Review Article - (2022) Volume 13, Issue 5
Abstract
Hydralazine is antihypertensive agent which is smooth muscle relaxant helps to treat high blood pressure. Hydralazine comes in a class of medications called vasodilators. It works by relaxing the blood vessels so that blood can flow more easily through the body. Isosorbide Dinitrate is antianginal agent which is used to prevent chest pain in patients with a certain heart condition (coronary artery disease). This medication belongs to a class of drugs known as nitrates. It works by relaxing and widening blood vessels so blood can flow more easily to the heart. This is the literature review of developed various analytical method for validation and estimation of Hydralazine, Isosorbide Dinitrate in combination. The analytical method like RP-HPLC (Revere Phase High Performance Liquid Chromatography), quality by design, Ultraviolet (UV) Spectrophotometry, Simultaneous Equation Method (SEM), stability indicating method were reported for Hydralazine Hydrochloride, Isosorbide Dinitrate. These analytical methods can be used for qualitative and quantitative estimation of Isosorbide Dinitrate and Hydralazine Hydrochloride in single dosage form as well as in combination with other drugs.
Keywords
Antianginal, Antihypertensive, HPLC (High Performance Liquid Chromatography), Hydralazine, Isosorbide nitrate
Abbreviations
HLZ: Hydralazine Hydrochloride; ISD: Isosorbide Dinitrate; RP- HPLC: Revere Phase High Performance Liquid Chromatography; CGMP: Cyclic Guanosine Mono Phosphate; PKG: Protein Kinase-G; NO: Nitric oxide; V-HeFT: Vasodilator-Heart Failure Trial; CHF: Cardiac Heart Failure; A-HeFT¬: African-American Heart Failure; DBH: Dopamine Beta-Hydroxylase
Introduction
In the 1950s Hydralazine was developed as a malaria treatment, along this hydralazine showed antihypertensive ability. Hydralazine chemically, 1-Hydrazinophthalazine and derived from Phthalazine. Hydralazine is orally bioavailable vasodilator. It relaxes (widens) veins and arteries, which make it easier for your heart to pump. It is sold under the brand name Apresoline to treat high blood pressure and heart failure. It also used as Antihypertensive agent in case of preeclampsia. It comes under the class of Smooth muscle relaxant. The mechanism of its action, Hydralazine is direct arteriole vasodilator. The mode of action is relates with intracellular calcium homeostasis. It inhibits Inositol trisphosphate (IP3)-induced release of calcium from the smooth muscle cells sarcoplasmic reticulum, it also inhibit myosin phosphorylation within the arterial smooth muscle. This results in the reduction in peripheral vascular resistance and leads to a compensatory baroreceptor-mediated release of epinephrine and norepinephrine, this cause increase in venous return and cardiac output. Hydralazine may cause stimulation of the sympathetic nervous system and that may cause tachyphylaxis and tachycardia, so it is sometimes given with a beta-blocker or diuretic for better patient tolerance (McComb MN, et al.,2016).
The metabolism of Hydralazine takes place in liver via polymorphic acetylation. Slow acetylators needs lower doses of the drug. Both the acetylated drug and unchanged drug are excreted in the urine and feces (Kirsten R, et al., 1998). In the clinical studies it was found to be HLZ shows more effective together with Isosorbide Dinitrate, for the treatment of people of African descent. HLZ shows instant effect within the time period of 15 min and last up to 6 hours. It is administered by not only orally but also injected into vein (Stuart MC, et al., 2009).
Isosorbide Dinitrate chemically known as 1, 4:3, 6-dianhydro-D-glucitol-2, 5-dinitrate, which is a nitrate ester and a glucitol derivative. Isosorbide Dinitrate is antianginal agent used to prevent chest pain from less supply of blood to heart i.e. angina pectoris and also used to treat heart failure and esophageal spasms. It is sold under the brand name Isordil and Sorbitrate. The clinical study says that ISD is useful in heart disorder due to systolic dysfunction along with hydralazine (Chavey WE, et al., 2008). It can be used sublingually at the time of attack as well as orally for chronic prophylaxis.
Isosorbide is a nitrate that shows its pharmacologic activity by releasing Nitric Oxide (NO) and an Endothelium-Derived Relaxing Factor (EDRF). Nitric oxide is endogenously produced in the cell in endothelium to dilate the blood vessels. Isosorbide Dinitrate undergoes bioactivation process in the cell organelle endoplasmic reticulum via cyt-P450 (cytochrome P450) enzymes to release nitric oxide (Daiber A and Münzel T, 2015), this NO activates the enzyme soluble guanylyl cyclase in the vascular smooth muscles, this result in increasing the levels of intracellular cyclic Guanosine Monophosphate (cGMP) and the associated protein kinases such as cGMP-dependent protein kinases (cGK-I). The cGMP activates the Myosin Light Chain Phosphatase (MLCP). This MLCP cause dephosphorylation of the myosin light chain. CGMP-cGK-I inhibits the Inositol-1, 4, 5-trisphosphate (IP3)-dependent calcium release, so intracellular calcium level is decreased (Etter EF, et al., 2001; Lincoln TM, et al., 1994). Due to the decreased level of intracellular calcium inhibits the Myosin Light Chain Kinase (MLCK) (Divakaran S and Loscalzo J, 2017). The MLCK with the unphosphorylated myosin light chain, causes the myosin head to detach from the actin component of the smooth muscle, resulting in smooth muscle relaxation and causing widening of vessel (Daiber A and Münzel T, 2015).
Literature Review
History of BiDil
BiDil is the first Drug combination approved by Food and Drug Administration (FDA) marketed for a single racial-ethnic group. Hydralazine and Isosorbide Dinitrate is the fixed dose combination sold under the brand name BiDil. The BiDil is approved by FDA by 23-Jun-2005 to treat and prevent heart failure. In 1980-1985, Dr. Jay Cohn from the University of Minnesota led a clinical trial in collaboration with the US Veterans Administration called the Vasodilator-Heart Failure Trial (V-HeFT I), in this trial it was tested whether Hydralazine and Isosorbide Dinitrate combination increases survival rate of patients with heart failure, the results was positive and next trial was held under V-HeFT II, which tested the combination of Hydralazine and Isosorbide Dinitrate against drug enalapril (Krimsky S and Sloan K, 2011). In 1989, Scientist Cohn got patent for combination treatment of Hydralazine and Isosorbide Dinitrate as US patent 4868179. Cohn licensed his patent with Medco Company. In early 1990s Medco prepared New Drug Application (NDA) for FDA’s approval for BiDil on the basis of V-HeFT trials. But the trials were not designed to support NDA and FDA rejected the application in 1997 because statistical power was not enough to show whether the combination really worked (Krimsky S and Sloan K, 2011).
The scientist Cohn again analysed the data and found a signal that drug combination of Hydralazine and Isosorbide Dinitrate may work better than previous, he made V-HeFT trials on self-identified African-American patients and published a paper on his work and applied for new patent on the use of BiDil in black patients (Krimsky S and Sloan K, 2011). It had already known that African-Americans with Congestive Heart Failure (CHF) respond less effectively to conventional CHF treatments (Exner DV, et al., 2001).
Cohn licensed new patent and old patent to the NitroMed Company, this company ran a clinical trial called the African-American Heart Failure Trial (A-HeFT), in 2004 the result of this trial were published in in the New England Journal of Medicine (Taylor AL, et al., 2004). The results were reduced mortality rate by 43%, reduced hospitalizations by 39% (Taylor AL, et al., 2004; PubChem, 2022). In June-2005, FDA approved BiDil on the basis of A-HeFT. Heart Failure Society of America included the use of the fixed dose combination of Hydralazine and Isosorbide Dinitrate as the standard of care in the treatment of heart failure in blacks in 2006 (Figures 1 and 2).
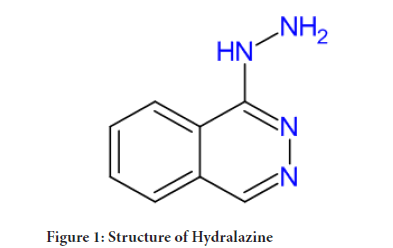
Figure 1: Structure of Hydralazine
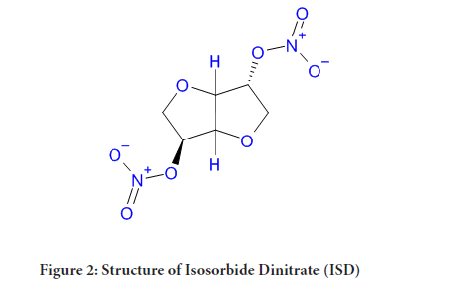
Figure 2: Structure of Isosorbide Dinitrate (ISD)
Mode of action
BiDil is a vasodilator, shows effect on both arteries and veins, BiDil on administration, which releases NO causing activating guanylyl cyclase to relax vascular smooth muscle. It also helps to normalize NO levels, wide the veins and arteries to increase productive blood flow, and decrease workload on the heart (Table 1).
| S. No | Properties | Hydralazine | Isosorbide Dinitrate (ISD) |
|---|---|---|---|
| 1. | Color | Orange solid | White solid |
| 2. | Molecular weight | 160.18 gm/mol | 236.14 gm/mol |
| 3. | Molecular formula | C8H8N4 | C6H8N2O8 |
| 4. | Category | Vasodilator | Organic nitrates |
| 5. | Melting Point (MP) | 172°C-173°C | 70°C |
| 6. | pKa value | -3.9 | 7.1 |
| 7. | Solubility | Acetic acid, Methanol, Ether | Acetone, Alcohol, Ether |
| 8. | pH | 3.5-4.5 | 9-10 |
Table 1: Drug profile (Pubchem, 2022)
Medical use of Hydralazine and Isosorbide Dinitrate
Along with antihypertensive activity, Hydralazine may shows following activity (Drug Bank, 2007):
• Beta-Lysine 5, 6-aminomutase inhibitor.
• Cyclin-Dependent Kinase 9 (CDK9)/cyclin T1 inhibitor.
• Thromboxane B2 antagonist.
• Glucose oxidase inhibitor.
• Arylacetonitrilase inhibitor.
• Guanylate cyclase stimulant.
• Aryl-alkyl acylamidase inhibitor.
• Aspartate-phenylpyruvate transaminase inhibitor.
• Aldehyde dehydrogenase (pyrroloquinoline-quinone) inhibitor.
• N-methylhydantoinase (ATP-hydrolysing) inhibitor.
Inhibitory action of hydralazine on catecholamine-synthesizing enzymes
The Hydralazine cause direct effect on catecholamine-synthesizing enzymes, it causes concentration-dependent inhibition of Tyrosine Hydroxylase (TH) obtained from bovine adrenal medulla and more promisable effect was shown when enzyme are incubated with the drug before the enzymatic assay (Morita K, et al., 1986).
The pharmacokinetic study showed that hydralazine increases the apparent Km value of the enzyme for tyrosine and cofactor, 6, 7-dimethyl-5, 6, 7, 8-tetrahydropterin (DMPH4), without changing Vmax. The inhibitory effect of hydralazine was irreversible and excessive amount of FeSO4 were unable to restore the enzyme activity inhibited by this drug. Hydralazine also inhibits the Dopamine Beta-Hydroxylase (DBH) in chromaffin granule membranes. This kinetic study has also shown that Hydralazine increased the apparent Km value of DBH for ascorbic acid without changing in the Vmax but it decreased the Vmax of the tyramine enzyme without any change in the apparent Km value. The observation of this study concludes hydralazine presumably causes the inhibition of catecholamine-synthesizing enzymes this result into allosteric alterations in the molecular structures of these enzymes. Thus it was confirmed that inhibitory action of hydralazine is due to its metal-chelating activity (Morita K, et al., 1986).
Along with antianginal activity, Isosorbide Dinitrate may show following activity:
• Nitric oxide donor.
• Myocardial ischemia treatment.
• Mannotetraose 2-alpha-N-acetylglucosaminyltransferase inhibitor.
• Beta glucuronidase inhibitor.
• Saccharopepsin inhibitor.
• Histidine kinase inhibitor.
• Sugar-phosphatase inhibitor.
• Ribulose-phosphate 3-epimerase inhibitor.
• Aspulvinone dimethylallyltransferase inhibitor
• ISD used in phobic disorders treatment.
Isosorbide Dinitrate a guanylate cyclase stimulant: The guanylyl cyclase is soluble in the heme-protein. The heme-protein soluble guanylyl cyclase is the intracellular receptor for nitric oxide. Guanylyl cyclase is heterodimetric enzyme with α and β subunits having heme moiety which is essential for binding of NO and activation of enzyme. The physiological responses like smooth muscle relaxation, inhibition of inflammation, and thrombosis are mediated by stimulation of guanylyl cyclase (Nossaman B, et al., 2012).
Methodology
Different analytical methods like UV spectrophotometry, HPLC, High-Performance Thin-Layer Chromatography (HPTLC) and bio-analytical methods are developed for simultaneous estimation of Hydralazine and Isosorbide Dinitrate.
UV spectroscopy: The principle of UV-Visible spectroscopy is based on the absorption of ultraviolet light by drugs which produce different spectra. Spectroscopy deals with interaction of light and matter. When drug sample is bombarded with light then it absorb the light and get excited and after some instant come ground state, this result in the production of a spectrum.
When drug sample absorb UV radiation the electron undergoes excitation, followed by relaxation i.e. jump to ground state. It is important to know that the energy difference between ground state and excited state is amount of UV radiation absorbed by sample, this is how UV spectroscopy works.
The different researchers developed and validate UV spectroscopic method for Hydralazine and Isosorbide Dinitrate.
Mastannama S, et al. the objective of this study was to present a simple, accurate and precise UV spectrophotometric method for simultaneous estimation of HLZ and ISD in bulk and their combined dosage form (Mastannama SK and Sridhar TA, 2015). In this study, the drug concentration is estimated by using Simultaneous Equation Method (SEM) at 232 nm and 208.28 nm (i.e., λmax values of HLZ and Histidinol Dehydrogenase (HISD) respectively). The corrected absorbance was carried at 208 nm and calibration curves were linear with correlation coefficient 0.999 over the concentration range of 2-10 μg/mL for both HLZ and ISD (Mastannama SK and Sridhar TA, 2015).
HPLC method: HPLC is the High Performance Liquid Chromatography, the chromatographic separation is done on the basis of distribution of the analyte i.e. sample between a mobile phase i.e. eluent and stationary phase (packing material of the column). On the basis of chemical structure of the analyte, the molecules are retarded while passing the stationary phase. The specific intermolecular interaction between the molecules of a sample and the packing material define their on column. So the different constituents of sample are eluted at different time, that’s how the separation of the sample ingredients is achieved.
The separation takes place on column in two ways i.e. normal phase and reverse phase. Normal phase is called Normal phase high performance liquid chromatography, reverse phase is termed as reverse phase high performance liquid chromatography.
The RP-HPLC, UPLC (Ultra Performance Liquid Chromatography) and various stability determining methods are reported for simultaneous estimation of HLZ and ISD.
Neelima K, et al. work in this paper is focusing on development of simple, sensitive, linear, precise and accurate RP-HPLC method for simultaneous estimation of Hydralazine, Isosorbide Dinitrate in bulk and tablet formulation. The Zodiac C18 (250 mm × 4.6 mm) 5 μ column is used for chromatographic separation of drug in an isocratic mode. The mobile phase consists of 0.01 M Ammonium acetate: Acetonitrile: Methanol in the ratio of 50:30:20 v/v and pH adjusted to 3 using ortho phosphoric acid was delivered at a flow rate of 1 ml/ min and effluents were monitored at 270 nm. The Retention Factor (RF) value of Hydralazine, Isosorbide Dinitrate was found to be 2.337 and 3.413 min, respectively. Calibration curves were linear with a correlation coefficient of 0.994 for Hydralazine, and 0.997 for ISD over the concentration range of 45-105 μg/ml for Hydralazine, and 24-56 μg/ml for ISD and precise with (% Relative Standard Deviation (%RSD)<2). This method was used for analysis of Isolazine tablet sample and the conclusion of this assay was obtained within specification limit (Neelima K and Prasad YR, 2014).
Mastanamma S, et al. in this article the chromatographic separation of Hydralazine and Isosorbide Dinitrate from their degradation products using Zorbax C18 (250 mm × 4.6 mm I.D; 5 μm) column at detection wavelength 278 nm, using a mobile phase consists of Orthophosphoric acid (0.1%) pH 2.1 and Methanol (60/40) in an isocratic elution mode at a flow rate of 1 ml/min. The retention times for Hydralazine HC l and Isosorbide Dinitrate were found to be 3.7 and 4.7 min respectively. The proposed method is suitable for application in quality-control laboratories for quantitative analysis of both the drugs individually and in combination dosage forms, since it is simple and rapid with good accuracy and precision (Mastanamma S, et al., 2016).
Kassey S, et al. found a simple, sensitive, linear, precise and accurate RP- HPLC method for simultaneous estimation of ISD and, Hydralazine Hydrochloride in tablet formulation was reported. The chromatographic separation of the two drugs was achieved on INERTSIL ODS C18 (150 × 4.6 × 5 μ) column in an isocratic mode. The mobile phase consisting of 0.1 M sodium dihydrogen phosphate: methanol in the ratio of 80:20 v/v and was delivered at a flow rate of 1 ml/min and effluents were monitored at 270 nm. The retention time of was found to be 1.679 and 3.430 min, respectively (Kassey S, et al., 2014).
Madhuri PL, et al. in this article the stability indicating Reversed-Phase Liquid Chromatography (RP-LC) method for simultaneous quantitative estimation of Hydralazine Hydrochloride and Isosorbide Dinitrate in bulk drug and combined tablet dosage form was reported. Chromatographic separation was done in isocratic mode at ambient temperature on hypersil C18 column having size 150 × 4.6 mm and 5 μ particle size using mobile phase comprising of potassium dihydrogen orthophosphate (0.01 M, pH 5.0 adjusted with dilute ammonia solution) and acetonitrile in the proportions of 50:50 V/V. The developed method could separate HLZ and ISB from its degradation products with good resolution (Madhuri PL and Gowri S, 2015).
Santhosh G, et al. found a simple, precise, rapid, specific and accurate RP- HPLC method was reported for simultaneous estimation of Isosorbide Dinitrate and Hydralazine HCl in pharmaceutical dosage form. Separation of drug was carried on Agilant Zorbax (C18) (4.6 mm × 250 mm, 5 μm) column, with mobile phase consist of mixture of buffer (pH 6.5, adjusted with potassium dihydrogen phosphate), acetonitrile in the ratio of 70:30 v/v, at the flow rate 0.8 ml/min. The detection was carried out at 274 nm. The retention times of Hydralazine HCl and Isosorbide Dinitrate was found to be 2.7 and 3.6 min respectively. The linearity, accuracy, precision, limit of detection and limit of quantitation, robustness and ruggedness were validated according ICH guidelines. Linearity of Isosorbide Dinitrate was found in the range of 50-150 μg/mL and that for Hydralazine HCl was found to be 50-150 μg/ml. The correlation coefficient for Isosorbide Dinitrate and Hydralazine HCl were 1 and 0.999 respectively (Santosh G, et al., 2014) (Table 2).
| S. No | Method | Parameter | Author |
|---|---|---|---|
| 1. | RP-HPLC | SP: Zodiac C18 column | Neelima K and Prasad YR, 2014 |
| MP: Ammonium acetate:Acetonitrile:Methanol | |||
| (50:30:20 v/v) | |||
| Wavelength: 270 nm | |||
| Flow rate: 1 ml/min | |||
| Temp: Ambient | |||
| 2. | Stability determining-RP-HPLC | SP: Zorbax C18 column | Mastanamma S, et al., 2016 |
| MP: Orthophosphoric acid:Methanol (60/40) | |||
| Wavelength: 278 | |||
| Flow rate: 1 ml/min | |||
| Temp: Ambient | |||
| 3. | RP-HPLC | SP: Inertsil ODS C18 column | Kassey S, et al., 2014 |
| MP: Sodium dihydrogen phosphate:Methanol (80:20 v/v) | |||
| Wavelength: 270 nm | |||
| Flow rate: 1 ml/min | |||
| Temp: Ambient | |||
| 4. | Stability indicating RP-LC | SP: Hypersil (BDS) C18 column | Madhuri PL and Gowri S, 2015 |
| MP: Potassium dihydrogen orthophosphate:Acetonitrile (50:50 v/v) | |||
| Wavelength: 275nm | |||
| Flow rate: 1 ml/min | |||
| Temp: Ambient | |||
| 5. | RP-HPLC | SP: Agilant Zorbax C18 column | Santosh G, et al., 2014 |
| MP: Potassium dihydrogen phosphate:Acetonitrile (70:30 v/v) | |||
| Wavelength: 274 nm | |||
| Flow rate: 0.8 ml/min | |||
| Temp: Ambient |
Note: SP: Stationary Phase; MP: Mobile Phase; RP-HPLC: Revere Phase High Performance Liquid Chromatography; RP-LC: Reversed-Phase Liquid Chromatography
Table 2: High Performance Liquid Chromatography (HPLC) methods
Green-Analytical Method: Chanduluru HK, et al. the objective of this study an eco-friendly determination of Isosorbide Dinitrate and Hydralazine Hydrochloride using a green analytical quality by design-based UPLC Method. The separation of Hydralazine Hydrochloride and Isosorbide Dinitrate, as well as their degradation product was reported with the help of Phenomenex C18 (50 × 2.1 mm, 2 μm) column containing ethanol and 0.1% trifluoroacetic acid (60%:40% v/v) at a flow rate of 0.5 mL min-1. The linearity range of 10-60 mg/mL-1 and 18.75-112.5 mg/ml with R2 of 0.9998 and 0.9992 for ISD and HLZ, respectively along with accuracy, reproducibility and selectivity (Chanduluru HK and Sugumaran A, 2021).
Discussion
Hydralazine is an orange crystalline powder, whereas Isosorbide Dinitrate is the white colored odorless crystalline powder. HLZ has lower molecular weight than ISD. The melting point difference is also large between HLZ and ISD. As we know that lower the pKa value stronger the acid, so the pKa value of HLZ is higher than ISD, since HLZ is more acidic than ISD. Both above mentioned drugs are soluble in organic solvent. In HPLC the chromatographic separation is carried using C18 column as stationary phase and various mobile phase in different proportion at different concentration the flow rate monitored at specific time interval in specific amount.
Conclusion
The above-mentioned data is concise collective information about the analysis of the Hydralazine and Isosorbide dinitrate in combination. All the methods mentioned are validated according to the ICH guidelines and are useful in the analysis of the mentioned drugs. The prominent method for the analysis of the above mentioned drugs is carried out by RP-HPLC with mobile phase phosphate buffer and acetonitrile at wavelength ranging from 270-280 nm. There is single eco-friendly method carried out by UPLC, degradation study also carried.
References
- McComb MN, Chao JY, Ng TM. Direct vasodilators and sympatholytic agents. J Cardiovasc Pharmacol Ther. 2016; 21(1): 3-19.
[Crossref] [Google Scholar] [PubMed]
- Kirsten R, Nelson K, Kirsten D, Heintz B. Clinical pharmacokinetics of vasodilators: Part I. Clin Pharmacokinetic. 1998; 34(6): 457-482.
[Crossref] [Google Scholar] [PubMed]
- Stuart MC, Kouimtzi M, Hill SR. WHO model formulary 2008. World Health Organization. 2009.
- Chavey WE, Bleske BE, van Harrison R, Hogikyan RV, Kesteron S, Nicklas JM. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008; 77(7): 957-964.
[Google Scholar] [PubMed]
- Daiber A, Münzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: Emphasis on redox biology and oxidative stress. Antioxid Redox Signal. 2015; 23(11): 899-942.
[Crossref] [Google Scholar] [PubMed]
- Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem. 2001; 276(37): 34681-34685.
[Crossref] [Google Scholar] [PubMed]
- Lincoln TM, Komalavilas P, Cornwell TL. Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension. 1994; 23(6): 1141-1147.
[Crossref] [Google Scholar] [PubMed]
- Divakaran S, Loscalzo J. The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J Am Coll Cardiol. 2017; 70(19): 2393-2410.
[Crossref] [Google Scholar] [PubMed]
- Krimsky S, Sloan K. Race and the genetic revolution: Science, myth, and culture. Columbia University Press. 2011.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med. 2001; 344(18): 1351-1357.
[Crossref] [Google Scholar] [PubMed]
- Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004; 351(20): 2049-2057.
[Crossref] [Google Scholar] [PubMed]
- PubChem. Isosorbide Dinitrate Hydralazine compound summary. National Library of Medicine. 2022.
- Drug Bank. Hydralazine. Drug Bank. 2007.
- Morita K, Houchi H, Nakanishi A, Minakuchi K, Oka M. Inhibitory action of hydralazine on catecholamine-synthesizing enzymes prepared from bovine adrenal medulla. Jpn J Pharmacol. 1986; 40(3): 445-453.
[Crossref] [Google Scholar] [PubMed]
- Nossaman B, Pankey E, Kadowitz P. Stimulators and activators of soluble guanylate cyclase: Review and potential therapeutic indications. Crit Care Res Pract. 2012.
[Crossref] [Google Scholar] [PubMed]
- Mastannama SK, Sridhar TA. Simultaneous equation method and absorption correction method for the estimation of isosorbide dinitrate and hydalazine hydrochloride in bulk and their combined tablet dosage form using UV Spectrophotometry. Res J Pharm Technol. 2015; 8(1): 59.
- Neelima K, Prasad YR. Analytical method development and validation for simultaneous estimation of hydralazine, isosorbide dinitrate in bulk and tablet formulation by RP-HPLC. Int J Pharm Sci Res. 2014; 5(4): 1290.
- Mastanamma S, Saidulu P, Sravanthi A, Rajitha E. Stability indicating validated RP-HPLC method for simultaneous determination of hydralazine hydrochloride and Isosorbide dinitrate in bulk and pharmaceutical dosage form. Int J Pharm Sci Rev Res. 2016; 28: 141-148.
- Kassey S, Pulla RP, Prakash KV. Development and validation of stability indicating RP-HPLC method for simultaneous estimation of Isosorbide Dinitrate and Hydralazine Hydrochloride in combined dosage form. Indo Am J Pharm Sci. 2014; 4: 219-229.
- Madhuri PL, Gowri S. A novel validated stability indicating RP-LC method for simultaneous quantitative estimation of hydralazine hydrochloride and Isosorbide dinitrate in bulk drug and combined dosage form. World J Pharm Pharm Sci. 2015; 4(2): 1154-1169.
- Santosh G, Nagasowjanya G, Ajitha A, Umamaheshwararao V. Analytical method development and validation of simultaneous estimation of isosorbide dinitrate and hydralazine HCl in tablet dosage form by RP-HPLC. International Journal of Pharmaceutical Research and Analysis. 2014; 4: 307-311.
- Chanduluru HK, Sugumaran A. Eco-friendly estimation of isosorbide dinitrate and hydralazine hydrochloride using Green Analytical Quality by Design-based UPLC Method. RSC Advances. 2021; 11(45): 27820-27831.
Author Info
Laxman D Bulbule*, RK Godge and Sagar MagarCitation: Bulbule LD: Hydralazine and Isosorbide Dinitrate: An Analytical Review
Received: 01-Apr-2022 Accepted: 29-Apr-2022 Published: 06-May-2022, DOI: 10.31858/0975-8453.13.5.291-295
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






