Research Article - (2025) Volume 16, Issue 10
Investigation of microbiological and organoleptic properties of bee products (bee venom, solid pollen, and royal jelly) through water activity quantification during 8 days of storage
Harold A. Prada-Ram�rez1*, Raquel G�mez-Pliego4, Willy-F Cely-V2,5, Ericsson Coy-Barrera5, Sandra Gonzalez-Alarcon1, Rodrigo Palacio-Beltr�n1, Juan Pablo Montes-Tamara1, Romel Pe�a-Romero1, David D�az-Baez2, Gloria In�s Lafaurie2 and Humberto Zardo3Abstract
The aim of this investigation was to assess the stability of biological bioregulators for a maximum holding time of 8 days through water activity measurements. Microbiological and organoleptic measurements were carried out in parallel and simultaneously in order to experimentally establish a relationship between the status of the water activity and the microbiological and organoleptic characteristics of the tested bee-derived materials. Bee venom, solid pollen and royal jelly were stored for a maximum holding time of 8 days at specific storage conditions such as light-resistance glass containers and under refrigerated storage. For all the bioregulators tested, water activity measurements were performed on days 0, 5 and 8. On days 0 and 8, microbiological and organoleptic assessments were performed. Based on the scientific literature, it was established that under these storage conditions, bee venom, solid pollen and royal jelly exhibited water activity of 0.5278, 0.3088 and 0.9766, respectively, during the entire holding time. The results indicate that water activity can be used as a quality indicator for the microbiological and organoleptic stability of raw bee-derived materials intended to be used for the development of new dietary and nutraceutical formulations in different pharmaceutical forms such as tablets, capsules, lozenges, powder, granulated and oral suspension.
Keywords
Bee venom, Solid pollen, Royal jelly, Chilled-mirror dew point method
Introduction
The scientific evidence and USP general chapters reviewed supported the claim that water Activity (Aw) could be used as a quality indicator for microbiological assessment of food and pharmaceutical articles for final-product release, since low water activity (below 0.60) prevents the growth of objectionable pathogens, mesophiles, yeasts and molds (United States Pharmacopeia Convention 43., 2021; United State Pharmacopeia Convention 43., 2021; Prada HA, et al., 2024; Prada HA, et al., 2024).
Indeed, currently in the pharmaceutical industry Aw is often used as part of an integral risk-based approach for product release, since low Aw levels function not only as a microbiological gauge but also as an organoleptic and physicochemical indicator. Therefore, low Aw (Aw<0.60) prevents chemical reactions such as autohydrolysis and photosensitivity and also protects the products from spoilage due to microorganism activity (Prada HA, et al., 2024; Prada HA, et al., 2024).
However, in order to consider Aw a direct measure of the microbiological burden of a functional food or nutraceutical or pharmaceutical products, several aspects should be taken into account, such as historical microbiological data (using the plate-count method), validation of the manufacturing process, cold chain transportation, primary packaging container that prevents oxygen and water vapour exchange and robust microbiological skip-lot testing for carrying out standard reference microbiological tests (Prada HA, et al., 2023; Prada HA, et al., 2023; Prada HA, et al., 2023; Prada HA, et al., 2023).
Despite the well-known Aw applications, its use remains unexplored in the microbiological and organoleptic assessments of bee-derived products such as bee venom, solid pollen and royal jelly intended to be used as active principal ingredients in nutraceutical, dietary supplement and prebiotic treatments (Kim DH, et al., 2018; Ghadimi-Garjan R, et al., 2023; Chiacchio I, et al., 2021; MÃÂÂÂrgÃÂÂÂoan R, et al., 2019; Bagameri L, et al., 2022).
Bee-derived products are increasingly being used as part of functional food, nutraceuticals and dietary supplements because of their health-promoting properties and high nutritional value associated with bioactive compounds such as minerals, vitamins, lipids, proteins, carbohydrates, hormones, flavonoids and phenols, which have great therapeutic potential for the treatment of worldwide diseases such as gastrointestinal disorders, skin allergies and breast cancer, among others (Buitrago D, et al., 2024; Abdelwahab K, et al., 2021; Rim W, et al., 2019; Aida E, et al., 2021; Dong S, et al., 2007; Jagua-Gualdrón A, et al., 2020; Collazo N, et al., 2021; Hu F, et al., 2005; Eslam O, et al., 2019).
Therefore, bee-derived products have become essential for designing new formulations as an integral part of functional and nutraceutical food intended to be used for therapeutic purposes.
In order to shed light on maximum 8-day holding times for the raw materials, Aw quantifications were performed on bee venom, bee pollen and royal jelly samples, since Aw measurements using the validated chilled-mirror dew point method have become a key tool for assessing chemical degradation, organoleptic stability, microbiological susceptibility and standardization of storage conditions of biologically active principal ingredients and raw materials (United States Pharmacopeia Convention 43., 2021; United State Pharmacopeia Convention 43., 2021).
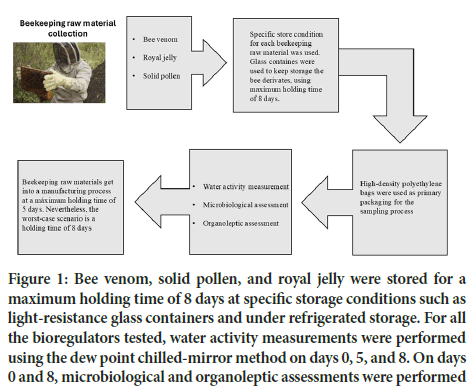
Therefore, stability studies of raw bee-derived materials are worth carrying out, since the waiting time may have a strong effect on the quality attributes of bee-derived raw materials prior to being used in a manufacturing process. Normally, biological raw materials remain in storage rooms not more than 3 days prior to compounding bulks. Hence a maximum holding time of 8 days might represent the worst-case scenario for providing evidence that the waiting times do not affect raw material’s quality (Figure 1). In this way, specific storage conditions such as temperature, humidity and primary packaging should be carefully controlled for each raw material tested, in accordance with the scientific literature, in order to prevent the holding time’s having an adverse impact on the original organoleptic and microbiological features of the bee venom, bee pollen and royal jelly. For this reason, it is important to test the allowable time for which the raw material may be stored under suitable storage conditions without negatively impacting their quality attributes.
Figure 1: Bee venom, solid pollen, and royal jelly were stored for a maximum holding time of 8 days at specific storage conditions such as light-resistance glass containers and under refrigerated storage. For all the bioregulators tested, water activity measurements were performed using the dew point chilled-mirror method on days 0, 5, and 8. On days 0 and 8, microbiological and organoleptic assessments were performed
Materials and Methods
Collection of bee products
The collection of the honeybee products bee venom, bee pollen and royal jelly was done in the Huila, Colombia department (southern region) in the municipality of Algeciras (2°31′19″ N 75°18′52″ W) during the 2025 Colombian summer season. The Huila region is mainly dominated by oak trees and a wide spectrum of flowering plants.
Storage and sampling of bee venom, pollen and royal jelly
The raw bee-derived samples were immediately transported in light-resistance screw cap glass containers in order to proceed with the quantification of the water activity. The samples were taken from the top of the containers in order to prevent further handling of the bee derivates. Sterile sampling devices were used and steps were taken to ensure that the primary packaging used for the sampling was similar to the original packaging in which the raw material arrived. Light-resistant screw-cap glass bottles and high-density polyethylene bags were deemed appropriate for storing the samples during the holding time assay. According to the scientific literature, high-density polyethylene bags are suitable sampling containers, because they have reduced oxygen and water vapor permeability (density 0.945-0.964 g/cm3) (DeLassus P, et al., 2000).
Reagents
The bee derivatives bee venom, royal jelly and solid pollen, were supplied by Vital Healthy Solution S.A.S and Laboratorios Coaspharma S.A.S in Bogotá, Colombia. For the construction of the calibration curve and the determination of the operating range and linearity of the chilled-mirror dew point method, standard saturated salt solutions with known water activity were used. The five standard reference materials used were sourced from METER group, Inc USA, SRM: Lithium chloride 13.41 MW ± 0.5% aw=0.25, lithium chloride 8.57 MW ± 0.5% aw=0.50, sodium chloride 6.0 MW ± 0.5% aw=0.76, sodium chloride 2.33 MW ± 0.5% aw=0.92 and USP purified water aw=1.00 ± 0.003.
Aqualab 4TE
Aqualab 4 TE is an instrument used to quantify water activity by means of the chilled-mirror dew point method. The system includes a dew point instrument with a precise temperature measurement chamber and Skala control software for carrying out all the administrative functions, such as the identification of samples. All water activity measurements were recorded and stored on the Amazon web site of the Skala control software. For routine water activity measurements, verifications were performed before their use in order to guarantee the precision and accuracy of the measurements. Verifications were performed using standards with known Aw in accordance with the guidelines outlined in Chapter 922 of the USP.
Quantification of water activity
3-gram samples of were taken on days 0, 5 and 8. These samples were used to quantify the water activity of the raw material throughout the maximum holding time of 8 days. The quantification of the Aw was carried out on days 0, 5 and 8 in order to determine the behavior of the Aw during the holding time. On days 0 and 8 of the holding time for each analyzed sample, microbiological and organoleptic tests were done to assess the quality attributes of the raw material.
To perform the Aw measurement, a representative amount (1 gram) of bee venom, bee pollen and royal jelly were placed into the 15 ml plastic disposable sample container provided by the Aqualab supplier. Laboratory conditions were minimum 21.6-maximum 34.4°C and 14.9%-69.4% relative humidity.
Microbiological and organoleptic testing
Microbiological and organoleptic tests were conducted for the materials taken on days 0 and 8 to determine the microbial burden and organoleptic characteristics (appearance, color and smell) during the holding time. The microbiological analysis included testing for mesophile fungi and yeasts, using conventional methods such as direct plating on agar plates in accordance with USP <61> (U.S. Pharmacopeial Convention, et al., 2020). For all the raw material tested, antimicrobial substances were neutralized in order to obtain successful microbiological determination. Accordingly, 10 g of the corresponding raw material was added to 90 mL of tryptic soy broth (Merck) SKU No. NCM0004A (dilution 1:10) with tryptic soy broth in the presence of the selected neutralizing agent (1 mL/L of Tween® 80 in tryptic soy broth) and then vigorously shaken in order to ensure homogenization of the sample. Agar plates (tryptic soy agar and sabouraud dextrose agar) were inoculated in duplicate with 1 mL of this dilution by means of the pour-plate technique. The plates were incubated at 20°C-25°C for 7 days for the yeast and mold count and at 30°C-35°C for 5 days for the mesophiles total count.
The organoleptic testing involved evaluating the appearance, colour and odour of the bee derivatives.
Results
Calibration curve using the saturated salt solution check standard
As is depicted in Table 1, calibration curves were constructed from known water activity standard checkpoints in order to confirm that the Aqualab 4TE provides a measurement result that is statistically equivalent to the true value of the saturated salt solution check standard. For this reason, five data points were plotted for the calibration curve (aw=0.25, 0.50, 0.76, 0.92 and 1). For each salt check point, six replicates were performed. As outlined in USP chapter <922>, an acceptance criterion is that the absolute error must be less than the sum of the instrument’s repeatability and the uncertainty in the standard solution (Table 1). The calibration curves have a validity of one year.
| Saturated salt check standards used to build up the calibration curves at 25°C | Calibration curve | ||||
|---|---|---|---|---|---|
| 0.25 | 0.5 | 0.76 | 0.92 | 1 | |
| Replicate 1 | 0.2501 | 0.5002 | 0.7601 | 0.9218 | 1.0026 |
| Replicate 2 | 0.2496 | 0.5006 | 0.7611 | 0.9224 | 0.9999 |
| Replicate 3 | 0.2498 | 0.4999 | 0.7609 | 0.9224 | 1.0043 |
| Replicate 4 | 0.2497 | 0.4999 | 0.7609 | 0.9223 | 0.9995 |
| Replicate 5 | 0.2498 | 0.5 | 0.7613 | 0.9234 | 1.0001 |
| Replicate 6 | 0.2499 | 0.4997 | 0.7607 | 0.9197 | 1.001 |
| Average (Aw) | 0.2498 | 0.5001 | 0.7608 | 0.922 | 1.0012 |
| Standard deviation | 0.0002 | 0.0003 | 0.0004 | 0.0012 | 0.0019 |
| Relative standard deviation | 0.0689 | 0.0629 | 0.0543 | 0.1346 | 0.1863 |
| Saturated salt check standard (Aw°) | 0.25 | 0.5 | 0.76 | 0.92 | 1 |
| Aw° (water activity expected)-Aw (water activity experimentally measure) | -0.0002 | 0 | 0.0008 | 0.002 | 0.0012 |
| Repeatability of the instrument (2*SD) | 0.0003 | 0.0006 | 0.0008 | 0.0025 | 0.0037 |
| Uncertainty of standadrd solution | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Reproducibility aqualab+standard deviation expected according to supplier | 0.0033 | 0.0036 | 0.0038 | 0.0055 | 0.0067 |
| <922> chapter USP | Absolute error=Aw°-Aw ≤ repeatability of the instrument+uncertainty of standard solution | ||||
Table 1: Overview of psychosocial domains impacted by endometriosis-related infertility
Storage and sampling of the bee venom, royal jelly and pollen
The analyzed raw materials, i.e., bee venom, royal jelly and bee pollen, were stored accordance with the scientific literature. As depicted in Table 2, royal jelly and bee venom were kept under refrigerated storage conditions, while solid pollen was appropriately stored at controlled room temperature (20°C-30°C). The samples were kept in light-resistant screw cap glass containers to prevent water humidity exchange and sample degradation.
| Raw material | Storage conditions |
|---|---|
| Bee pollen | Stored in tightly closed glass containers at controlled room temperature (20°C-30°C) and 30%-70% RH. |
| Royal jelly | Stored in closed glass containers at 2°C-6°C and 30%-70% RH. |
| Bee venom | Stored in waterproof, light-resistant glass containers at 2°C-6°C and 30%-70% RH. |
Table 2: Bee-derived raw materials and their storage conditions during the 8-day holding period
Microbiological and organoleptic assessment
Microbiological assessment of the bee venom, solid pollen and royal jelly was performed using the standard reference method based on the plate-count method for the determination of the total mesophilic, yeast and mold counts. As shown in Table 3, the microbiological and organoleptic characteristics of the materials remained within specifications during the maximum 8-day holding period (Table 3). Similarly, the materials tested maintained their colour, smell and appearance unaffected, exhibiting the same texture as the original sample (Table 3).
| Raw material | Organoleptic characteristics | Total mesophilic count | Total yeast and mold count |
|---|---|---|---|
| Bee pollen | Yellow, viscous jelly with a waxier texture than honey | 120 cfu/g | 60 cfu/g |
| Bee venom | White solid powder (apitoxin) | <10 cfu/g | <10 cfu/g |
| Royal jelly | Irregular yellow to brown granules; sweet taste | <10 cfu/ml | <10 cfu/ml |
Table 3: Microbiological and organoleptic characteristics of bee venom, bee pollen, and royal jelly remained within specification during the maximum 8-day holding period
Quantification of water activity during the maximum 8-day holding period
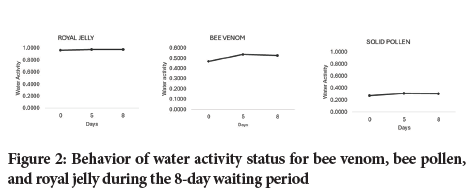
As is shown in Table 4, water activity measurements were performed on days 0, 5 and 8 for the bee venom, royal jelly and solid pollen. For each raw bee-derivate material, 6 replicates were performed on days 0, 5 and 8. The average, standard deviation and coefficient of variation were calculated (Table 4). The water activity behavior during the maximum 8-day holding period for the tested materials is depicted in Figure 2.
| Water activity | ||||
|---|---|---|---|---|
| Day 0 (n=6) | Day 5 (n=6) | Day 8 (n=6) | ||
| Bee pollen | Average | 0.27493 | 0.3115 | 0.3088 |
| Standard deviation | 0.0007 | 0.0015 | 0.0007 | |
| Coefficient variation | 0.2376 | 0.4719 | 0.2262 | |
| Royal jelly | Average | 0.9679 | 0.9808 | 0.9766 |
| Standard deviation | 0.0013 | 0.0019 | 0.0026 | |
| Coefficient variation | 0.1343 | 0.1912 | 0.2668 | |
| Bee venom | Average | 0.4723 | 0.5395 | 0.5278 |
| Standard deviation | 0.0009 | 0.001 | 0.0026 | |
| Coefficient variation | 0.1965 | 0.1819 | 0.4925 | |
Table 4: Water activity (aw) measurements of bee venom, bee pollen, and royal jelly on days 0, 5, and 8 of the holding period. Statistical differences in measured parameters for bee venom, bee pollen, and royal jelly on days 0, 5, and 8 of the holding period (ANOVA, P<0.05)
Figure 2: Behavior of water activity status for bee venom, bee pollen, and royal jelly during the 8-day waiting period
Discussion
The calibration curve showed that the chilled-mirror dew point method provides precise and accurate results that are statistically equivalent to the true values of the standard solutions, since the absolute error was within the specification, according to USP <922> requirements (Table 1). As shown in Table 2, the bee venom, bee pollen and royal jelly were stored under specific controlled storage conditions using light-resistant glass containers as the primary packaging in order to prevent vapor water exchange that could have affected Aw status on the bee-derived products tested (Table 2). Consequently, the royal jelly and bee venom were kept under refrigeration to prevent autohydrolysis and microbiological contamination, while the bee pollen was stored at room temperature. As for the Aw outcomes during the entire holding time, it was possible to demonstrate that the storage conditions used to keep the materials were suitable for preserving their original characteristics prior to becoming involved in a manufacturing process. This research supplied supporting evidence that demonstrates that under the tested storage conditions, the initial microbiological and organoleptic attributes of the bee venom, bee pollen and royal jelly were unperturbed during the entire holding time (Table 3 and Figure 2). It should be noted that bee pollen had an initial bioburden, but it was under acceptable microbiological levels (Table 3), while bee venom and royal jelly do not have an initial bioburden.
In this regard, the royal jelly exhibited average Aw levels of 0.9679, 0.9808 and 0.9766 on days 0, 5 and 8 respectively (Table 4). At these water activity levels, royal jelly becomes prone to being spoiled by the activity of microorganisms, since objectionable Gram-negative bacteria such as Pseudomonas aeruginosa, Escherichia coli and Salmonella as well as Gram-positive ones can survive at water activity between 0.95 and 0.97 (United States Pharmacopeia Convention 43, 2021). Bacteria such as Staphylococcus aureus can survive at a water activity level higher than 0.86 (United States Pharmacopeia Convention 43, 2021), while yeast and mold can grow at water activity levels greater than 0.75 (United States Pharmacopeia Convention 43, 2021). Regarding the low bioburden (<10 cfu/g) found in royal jelly, it is noteworthy that royal jelly contains a natural mixture of different secondary metabolites, major royal jelly proteins, phenols, flavonoids and fatty acids such as 10-Hydroxy-2-Decenoic Acid (10-HDA) that are responsible for antimicrobial activity that do not allow microbial harvesting.
The bee venom and solid pollen exhibited water activity of 0.3088 and 0.5278, respectively, during the holding time of 8 days (Table 4). It is well known that at these Aw levels (Aw <0.60), microorganisms are unable to grow, thus avoiding any contamination (United States Pharmacopeia Convention 43, 2021; Prada HA, et al., 2024). However, solid pollen had a steady bioburden during the entire holding time (mesophile 120 cfu/g and yeast mold <60 cfu/g, Table 3). However, the powdered bee venom did not show any growth of mesophile, yeast or mold counts during the entire holding time (<10 cfu/g, Table 3).
Recent publications have shown the preservative potential of bee derivative products, which exhibit excellent activity against a wide range of microorganisms, including bacteria, yeasts and molds (Ghadimi-Garjan R, et al., 2023; Chiacchio I, et al., 2021; MÄrgÄoan R, et al., 2019; Bagameri L, et al., 2022; Silva LR, et al., 2009). Regarding the low bioburden (<10 cfu/g) found in bee venom, it is possible that some natural antimicrobial agents prevent the proliferation of mesophilic yeasts and molds in these bee derivatives (Kim DH, et al., 2018; Ghadimi-Garjan R, et al., 2023; Chiacchio I, et al., 2021). In this regard, bee venom contains a well-known peptide, so called melittin, whose cytolytic potential has been widely proven to provide an intrinsic preservative potential against bacteria, yeasts and fungi (Ghadimi-Garjan R, et al., 2023). Indeed, pure bee venom has been proposed as an alternative to antibiotics in broiler chicken (Kim DH, et al., 2018). Moreover, scientific evidence supports the claim that melittin exerts its wide-spectrum lytic activity through its nonspecific membrane-binding capacity, which causes membrane disruption and bacterial lysis (Kim DH, et al., 2018; Ghadimi-Garjan R, et al., 2023; Chiacchio I, et al., 2021). In the same way, although solid pollen has an initial bioburden, it was steady during the entire holding time assay. This may be explained by its antimicrobial properties due to the presence of multiple bioactive compounds (MÄrgÄoan R, et al., 2019).
Nonetheless, special attention needs to be paid to royal jelly, because when water activity is >0.86, the bioregulators might harbor the growth of microorganisms. However, water activity status could be utilized as a useful tool for assessing the microbiological and organoleptic properties of royal jelly during the holding time trials. For bee venom, solid pollen and royal jelly, it would be interesting to further assess the preservative potential against several pathogens routinely tested in the pharmaceutical industry, in order to shed light on which microorganisms may potentially contaminate the royal jelly (Pasupuleti VR, et al., 2017; Uthaibutra V, et al., 2023).
As shown in Table 4, the observed uncertainties of Aw for the 6 replicates from the bee venom, bee pollen and royal jelly exhibited a standard deviation below 0.003 (n=6), showing the high concordance precision of the chilled-mirror dew point method values (Table 4). In order to observe the effect of operational variables such as the effect of different days, a multi-factorial analysis of variance was performed (ANOVA P<0.05, Table 4). It is of vital importance to note that for all the analyzed raw bee materials, statistically significant differences in the Aw status were observed between days during the holding time assay (days 0, 5 and 8, ANOVA P<0.05). These variations higher than 0.003 can be considered to be normal, since they may be due to hygroscopic properties of the raw bee materials within the temperature and relative humidity ranges that may occur throughout the day in the storage areas. It is worthwhile mentioning that the glass containers and the primary packaging used for the sampling (high-density polyethylene bags) prevent oxygen and water vapor permeability, leading to keeping the water activity status steady during the maximum holding time of 8 days.
Conclusion
Currently in the food industry, water activity is used as an indicator of microbiological and organoleptic safety and shelf life for a large array of raw materials and products. However, there are no studies regarding the use of water activity as a microbiological assessment of raw bee materials such as bee venom, bee pollen and royal jelly. Thus this investigation seems to be the first scientific research project that documents the relationship between the water activity and the microbiological assessment of bee derivatives as an active principal ingredient of nutraceutical and dietary supplements. The results obtained for the raw bee venom and royal jelly indicate that the storage conditions (temperature 2°C-8°C and relative humidity 30%-70%) in the dispensing and raw materials storage areas are appropriate. The results obtained for the solid pollen indicate that the storage conditions (temperature 20°C-30°C and relative humidity 30%-70%) in the dispensing and raw materials storage areas are appropriate. The organoleptic and microbiological characteristics were not altered during the 8-day waiting period. For bee venom and solid pollen, water activity <0.50 supports a holding time of 8 days between dispensing raw material and bulk compounding. Royal jelly with Aw>0.95 remained stable the 8-day holding period and evidenced neither organoleptic nor microbiological changes, also supporting the 8-day holding period between raw material dispensing and bulk compounding.
This study shows that water activity could be used as a direct measurement of microbiological assessment for bee-derived products. However, we recommend that actual microbiological and organoleptic tests be performed as part of vendor qualification and quality audits.
Data Availability Statement
The data underlying this article will be shared at reasonable request to the corresponding author
Ethical Approval Statement
This article has been prepared in accordance with ethical standards and principles. All research conducted for this study was approved by the relevant ethics committee, ensuring that the rights and welfare of participants were safeguarded. Information consent was obtained from all participants involved in the study and confidentiality was maintained throughout the research process. The authors declare that there are no conflicts of interest related to this publication.
Funding
The authors would like to thank Laboratorios Coaspharma S.A.S for their financial support.
Conflicts of Interest
The authors declare no conflict of interest. All the research was funded by Laboratorios Coaspharma S.A.S.
Ethical Approval Statement
This article has been prepared in accordance with ethical standards and principles. All research conducted for this study was approved by the relevant ethics committee, ensuring that the rights and welfare of participants were safeguarded. Information consent was obtained from all participants involved in the study and confidentiality was maintained throughout the research process. The authors declare that there are no conflicts of interest related to this publication.
Consent Statements
All the authors have read and approved the submission of the manuscript to the Journal of Food Science and Technology.
Consent for Publication
All the authors give consent for the manuscript to be published, including individual’s data or image.
Author Info
Harold A. Prada-Ram�rez1*, Raquel G�mez-Pliego4, Willy-F Cely-V2,5, Ericsson Coy-Barrera5, Sandra Gonzalez-Alarcon1, Rodrigo Palacio-Beltr�n1, Juan Pablo Montes-Tamara1, Romel Pe�a-Romero1, David D�az-Baez2, Gloria In�s Lafaurie2 and Humberto Zardo32Unit of Basic Oral Investigation-UIBO, School of Dentistry, Universidad El Bosque, Bogotá, Colombia
3School of Pharmaceutical Sciences, University of São Paulo, São Paulo, Brazil
4Departamento de Ciencias Biológicas, Sección de Ciencias de la Salud Humana, Facultad de Estudios Superiores Cuautitlán-UNAM, Cuautitlán Izcalli, Estado de México, Mexico
5Bioorganic Chemistry Laboratory, Facultad de Ciencias Básicas y Aplicadas, Universidad Militar Nueva Granada, Cajicá, Colombia
Received: 30-Jul-2025, Manuscript No. SRP-25-168388; Accepted: 11-Aug-2025, Pre QC No. SRP-25-168388 (PQ); , Pre QC No. SRP-25-168388 (PQ); , QC No. SRP-25-168388; , Manuscript No. SRP-25-168388 (R); Published: 18-Aug-2025, DOI: 10.31858/0975-8453.16.8.1-6
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3