei, Taiwan
4Department of Nursing, Min-Sheng General Hospital, Taoyuan, Taiwan
5Department of Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
Citation: Shen MC: Evaluation of the Safety and Potential Therapeutic Effects of Hydrogen-Rich Coral Calcium on Autoimmune Diseases
Received: 29-Jun-2022 Accepted: 11-Oct-2022 Published: 18-Oct-2022, DOI: 10.31858/0975-8453.13.12.855-861
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3