Review Article - (2024) Volume 15, Issue 7
Abstract
Solid Lipid Nanoparticles (SLNs) represent an advanced pharmaceutical technology designed to address challenges related to drug bioavailability and drug delivery targeting. This detailed overview delves into the various methodologies used to create SLNs, providing insights into the formulation strategies, manufacturing processes and characterization methods used throughout their development. Important topics discussed are the choice of lipid matrix, emulsification techniques and how process variables affect the physical characteristics of solid lipid nanoparticles. Furthermore, the review explores the latest advancements in SLN preparation, including novel approaches such as nano-emulsion techniques, microfluidics and alternative lipid sources. By giving a complete overview of SLN production methods, this review aims to enhance knowledge of their potential for use in drug delivery applications and pharmaceutical research.
Keywords
Solid Lipid Nanoparticles (SLNs), Drug delivery, Nano-emulsion techniques, Lipid matrix, Formulation strategies
Introduction
Lipid-based nanosystems have been identified as a potential nanocarrier for encapsulating various active chemicals in recent years. SLNs offer excellent physical and colloidal stability and great biocompatibility. By carefully selecting the components and adhering to the preparation procedures, we may create carrier structures that have the required physicochemical attributes and biological qualities (Pucek-Kaczmarek A, 2021). In the early 1990s, SLNs were proposed as a potential alternative to existing lipid-based carriers in nanomedicine. They resemble to be oil in water emulsions but in contrast, SLNs substitute the liquid lipid with solid lipid at room temperature, allowing SLNs to incorporate both hydrophobic and hydrophilic drugs. The Active Pharmaceutical Ingredients (API) in SLNs are encapsulated in lipids. They are also called colloidal particles, 10-1000 nm in diameter. SLNs are composed of organic matter and carbon, as shown in Figure 1 (Mukherjee S, et al., 2009; Charcosset C and Fessi H, 2005).
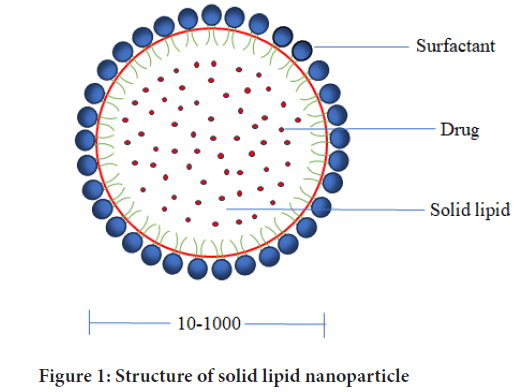
Figure 1: Structure of solid lipid nanoparticle
Recent years have highlighted the imperative understanding that the mere advancement of novel drugs is insufficient to guarantee progress in drug therapy (Charcosset C and Fessi H, 2005). The discovery of suitable drug delivery techniques become a strategic imperative as SLNs address issues with significant plasma level changes and limited solubility in water, while also combining the benefits of traditional systems with some of their main drawbacks, such as poor absorption, rapid metabolism and elimination. When compared to alternative distribution methods, nanoparticles have several benefits due to their special qualities such as nanoparticle size, huge surface area and the ability to modify their surface characteristics (Pandey S, et al., 2022). Nanotechnology is a rapidly growing field and SLNs are the core components for this new field of medicinal research (Abdelwahab SI, et al., 2023). This technique presents a potential option for the advancement of treatments since it has several potential uses in drug delivery and pharmaceutical research (Basha SK, et al., 2021). SLNs, stabilized by surfactants, offer a compelling profile, seamlessly integrating advantages from conventional carriers. Similar to polymeric nanoparticles, they feature a robust matrix, safeguarding chemically volatile API and allowing precise modulation of drug release (Khairnar SV, et al., 2022).
SLNs represent a nanoparticulate form of an active compound, characterized by the dispersion of a solid lipid in a water medium, facilitated by a surfactant. Moreover, SLN exhibits the potential to enhance the physical stability of the formulation owing to its diminutive size (Nugraha MW, et al., 2020). Nevertheless, there are several disadvantages to SLNs as well, such as the limited drug-loading ability for water-soluble drugs, low solubility in lipid melts and the potential for drug release and particle agglomeration while being stored (Pandey S, et al., 2022) (Table 1). One of the most significant drawbacks of SLNs is that unintentional and uncontrolled strengthening of the crystal structure during their formation and preservation may result in the release of the enclosed medication (Duan Y, et al., 2020). The utilization of ecologically safe, biodegradable components and production methods may be their main benefit. The majority of the nanoparticles in this category are classified as class I in the nanotoxicological categorization system due to their small size and biodegradable nature (Battaglia L, et al., 2014). A key benefit of SLNs is that the lipid core is composed of biological lipids, thereby mitigating the potential for toxicity (Table 2) (Mukherjee S, et al., 2009; Pandey S, et al., 2022; Akanda M, et al., 2023; Hernández-Esquivel RA, et al., 2022; Parhi R and Suresh P, 2012).
| Advantages | Disadvantages |
|---|---|
| SLNs offer enhanced stability and scalability | Inadequate drug loading capacity |
| Reduced toxicity risk | Drug expulsion post polymeric transition |
| Long-term stability | Elevated water content in dispersions |
| Ease of manufacturing | Limited hydrophilic drug loading capacity |
| Controlled release kinetics | |
| Enhances bioavailability of poorly water-soluble compounds | |
| Chemical protection for labile compounds | |
| Same raw material as emulsion | |
| SLNs enable the achievement of high concentrations of functional compounds |
Table 1: Advantages and disadvantages of SLNs
| S. No | Lipids | Examples |
|---|---|---|
| 1 | Fatty acids | Lauric acid, dodecanoic acid, eicosanoic acid, tetradecanoic acid, palmitic acid, oleic acid, (octadecanoic acid ) stearic acid, behenic acid, octadecadienoic acid (linoleic acid) |
| 2 | Triglycerides | Tripalmitin, trilaurin, trimyristin, tricaprin, hydrogenated coco glycerides, triolein, Tripalmitin Dynasan® 116, tri caprylate, Tristearin Dynasan® 118, Medium Chain Triglycerides (MCT), Trimyristin Dynasan®114, Glyceryl behenate |
| 3 | Diglycerides | Glyceryl distearate |
| 4 | Monoglycerides | Glyceryl hydroxyl stearate, glyceryl behenate, glyceryl monostearate |
| 5 | Mixed glycerides | Glyceryl palmitostearate |
| 6 | Hydrogenated glycerides | Hydrogenated coconut oil glycerides, hydrogenated palm kernel oil glycerides |
| 7 | Cyclic complexes | Para-acyl calixarenes, cyclodextrin |
| 8 | Waxes | Cetyl palmitate, beeswax, carnauba wax |
| 9 | Hard fat | Hydrogenated coco-glycerides, hydrogenated vegetable oil, polyethylene glycol glycerides |
| 10 | Cationic lipids | Cetrimide, Stearyl Amine (SA Dioleoyltrimethylammonium Propane (DOTAP), benzalkonium chloride, Cetylpyridinium Chloride (CPC), dimethyl dioctadecyl ammonium bromide |
| 11 | Others | Anhydrous milk fat, cocoa butter, soybean oil, castor oil, hydrogenated ricinus oil, arachis oil, cocoa butter, hydrogenated palm oil, goat fat fully hydrogenated soybean oil |
Table 2: Varieties of lipids utilized in SLN
Literature Review
High-energy methods
High-energy methods include High Pressure Homogenization (HPH), Ultrasonic/high-speed homogenization and supercritical fluid methods.
HPH: This method’s benefit is that it produces SLNs with tiny particle sizes and an excellent ability to entrap. HPH involves pumping a molten lipid quickly and at a pressure of 500-5000 bar through a small opening. Typically, a lipid content of 5%-10% is employed however studies have looked at up to 40%. Hot and cold homogenizations are the two techniques for HPH (Duong VA, et al., 2020).
SLN preparation using hot homogenization
The development of SLNs presents a compelling opportunity for the pharmaceutical industry due to their scalability, reduced manufacturing time, and the absence of organic solvents, making them particularly suitable for hydrophilic or heat-sensitive medications. The process begins by combining the lipid and drug above their melting point, followed by the addition of a surfactant in water at the same temperature. This mixture is then emulsified using a shear device to create a pre-emulsion. The next step involves cooling the mixture to room temperature to facilitate recrystallization. To achieve optimal particle sizes, typically less than 500 nm, 3-5 homogenization cycles are performed at pressures ranging from 500 to 1500 bar. It is crucial to optimize these parameters carefully, as over-homogenization can lead to an increase in particle size. Finally, the SLNs are characterized to ensure they meet the desired properties for effective drug delivery. Combine lipid and drug above melting point.
SLN preparation using cold homogenization
The process of creating SLNs using cold homogenization is ideal for medications that dissolve in water to avoid medication loss, particularly when dealing with huge particles and time-consuming procedures. This method begins by cooling a lipid melt that contains the drug to solidify it. Next, the solid lipids are ground into microparticles, which are then dispersed in a cold surfactant solution. The mixture is homogenized at room temperature or below, allowing for optimal particle formation. Subsequently, gravity is employed to break down the lipid microparticles into SLNs, achieving particle sizes that range from 50-1000 nm. This systematic approach not only enhances the bioavailability of water-soluble drugs but also streamlines the formulation process (Hernández-Esquivel RA, et al., 2022; Teja VC, et al., 2014).
Ultrasonic and high-speed homogenization
It offers an efficient and user-friendly approach to formulating SLNs and Nanostructured Lipid Carriers (NLCs), eliminating the need for organic solvents, high levels of surfactants, and minimizing medication exposure to high temperatures and metal pollution. The process begins by melting solid lipids and adding the medication, which is then combined with a warmed aqueous phase containing surfactant while maintaining a temperature 5°C above the lipid melting point. High-speed stirring is employed for homogenization, followed by further refinement through ultra-soni-cation. To ensure purity, the nanoemulsion is filtered to remove any foreign particles before cooling to facilitate the formation of SLNs or NLCs. For optimal particle size control, bath sonication can be considered, while continuous monitoring of surfactant and lipid concentrations is important. Once prepared, SLNs should be stored between 4°C and 5°C for stability, and freeze-drying with 5% mannitol as a cryoprotectant enhances their longevity. This meticulous process not only ensures high-quality formulations but also safeguards against thermal degradation and metal contamination (Mukherjee S, et al., 2009; Satapathy S and Patro CSP, et al., 2022).
Supercritical fluid method
It is a sophisticated technique that ensures uniform distributions of particle sizes and high extraction efficiency of solvents, albeit at the cost of utilizing expensive supercritical fluids and organic solvents. The process begins by dissolving lipids and drugs in chloroform, which is then transferred into a water phase along with a surfactant. This organic solution is subjected to HPH to create a stable emulsion. Subsequently, the emulsion is introduced to the top of an extraction column, while supercritical Carbon dioxide (CO2) is simultaneously introduced from the bottom. The supercritical CO2 rapidly extracts the organic solvent from the emulsion, facilitating the formation of SLNs or NLCs. Finally, the resulting SLNs or NLCs are collected from the bottom of the extraction column, yielding a highly efficient and effective formulation process (Mukherjee S, et al., 2009; Teja VC, et al., 2014; Trucillo P and Campardelli R, 2019).
Low energy methods
Microemulsion based method: It is an innovative and practical approach for producing SLNs and NLCs, offering advantages such as easy scalability, repeatability, and a solvent-free process. The procedure begins by dissolving the drug in molten lipids at temperatures above their melting point. While the lipids melt, an aqueous phase composed of water and surfactant is prepared and preheated to room temperature. To form a clear, stable microemulsion, the aqueous phase is gently mixed into the lipid phase. This microemulsion is then transferred into a cold aqueous solution (between 2°C-10°C) and mixed gently using machinery, ensuring that the volume of the heated emulsion is 25-50 times less than that of the cold aqueous phase. As dilution occurs, a nanoemulsion forms, leading to the crystallization of lipids into SLNs or NLCs. This method has successfully produced SLNs with small particle sizes of less than 200 nm and low polydispersity indices below 0.2, demonstrating enhanced efficacy compared to free drug molecules in various studies. For large-scale production, microemulsions can be prepared in a temperature-controlled tank and then pumped into a cold-water tank for lipid precipitation. Although the dilution process is simple and reproducible, it does require significant volumes of water and surfactant. To concentrate the final product, techniques such as lyophilization or ultra-filtration can be employed to remove excess water from SLN and NLC dispersions (Battaglia L, et al., 2014; Teja VC, et al., 2014).
Hot microemulsion technique/microemulsion dilution technique: It includes method 1 (direct method) and method 2 (stepwise method).
Method 1: To prepare a non-aqueous microemulsion, the first step involves accurately weighing each component selected from phase diagrams and placing them into glass vials. These vials are then filled with the required amount of water to facilitate the emulsion process. Next, the vials are placed in a jacketed vessel and stirred for 10 minutes at 1500 revolutions per minute (rpm), maintaining a temperature of either 65°C or 80°C, depending on the specific formulation. After this stirring period, the formulations are allowed to cool to room temperature while continuing to be stirred magnetically. This careful control of temperature and mixing ensures the formation of a stable microemulsion, setting the stage for subsequent processing steps.
Method 2: In the stepwise method for preparing a non-aqueous microemulsion, the process begins by measuring and filling glass vials with the selected non-aqueous components. Once the vials are prepared, the mixture is stirred at the appropriate temperature until the solid lipid melts, resulting in a homogeneous blend, which typically takes about 3-4 minutes. Following this, the hot water phase is gradually added in 200 μl increments to ensure proper integration. The mixture is then stirred for an additional 5 minutes to promote thorough mixing and emulsification. Finally, the formulation is allowed to cool to room temperature while being stirred magnetically at 1500 rpm, ensuring that the microemulsion remains stable and well-dispersed throughout the cooling process (Fadda P, et al., 2013; Negi JS, et al., 2013).
Microemulsion cooling technique: It is a systematic approach to producing SLNs that emphasizes efficiency and biocompatibility. The process begins by melting emulsifying wax at temperatures ranging from 37°C-55°C. While maintaining this temperature, water is added gradually to create a uniform slurry, with minimal swirling to ensure stability. A suitable polymeric surfactant is then incorporated into the water phase in predetermined proportions to enhance emulsification. Once the microemulsion is formed, it is cooled to room temperature or 4°C, prompting the precipitation of SLNs from the undiluted microemulsion. The choice of matrix material, derived from excipients such as waxes or polymeric surfactants, is important for the formulation’s performance. Operating at mild temperatures facilitates rapid, reproducible, and cost-effective production while ensuring that all ingredients are potentially biocompatible. This method achieves high entrapment efficiency, particularly for water-insoluble medications, and allows for the easy incorporation of cell-specific ligands on the SLN surface for targeted delivery, enhancing therapeutic efficacy and precision (Battaglia L, et al., 2014).
Dual/double emulsion method: Creating a double emulsion of water, oil, and water is an effective method particularly suited for hydrophilic medications, although it often results in large particle sizes and high drug loss. The process begins by dissolving the hydrophilic drug in an aqueous solvent to form the inner aqueous phase. This inner phase is then dispersed into a lipid-containing emulsifier, resulting in the formation of primary water-in-oil (w/o) emulsion. To create the double emulsion (w/o/w), an aqueous solution containing a hydrophilic emulsifier, such as poloxamer, is added to the primary emulsion. The mixture is stirred thoroughly to ensure uniformity before isolating the double emulsion through filtration. This technique allows for the production of lipid nanoparticles without the need to melt the lipid, which facilitates potential surface modifications that enhance stability. However, it is important to note that the final particles tend to be polydisperse, which can limit precise control and application in certain fields where uniformity is essential. (Akanda M, et al., 2023; Satapathy S and Patro CSP, et al., 2022).
Phase Inversion Temperature (PIT): This method utilizes surfactants made of non-ionic polyoxyethylene, which are devoid of solvents and require minimal energy, although it may lead to a low level of nanoemulsion stability. The process begins with the selection of solid lipids, non-ionic surfactants, and aqueous phase components. Both the oil phase and aqueous phase are heated separately to approximately 90°C, which is above the phase transition temperature. To create w/o emulsion, the aqueous phase is added dropwise to the oil phase while stirring continuously. After this addition, the emulsion is allowed to cool while stirring constantly at room temperature. During this cooling process, turbidity clearance is observed at the PIT. Once below the PIT, an oil-in-water (o/w) nanoemulsion is formed. Finally, the SLNs are characterized and stored appropriately to maintain their stability and efficacy (Battaglia L, et al., 2014; Gao S and McClements DJ, 2016; Singh R, 2019).
Membrane contactor method: The use of a particular membrane contactor for the formulation of SLNs offers significant advantages in terms of scale-up viability and controllability of particle size, although it also presents challenges such as potential membrane blockage. The process begins with a pressure vessel to raise the melting point of the lipid phase, followed by passing this heated lipid phase through a module containing a Kerasep clay film. As the lipid phase migrates dilatorily to the layer surface, the water phase is isolated within the module. To facilitate the formation of smaller particles, the lipid phase is pushed through the membrane’s openings, resulting in SLNs forming in the water phase after cooling. Adjusting process parameters such as lipid content, lipid phase pressure, and aqueous cross- flow velocity allows for effective control over particle size. Maintaining the temperature of the aqueous phase below the lipid melting point ensures rapid solidification of the lipidic phase, which contributes to reducing SLN size. Overall, utilizing membrane contactors for SLN formulation enhances feasibility and scalability while providing a method for precise particle size control, despite the complexities involved in managing a potentially blocked system (Battaglia L, et al., 2014; Battaglia L, et al., 2010).
Coacervation technique: The usage of fatty acids and alkaline salts in nanoparticle formulation presents a simple and solvent-free method, although it is not suited for medications sensitive to pH and is effective only with alkaline salt lipids. The process begins by creating an alkaline salt micellar solution using soap and a fatty acid, achieved by dissolving the chosen salt (e.g., sodium stearate or sodium palmitate) in water at a temperature above its Krafft point. To enhance micellization, the medication can be either directly dissolved into the micellar solution or pre-dissolved with a small amount of ethanol. Next, a stabilizing agent, such as dextran, hydroxypropyl methylcellulose, polyvinyl acetate/polyvinyl alcohol, or polyoxyethylene/polyoxypropylene polymers, is selected from non-ionic surface-active polymers. An acid solution, known as the coacervating solution, is then prepared and heated to a temperature between 40°C and 50°C, above the Krafft point of the fatty acid sodium salt-with higher temperatures potentially required for salts like sodium arachidate and behenate. Mixing the micellar solution with the acid solution results in the precipitation of fatty acid nanoparticles due to proton exchange. The obtained suspension is rapidly cooled to 15°C to stabilize the SLNs. To regulate SLN size, reaction conditions can be modified, such as adjusting lipid concentration in both the micellar solution and the type or quality of polymer stabilizer used. Enhancing the interaction between the coacervating solution and the fatty acid salt is important for achieving a homogeneous and stable nanoparticle suspension (Battaglia L, et al., 2014; Gallarate M, et al., 2011; Subroto E, et al., 2022).
Organic solvent-free melt dispersion technique: The process of synthesizing SLNs begins by heating the lipid phase, such as stearic acid, above its melting point. Once the lipid is sufficiently heated, it is dispersed in water using a low Hydrophilic-Lipophilic Balance (HLB) surfactant to create the initial water-in-oil (w1/o) emulsion. Next, a warm aqueous solution of a high HLB surfactant, such as tween 80, is combined with the first emulsion to form the second emulsion, known as water-in-oil-in-water (w1/o/ w2), which is then homogenized to ensure uniformity. The resulting double emulsion is subsequently added to cooled water while shaking, promoting the synthesis of solid lipid nanoparticles. Additionally, there is an option to incorporate desired substances, such as dyes, into either the internal aqueous phase or the lipid phase prior to forming the emulsions, allowing for further customization of the SLNs for specific applications (Peres LB, et al., 2016; Duong VA, et al., 2020).
Organic solvent approaches
Solvent evaporation emulsion method: This method is ideal for formulating medications that are extremely thermolabile, as it avoids exposure to extreme heat or physical strain while effectively removing toxic organic solvents. The process begins by dissolving the drugs and lipids in water-immiscible organic solvents, such as cyclohexane or chloroform. This solvent-drug-lipid solution is then emulsified in a phase of water to create nano-dispersions. Following emulsification, the organic solvent is evaporated using a rotary evaporator or mechanical stirring, which leads to lipid precipitation and the formation of SLNs and NLCs. To control the particle sizes of SLNs and NLCs, it is essential to maximize the amount of lipids in the organic phase. Additionally, care must be taken to avoid high temperatures during encapsulation to protect thermolabile drugs. Finally, further purification steps, such as additional evaporation or ultra-filtration, can be performed to concentrate the suspension and enhance the quality of the final product (Khairnar SV, et al., 2022; Parhi R and Suresh P, 2012).
Solvent emulsion and diffusion method: This method is particularly suited for formulating medications that are extremely thermolabile, as it avoids the use of extreme heat or physical strain, while also eliminating toxic chemical solvents and minimizing the volume of water used, resulting in a low concentration of SLNs. The process begins by achieving initial thermodynamic equilibrium through reciprocal saturation between water and an organic solvent. Medications and lipids are then dissolved in a water-soluble organic solvent, which is subsequently emulsified in the water phase containing a stabilizer under stirring to form an o/w emulsion. This emulsion is diluted with water at a ratio of 1:5 to 1:10, allowing the solvent to diffuse into the continuous aqueous phase, which leads to spontaneous formation of SLNs as lipid precipitation occurs. The organic solvent is removed via lyophilization or vacuum distillation, ensuring that the integrity of the thermolabile drugs is maintained. To achieve the desired particle sizes and polydispersity indices, formulation parameters such as lipid type and solvent choice can be optimized. Additionally, this method allows for the exploration of various drug and lipid combinations to encapsulate both hydrophilic and hydrophobic drugs. Careful attention is given to minimize exposure of drugs to extreme conditions by avoiding high temperatures and physical stress. The method can also be scaled up for industrial production, and purification of the SLN and NLC dispersions involves removing any residual organic solvent, similar to techniques used in microemulsion methods.
Solvent injection method (solvent-based method or solvent displacement): This method for producing SLNs is easy to use, facilitates quick manufacturing, and requires no complex equipment, all while effectively removing toxic organic solvents. The process begins by dissolving the lipid and active ingredients in a water-miscible or organic solvent. A syringe needle is then used to inject this organic solvent solution into a vigorously stirred water phase, typically distilled water. Upon contact with water, the lipid precipitates, leading to the formation of nanoparticles. As the organic solvent rapidly dissolves in the water, the lipid molecules self-assemble into nanoparticles due to hydrophobic interactions. After the formation of the nanoparticles, the SLN dispersion is collected. Optionally, the dispersion can be filtered to remove any large particles or aggregates, ensuring a more uniform and stable final product (Charcosset C, et al., 2006).
Preparation of SLN using membrane contactor method: In this innovative method for producing SLNs, a specialized membrane contactor module is utilized, featuring a ceramic membrane with pore diameters typically ranging from 0.1-0.45 μm, which effectively separates the water phase from the lipid phase. The process begins by heating the lipid phase, which contains the solid lipid. This heated lipid phase is then allowed to flow parallel across the surface of the ceramic membrane, while the water phase circulates on the opposite side. As the lipid phase makes contact with the membrane, small droplets are formed due to the temperature difference between the two phases. These droplets subsequently solidify into SLNs, resulting in a highly efficient and controlled nanoparticle formation process. This method not only enhances the stability of the SLNs but also allows for precise control over their size and distribution (Steiner D and Bunjes H, 2021).
Other scale-up methods
Hot melt extrusion coupled with HPH: Combining hot melt extrusion with HPH significantly enhances the scalability of SLN preparation. The process begins by pumping raw materials into the extruder barrels at elevated temperatures that exceed the lipid melting point. An insulated connector is used to connect the high-pressure homogenizer at the end of the extruder barrel, ensuring efficient transfer of materials. To achieve smaller particle sizes, it is important to maximize process variables such as lipid content, screw design, and residence time within the extruder. By carefully varying parameters like screw speed, barrel temperature zones, and liquid addition zones, SLNs can be produced with sizes below 200 nm. It is essential to ensure complete melting of the materials and liquefaction of drug particles before they come into contact with the emulsifier. Additionally, controlling the barrel temperature and screw speed is vital for facilitating emulsion formation between the melted medication and surfactant, leveraging the shear generation within the extruder space to create stable and uniform nanoparticle dispersion. This integrated approach not only improves production efficiency but also enhance the quality and consistency of the final SLN product (Khairnar SV, et al., 2022).
Liquid flow-focusing and gas displacing method in microchannel: The process of synthesizing SLNs begins by dissolving lipids in a water-based surfactant and organic solvent to create a lipid solution. Microchannels are then configured with specific junctions designed for lipid and aqueous solutions, as well as for gas insertion. Simultaneously, lipid and aqueous solution streams are injected into the cross-junction of the microchannel to focus the liquid flow. To facilitate SLN development, inert gas is injected into the main flow through a T-shaped connection, creating a slug flow of the gas-liquid combination. This hydrodynamic focusing technique allows for the formation of SLNs with narrow size distributions and small diameters. As the gas sparging continues alongside the liquid flow, it helps maintain solids in suspension within the microchannels. The diffusion of the organic solvent into the aqueous phase leads to supersaturation of lipids, resulting in SLN synthesis. Large gas bubbles split the upper liquid streams, forming Taylor bubbles that effectively suspend the gas-liquid SLNs. Importantly these Taylor bubbles create liquid slug flow motions that prevent the deposition of SLNs within the microchannels, ensuring continuous production. By maintaining a smooth and free flow of SLNs through the microchannels, this method enables efficient and scalable SLN manufacturing.
Nanoprecipitation using static mixers: The process of preparing SLNs begins with the careful selection of a suitable lipid and organic solvent for dissolving the lipid. Once chosen, the lipid is dissolved in the selected organic solvent to create a homogeneous lipid solution. Next, the aqueous phase is prepared using deionized water or an aqueous buffer solution. A static mixer is then installed in the system, ensuring proper connection and secure placement. The lipid solution and water phase are simultaneously injected into the static mixer at controlled flow rates. The static mixer facilitates efficient mixing of the lipid solution and water phase, promoting rapid and homogeneous blending. The resulting SLN suspension is collected at the outlet of the static mixer. Following collection, it is essential to characterize the obtained SLNs for parameters such as particle size, size distribution, morphology, and stability. To optimize the process, fine-tuning of variables such as lipid concentration, mixing conditions, and flow rate may be necessary. Finally, this nanoprecipitation process can be scaled up using static mixers for large-scale production of SLNs while maintaining efficient mixing and precise control over particle size (Khairnar SV, et al., 2022).
Dual centrifugation: SLNs are usually made by HPH above the lipid’s melting temperature. This method is resource-intensive and not suitable for processing multiple samples simultaneously. An alternative method for preparing SLNs involves dual centrifugation, which offers a streamlined approach to emulsification (Figure 2). The process begins by weighing the necessary ingredients into 2 ml vessels, which are then placed in a heated centrifuge. Additional rotation is applied to create intensive stressing, enhancing the emulsification process. Grinding media is added to the vessels to facilitate this process further. The emulsification is initiated and maintained for 10 minutes, during which it is important to monitor the sample temperatures, ensuring they remain below 90°C to protect the integrity of the lipids. Throughout the procedure, adjustments to process variables can be made as needed to optimize outcomes.
Figure 2: Emulsification diffusion technique (Teja VC, et al., 2024)
After emulsification, particle sizes are analysed to confirm they are below 200 nm with a narrow size distribution, ensuring high quality of the SLNs. Finally, all process parameters and results are documented meticulously for future reference and reproducibility. This method not only enhances efficiency but also contributes to the consistent production of high-quality nanoparticles (Khairnar SV, et al., 2022; Battaglia L, et al., 2014; Üner M, 2016).
Spray drying: The preparation of SLNs begins with the creation of an ethanolic solution containing the selected lipids and the desired drug. This solution is then atomized using methods such as centrifugal, ultrasonic, or electrostatic atomization to create fine droplets. These droplets are subsequently subjected to hot gas, which induces rapid solvent evaporation and facilitates the formation of SLNs. To separate the hot air from the newly formed SLNs, a cyclone separator or electrostatic precipitator is employed. Throughout this process, it is essential to control various parameters, including temperature, humidity, feed composition, rate of drying, and gas flow rate, to ensure optimal conditions for nanoparticle formation. Further optimization of SLN characteristics is conducted to refine dimensions, dispersion, form, density and morphology.
Finally, it is important to ensure that the macroscopic powder qualities meet industry standards for bulk density, dispersibility, tapped density and flowability, thereby guaranteeing the suitability of the SLNs for their intended applications. This method not only enhances efficiency but also contributes to the production of high-quality lipid nanoparticles (Khairnar SV, et al., 2022; Battaglia L, et al., 2014).
Examples of applications of solid lipid nanoparticles
Drug delivery: SLNs have been extensively explored as carriers for drug delivery systems (Paliwal R, et al., 2020; Müller RH, et al., 2000; Wissing SA and Müller RH, 2003).
Cosmetics: SLNs are utilized in cosmetic formulations for delivering active ingredients such as vitamins, antioxidants and UV filters. They provide improved stability and skin penetration, enhancing the efficacy of cosmetic products (McClements DJ and Xiao H, 2012).
Food industry: SLNs have emerged as potential carriers for bioactive compounds in food products. They can encapsulate lipophilic substances, enhancing their solubility, bioavailability and stability in food matrices (Musielak E, et al., 2022).
Factors affecting solid lipid nanoparticles
Type of lipid: The choice of lipid affects the particle size, stability, crystallinity and drug-encapsulating capacity of SLNs. Different lipids have different melting points, solubility and compatibility with drugs.
Type of surfactant: The types, concentration and ratio of surfactant to lipid can influence the size, zeta potential and drug release of SLNs.
Method of preparation: There are various methods to prepare SLNs, such as high-pressure homogenization, microemulsion, solvent injection and sonication. Every approach has benefits and drawbacks and it might have an impact on the physicochemical characteristics and functionality of SLNs.
Process parameters: Parameters, such as temperature, pressure, speed, time and solvent type, can also affect the quality and characteristics of SLNs. The optimal process parameters depend on the type of lipid, surfactant and method used (Duan Y, et al., 2020).
Drug-lipid compatibility: The drug’s compatibility with the lipid matrix affects drug encapsulation efficiency and release profile (Shirodkar RK, et al., 2019; Sastri KT, et al., 2020).
Solvent choice: The choice of solvent(s) used in the preparation process can influence SLN characteristics. Solvents should be carefully selected to ensure lipid solubility and compatibility with the drug and other formulation components (Battaglia L, et al., 2014; Khairnar SV, et al., 2022; Sastri KT, et al., 2020; Oehlke K, et al., 2017).
Antioxidants/stabilizers: Incorporating antioxidants or stabilizers can enhance SLN stability by preventing lipid oxidation and particle aggregation during storage (Subroto E, et al., 2023; Shah R, et al., 2014).
Particle size and distribution: For SLN performance, size distribution and particle size control are essential. Particle size and distribution are affected by variables such as lipid content, surfactant-to-lipid ratio and processing factors (Hernández-Esquivel RA, et al., 2022; Parhi R and Suresh P, 2012).
Sterilization and storage conditions: Sterilization methods and storage conditions (e.g., temperature and humidity) can affect SLN stability and shelf-life (Hernández-Esquivel RA, et al., 2022).
Scale-up considerations: Sterilization methods and storage conditions (e.g., temperature and humidity) can affect SLN stability and shelf-life (Khairnar SV, et al., 2022; Sastri KT, et al., 2020).
Recent advances and future perspectives
SLNs for theranostics: Solid lipid nanoparticles, which are tiny vesicles made of lipids (fats), can also be used for theranostics. They have the potential to deliver drugs and imaging agents simultaneously. They can deliver drugs and imaging agents non-invasively (Duan Y, et al., 2020).
Silence Therapeutics (ST): ST utilizes SLN technologies to create precise medicines targeting specific disease-related genes for unmet medical needs. The progress in SLN technologies also positions ST for wider acceptance in the pharmaceutical sector, improving the effectiveness of Ribonucleic Acid (RNA) interference therapies (Silence Therapeutics, 2023; Nguyen TT and Duong VA, 2022).
Industrial significance: SLNs are gaining industrial attention due to their potential for large-scale manufacturing. Several SLN-based formulations are currently in clinical trials, hinting at their increasing presence in the market (Khairnar SV, et al., 2022; Üner M, 2016).
Continuous processing: In the pharmaceutical industry, continuous processing aims to minimize batch-to-batch variations. Implementing continuous methods ensures consistent SLN production (Khairnar SV, et al., 2022).
SLNs as drug delivery systems: SLNs are effective for delivering drugs with low solubility in water, short half-life and low chemical stability (Shirodkar RK, et al., 2019). Quality of production: By altering hardware aspects and formulation processing components, consistent and repeatable quality of SLN production can be achieved (Shirodkar RK, et al., 2019; Sailaja AK, et al., 2011; Lee SY, et al., 2020).
Production challenges: There are difficulties like phase separation, sterilization and polymorphism. Super Critical Fluid (SCF) and other temperature-controlled techniques can be used to address polymorphism.
Phase separation can be managed with optimized lyophilization processes, enhancing stability and reducing microbial growth incidents (Khairnar SV, et al., 2022).
Sterilization: Sterilization remains a challenge due to the sensitivity of lipids to gamma irradiation and thermolabile substances. Filtration methods offer a solution, although batch capacity and filter pore size are considerations.
Protein corona formation and enzyme degradation: Translation is hindered by the development of protein corona and the breakdown of enzymes. Pre-clinical research to address these issues is made easier by developments in High Throughput Screening (HTS) and bio-corona characterization estimating techniques (Wang W, et al., 2021; Lombardo D and Kiselev MA, 2022) (Table 3).
| Parameters | Characterization |
|---|---|
| Polydispersity index and particle size | Dynamic Light Scattering (DLS)/Photon Correlation Spectroscopy (PCS) and laser diffraction |
| Shape, morphology and imaging | Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM) and Transmission Electron Microscopy (TEM) |
| Zeta potential | PCS, Electrophoretic Light Scattering (ELC) |
| Crystallinity | Differential thermal analysis/Differential scanning calorimetry and X-Ray Diffraction (XRD) |
| Encapsulation efficiency | Size exclusion/gel filtration chromatography, ultracentrifugation, filter membrane, dialysis, etc. |
| Structure and drug-lipid interaction | NMR spectroscopy, Fourier Transform Infrared (FTIR) spectroscopy, Raman spectroscopy |
Table 3: Characterization methods of SLNs
Scale-up efforts: Encouraging scale-up efforts is important for bringing SLNs to market. Like nanosuspensions, SLNs can go through phases of processing like spray drying and lyophilization.
Industrial benchmark and regulatory guidelines: Adherence to commercial standards, including clean rooms, distinct production facilities, validated machines, safety protocols and employee education, is essential. Clear regulatory guidelines, similar to those for liposomes, provide a framework for SLN development and approval (Khairnar SV, et al., 2022).
Clinical studies and market adoption: Ongoing clinical studies indicate a positive trajectory for SLN technologies in reaching the pharmaceutical market.
With advancements in research and regulatory clarity, SLNs are used for broader adoption in the pharmaceutical industry (Khairnar SV, et al., 2022, Lombardo D and Kiselev MA, 2022).
Conclusion
This review comprehensively examines the innovative approaches in the formulation of SLNs and their advancements in drug delivery for modern medicine. The abstract succinctly introduces the key topics covered, including types of lipids used in SLNs, various preparation methods, characterization techniques, factors influencing SLN preparation, applications of SLNs, recent advances and future directions. Through a thorough analysis of these aspects, it is evident that SLNs hold significant potential as a drug delivery system, offering enhanced durability, sustained release and targeted delivery capabilities. Furthermore, the exploration of recent advances highlights the continuous evolution of SLN technology, resulting in more efficient and effective pharmaceutical therapies. Looking ahead, future directions suggest further research into optimizing SLN formulations, exploring novel applications and addressing challenges to facilitate their widespread adoption in modern medicine. Overall, this review highlights the importance of SLNs as an adaptable medication delivery platform, leading to advancements in therapeutic strategies and ultimately improving patient outcomes.
References
- Pucek-Kaczmarek A. Influence of process design on the preparation of solid lipid nanoparticles by an ultrasonic-nanoemulsification method. Processes. 2021; 9(8): 1265.
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009; 71(4): 349-358.
[Crossref] [Google Scholar] [PubMed]
- Charcosset C, Fessi H. Preparation of nanoparticles with a membrane contactor. J Control Release. 2005; 266(1-2): 115-120.
[Crossref] [Google Scholar] [PubMed]
- Pandey S, Shaikh F, Gupta A, Tripathi P, Yadav JS. A recent update: Solid lipid nanoparticles for effective drug delivery. Adv Pharm Bull. 2022; 12(1): 17.
[Crossref] [Google Scholar] [PubMed]
- Abdelwahab SI, Taha MM, Moni SS, Alsayegh AA. Bibliometric mapping of solid lipid nanoparticles research (2012-2022) using VOSviewer. Med Novel Technol Devices. 2023; 17: 100217.
- Basha SK, Dhandayuthabani R, Muzammil MS, Kumari VS. Solid lipid nanoparticles for oral drug delivery. Mater Today Proc. 2021; 36: 313-324.
- Khairnar SV, Pagare P, Thakre A, Nambiar AR, Junnuthula V, Abraham MC, et al. Review on the scale-up methods for the preparation of solid lipid nanoparticles. Pharmaceutics. 2022; 14(9): 1886.
[Crossref] [Google Scholar] [PubMed]
- Nugraha MW, Iswandana R, Jufri M. Preparation, characterization, and formulation of solid lipid nanoparticles lotion from mulberry roots (Morus albaL.). Int J Appl Pharm. 2020; 12: 182-186.
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, et al. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020; 10(45): 26777-26791.
[Crossref] [Google Scholar] [PubMed]
- Battaglia L, Gallarate M, Panciani PP, Ugazio E, Sapino S, Peira E, et al. Techniques for the preparation of solid lipid nano and microparticles. Application of nanotechnology in drug delivery. 2014.
- Garud A, Singh D, Garud N. Solid Lipid Nanoparticles (SLN): Method, characterization and applications. Int Curr Pharm J. 2012; 1(11): 384-393.
- Akanda M, Mithu MS, Douroumis D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J Drug Deliv Sci Technol. 2023: 104709.
- Hernández-Esquivel RA, Navarro-Tovar G, Zárate-Hernández E, Aguirre-Bañuelos P. Solid Lipid Nanoparticles (SLN). Nanocomposite Materials for Biomedical and Energy Storage Applications. 2022.
[Crossref]
- Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles-A review. Curr Drug Discov Technol. 2012; 9(1): 2-16.
[Crossref] [Google Scholar] [PubMed]
- Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020; 25(20): 4781.
[Crossref] [Google Scholar] [PubMed]
- Teja VC, Chowdary VH, Raju YP, Surendra N, Vardhan RV, Reddy BK. A glimpse on solid lipid nanoparticles as drug delivery systems. J Glob Trends Pharm Sci. 2014; 5(2): 1649-1657.
- Satapathy S, Patro CSP. Solid lipid nanoparticles: Formulation, preparation, and characterization: A review. J Heal Sci. 2022: 9(4): 46-55.
[Crossref]
- Trucillo P, Campardelli R. Production of solid lipid nanoparticles with a supercritical fluid assisted process. J Supercrit Fluids. 2019; 143: 16-23.
- Fadda P, Monduzzi M, Caboi F, Piras S, Lazzari P. Solid lipid nanoparticle preparation by a warm microemulsion based process: Influence of microemulsion microstructure. Int J Pharm. 2013; 446(1-2): 166-175.
[Crossref] [Google Scholar] [PubMed]
- Negi JS, Chattopadhyay P, Sharma AK, Ram V. Development of Solid Lipid Nanoparticles (SLNs) of lopinavir using hot Self Nano-Emulsification (SNE) technique. Eur J Pharm Sci. 2013; 48(1-2): 231-239.
[Crossref] [Google Scholar] [PubMed]
- Gao S, McClements DJ. Formation and stability of solid lipid nanoparticles fabricated using phase inversion temperature method. Colloids Surf A. 2016; 499: 79-87.
- Singh R. Preparation of solid lipid nanoparticles through various methods using different precursors. J Drug Deliv Ther. 2019; 9(2): 415-419.
- Battaglia L, Gallarate M, Cavalli R, Trotta M. Solid lipid nanoparticles produced through a coacervation method. J Microencapsul. 2010; 27(1): 78-85.
[Crossref] [Google Scholar] [PubMed]
- Gallarate M, Battaglia L, Peira E, Trotta M. Peptide‐loaded solid lipid nanoparticles prepared through coacervation technique. Int J Chem Eng. 2011; 2011(1): 132435.
- Subroto E, Andoyo R, Indiarto R, Wulandari E, Wadhiah EF. Preparation of solid lipid nanoparticle-ferrous sulfate by double emulsion method based on fat rich in monolaurin and stearic acid. Nanomaterials. 2022; 12(17): 3054.
[Crossref] [Google Scholar] [PubMed]
- Peres LB, Peres LB, de Araújo PH, Sayer C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloid Surf. 2016; 140: 317-323.
[Crossref] [Google Scholar] [PubMed]
- Charcosset C, El-Harati AA, Fessi H. A membrane contactor for the preparation of solid lipid nanoparticles. Desalination. 2006; 1(200): 570-571.
- Steiner D, Bunjes H. Influence of process and formulation parameters on the preparation of solid lipid nanoparticles by dual centrifugation. Int J Pharm: X. 2021; 3: 100085.
[Crossref] [Google Scholar] [PubMed]
- Üner M. Characterization and imaging of solid lipid nanoparticles and nanostructured lipid carriers. InHandbook of nanoparticles. 2016: 117-141.
- Paliwal R, Paliwal SR, Kenwat R, Kurmi BD, Sahu MK. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin Ther Pat. 2020; 30(3): 179-194.
[Crossref] [Google Scholar] [PubMed]
- Müller RH, Mäder K, Gohla S. Solid Lipid Nanoparticles (SLN) for controlled drug delivery-A review of the state of the art. Eur J Pharm Biopharm. 2000; 50(1): 161-177.
[Crossref] [Google Scholar] [PubMed]
- Wissing SA, Müller RH. Cosmetic applications for Solid Lipid Nanoparticles (SLN). Int J Pharm. 2003; 254(1): 65-68.
[Crossref] [Google Scholar] [PubMed]
- McClements DJ, Xiao H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012; 3(3): 202-220.
[Crossref] [Google Scholar] [PubMed]
- Musielak E, Feliczak-Guzik A, Nowak I. Optimization of the conditions of Solid Lipid Nanoparticles (SLN) synthesis. Molecules. 2022; 27(7): 2202.
[Crossref] [Google Scholar] [PubMed]
- Shirodkar RK, Kumar L, Mutalik S, Lewis S. Solid lipid nanoparticles and nanostructured lipid carriers: Emerging lipid based drug delivery systems. Pharm Chem J. 2019; 53: 440-453.
- Sastri KT, Radha GV, Pidikiti S, Vajjhala P. Solid lipid nanoparticles: Preparation techniques, their characterization, and an update on recent studies. J Appl Pharm Sci. 2020; 10(6): 126-141.
- Oehlke K, Behsnilian D, Mayer-Miebach E, Weidler PG, Greiner R. Edible Solid Lipid Nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PloS One. 2017; 12(2): e0171662.
[Crossref] [Google Scholar] [PubMed]
- Subroto E, Andoyo R, Indiarto R. Solid lipid nanoparticles: Review of the current research on encapsulation and delivery systems for active and antioxidant compounds. Antioxidants. 2023; 12(3): 633.
[Crossref] [Google Scholar] [PubMed]
- Shah R, Eldridge D, Palombo E, Harding I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J Phys Sci. 2014; 25(1): 59-75.
- Silence therapeutics completes enrollment in phase 2 study of zerlasiran (SLN360) in subjects with elevated lipoprotein(a) at high risk of atherosclerotic cardiovascular disease events. Silence Therapeutics. 2023.
- Nguyen TT, Duong VA. Solid lipid nanoparticles. Encyclopedia. 2022; 2(2): 952-973.
- Sailaja AK, Amareshwar P, Chakravarty P. Formulation of solid lipid nanoparticles and their applications. J Curr Pharm Res. 2011; 1(2): 197.
- Lee SY, Son JG, Moon JH, Joh S, Lee TG. Comparative study on formation of protein coronas under three different serum origins. Biointerphases. 2020; 15(6): 061002.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Huang Z, Li Y, Wang W, Shi J, Fu F, et al. Impact of particle size and pH on protein corona formation of solid lipid nanoparticles: A proof-of-concept study. Acta Pharm Sin B. 2021; 11(4): 1030-1046.
[Crossref] [Google Scholar] [PubMed]
- Lombardo D, Kiselev MA. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022; 14(3): 543.
[Crossref] [Google Scholar] [PubMed]
Author Info
Jyoti S Kolapkar*, Sandip B Ahire, Vinod A Bairagi and Avinash B GangurdeCitation: Kolapkar JS: Investigating Novel Methods for Formulating Solid Lipid Nanoparticles
Received: 03-Jul-2024 Accepted: 17-Jul-2024 Published: 24-Jul-2024, DOI: 10.31858/0975-8453.15.7.230-237
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






