Research Article - (2023) Volume 14, Issue 2
Abstract
Background: The aim of this study is to investigate the risk of lung cancer linked with the use of Angiotensin- Converting Enzyme Inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARB) and Overall Survival for lung cancer.
Methods: By using specific terminologies related to cancer and the inhibitors of ACEIs/ARBs, we searched on several research databases for studies that used to investigate the lung cancer risk associated with patients uses of ACEIs/ARBs.
Results: Thirty two studies met the inclusion criteria. In two investigations, individuals with advanced pancreatic cancer (HR=0.52, %CI 0.29-0.88) and non-small cell lung cancer (HR=0.56, %CI 0.33-0.95) had a substantial improvement in Overall Survival (OS).
Long-term usage of ACEIs/ARBs was linked to an elevated risk of lung cancer in six studies. While the other investigations concluded the use of ACEIs/ARBs was not significantly associated with increased risk of overall incident of lung cancer.
Conclusion: There is some evidence to suggest that ACEI or ARB use may be associated with lung cancer. Larger and more robust studies are required to explore this relationship further.
Keywords
Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin Receptor Blockers (ARBs), Lung cancer, Survival, Systematic review
Abbreviations
ACE: Angiotensin-Converting Enzyme; ACEIs: Angiotensin-Converting Enzyme Inhibitors; ARBs: Angiotensin Receptor Blockers; BB: Beta Blockers; BMI: Body Mass Index; HR: Hazard Ratio; OR: Odds Ratio; CI: Confidence Intervals; OS: Overall Survival
Introduction
Angiotensin Converting Enzyme Inhibitors (ACEIs) are frequently used as first-line drugs in the control of high blood pressure, management of different cardiovascular diseases and chronic kidney failure (Bhaskaran K, et al., 2012). ACE Inhibitors mainly act by inhibiting the generation of angiotensin-II results in decreasing the Peripheral Vascular Resistance (PVR) and blood pressure, also decrease the aldosterone production, therefore decrease in sodium and water retention will occur which result in lowering blood pressure (Deshayes F and Nahmias C, 2005). Therefore, its central mechanism of action operates by decreasing the activity of Renin Angiotensin System (RAS). Despite the fact that cancer survival rates are improving, it remains the leading cause of morbidity and death worldwide (Zhang W, et al., 2015). RAS plays a role in carcinogenesis through stimulation of angiogenesis, inflammation and tissue proliferation (Pinter M and Jain RK, 2017; Rosenthal T and Gavras I, 2009). Angiotensin receptors have also been discovered to be up-regulated in a variety of cancer tissues like Lung cancer. In addition increases the production of vascular endothelial growth factors. Based on these observations, it has been postulated that the pharmacotheraputic RAS modulation, may play a role on the development of a specific malignancies (Shen J, et al., 2016). Innovative approaches for targeted therapy are constantly being researched in order to improve cancer patients’ health and survival outcomes. Studies conducted more than three decades ago suggested that RAS may have a role in lung carcinogenesis, as patients with lung cancer had decreasing level of ACE (Rosenthal T and Gavras I, 2009; Hicks BM, et al., 2018). A study designed among 141 newly diagnosed individuals with primary lung cancer showed that they have significantly lower serum ACE concentration compared to healthy control individuals. Others investigations have found that patients with lung cancer has lower serum levels of ACE than those with other respiratory carcinomas (Rømer FK, 1981; Gallagher PE, et al., 2011). Furthermore, numerous investigations have discovered that ACE activity increases following radiation therapy or chemotherapy in patients with bronchial carcinoma (Pinter M and Jain RK, 2017; Meng L, et al., 2021), as well as in patients in clinical remission. In primary human lung cancer tissues, Prochazka J, et al., 1991 found a substantial drop in ACE activity when compared to normal lung tissue (Prochazka J, et al., 1991). Moreover, studies on gene polymorphisms found that the genotype distribution of the endothelial nitric oxide synthase gene was significantly different in lung cancer patients compared to the control population. As a result, a decreased in ACE serum in lung cancer patients is likely due to increased tumour burden, resulting in decreased pulmonary epithelial function (Peddireddy V, et al., 2018). The effect of ACE Inhibitors on cancer risk is still being controversial; previous in-vestigations have suggested increased, decreased or no association of ACEIs with the increased risk of lung cancer. In a retrospective cohort research, Lever AF, et al., 1998 proposed that the usage of ACE Inhibitors might prevent tumour progression. Long-term ACEI users with high blood pressure had a lower incidence of lung cancer, according to the study (Lever AF, et al., 1998; Friis S, et al., 2001). In a high-risk cohort study, users of ACEIs were found to have a lower chance of getting keratinocyte carcinoma (Christian JB, et al., 2008). Unfortunately, other studies failed to find an ACEI effect in this prospective (Friis S, et al., 2001; Lindholm LH, et al., 2001; Pasternak B, et al., 2011). Studies specifically assessing the risk of lung cancer associated with long term uses of ACEIs have conflicting and limited evidence (Azoulay L, et al., 2012; Hung JK, et al., 2021; Hallas J, et al., 2012). Study conducted by Azoulay L, et al., 2012 reported an increased risk of lung cancer associated with long term uses of ACEIs. However, Hallas J, et al., 2012 reported weak evidence between the long term uses of ACEIs and lung cancer (Hallas J, et al., 2012). However, a recent cohort study in UK reported the use of ACEIs associated with lung cancer; the association was particularly elevated among people using ACEIs for more than five years (Hicks BM, et al., 2018). Lin SY, et al., 2020, Cohort study reported ACEIs use associated with lung cancer compared with Angiotensin Receptor Blockers (ARBs) use. Coleman CI, et al., 2008 did a mixed treatment comparison meta-analysis of a variety of randomized control studies and did not report any association between ACEIs use and lung cancer. A more recent meta-analysis of randomized control trials were reported an increased risk among regular users of ACEIs (Hicks BM, et al., 2018). An observational cohort study conducted by Anderson JL, et al., 2021 reported a small increase in lung cancer risk with ACEIs compared with ARB. Furthermore, Connolly and colleagues reported that no increased risk for lung cancer with the users of ACEIs in 15 randomized trials were included in a meta-analysis (ARB Trialists Collaboration, 2011). Given the inconsistent findings on cancer incidence, as well as preclinical evidence indicating these treatments are implicated in tumor growth and vascularization (Deshayes F and Nahmias C, 2005). ACEIs are probable to play a significant role in cancer development. Despite mounting evidence of a link between ACEIs and carcinogenesis, their impact on those who already have cancer in unknown. A systematic analysis of existing epidemiologic studies trial data was done to determine whether the use of ACEIs increases the incidence of diseases, metastasis, and morality within cancer patients.
Methodology
Literature search
Studies published from 1 January to 31 March, 2021 without any language or data restriction were identified through electronic literature searches of the Ovid MEDLINE (US National library of medicine, Bethesda, MD, USA); EMBASE (Reed Elsevier PLC, Amsterdam, The Netherlands), SCOPUS, PubMed and Web of Science (Thompson Reuters) databases. The electronic search combined terms, related to “Lung cancer” OR “Lung carcinoma” in various combinations as well as “Angiotensin Converting Enzymes Inhibitors “, “ACE Inhibitors” and with the names of individual medications for example; ‘Captopril’, ‘Enalapril’ and ‘Fosinopril’. An epidemiology study search filter was also used. The overall search was limited on humans only.
Criteria for inclusion
By removing duplicates, papers were assessed for inclusion based on pre-determined eligibility conditions using the title and abstract. All of the articles were screened separately by the authors. When the title or abstract of an article did not indicate whether it was relevant to the review, the full text was obtained for further examination. Only papers that met the full criteria were considered for this review: (a) the study evaluated the use of ACE Inhibitors and ARBs in the study subjects; (b) an experimental and control has been used; (c) the design of the study was interventional (randomised control trial) or observation-based (cohort or case control study); and (d) the study used clinically related outcomes.
Data extraction and quality assessment
The researcher extracted the information based on the following parameters: First author, year of publication, location, study period, classification of drug use, exposures assessment, outcome determination, risk measurement, and confounder adjustment was obtained from each of the included reference articles. Wherever possible, Confidence Intervals were generated using data from individual studies if Confidence Intervals were not supplied. All of the included studies were evaluated for potential bias. This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) Statement for systematic review and meta-analysis reporting (Moher D, et al., 2009).
Results
Description of included studies
The search retrieved 3,809 studies and this number became 3,509 after duplicates have been removed (Figure 1). A total 32 articles relevant to ACEIs/ARBs use and cancer progression were identified from the online databases search and met the inclusion criteria for the review. Characteristics of studies included in the systematic review are outlined in Table 1. Twenty cohort studies, seven case control studies, two hospital retrospective trial, two Hospital-based prospective trial and one randomized control trial, were carried out between 1980 and 2021 were included in the systematic review (Bhaskaran K, et al., 2012; Lever AF, et al., 1998; Friis S, et al., 2001; Pasternak B, et al., 2011; Azoulay L, et al., 2012; Hallas J, et al., 2012; Hicks BM, et al., 2018; Lin SY, et al., 2020; Anderson JL, et al., 2021; Hsu HL, et al., 2020; Kristensen KB, et al., 2021; Lee SH, et al., 2021; Makar GA, et al., 2014; Kumar P, et al., 2021; Wei J, et al., 2019; Chae YK, et al., 2011; Ganz PA, et al., 2011; Keizman D, et al., 2011; Melhem-Bertrandt A, et al., 2011; Nakai Y, et al., 2010; Wilop S, et al., 2009; Heinzerling JH, et al., 2007; Buchler T, et al., 2005; Chiang YY, et al., 2014; Jick H, et al., 1997; Sipahi I, et al., 2010; van der Knaap R, et al., 2008; Rosenberg L, et al., 1998; Assimes TL, et al., 2008; Pahor M, et al., 1996; Yoshiji H, et al., 2009; Ronquist G, et al., 2009).
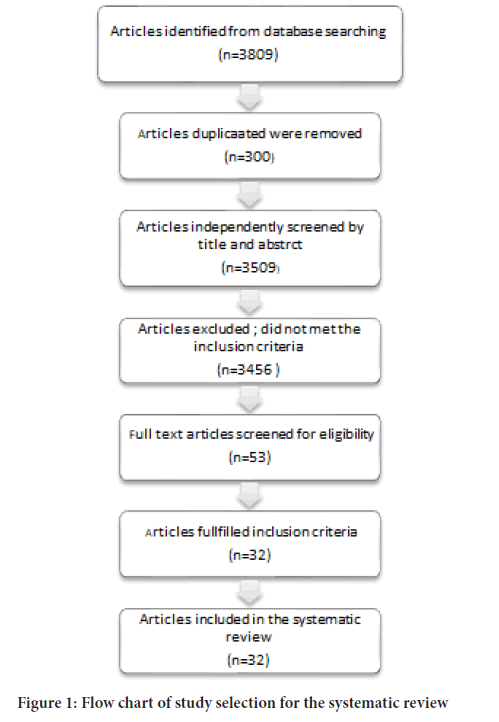
Figure 1: Flow chart of study selection for the systematic review.
| S/no | Author (Year) | Study Design | Study period | Exposure source | Name of institution (country) | Source of outcome(s) | Follow-up (years) |
|---|---|---|---|---|---|---|---|
| 1 | Anderson JL, et al., 2021 | Cohort | 1996-2018 | Intermountain Enterprise Data warehouse (EDW) database. | Intermountain Heart Institute, Intermountain Medical Centre (USA) | Intermountain Enterprise Data Warehouse (EDW) database | ns |
| 2 | Kristensen KB, et al., 2021 | Case control | 2000-2015 | Danish National Health and Administrative Registries | Danish National Health (Denmark) | Danish National Health and Administrative Registries | 3.2 years |
| 3 | Lee SH, et al., 2021 | Cohort | ns | Observational Health Data Sciences and Informatics (OHDSI) | Rehabilitation and Prevention Center, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine (Korea) | Hospital records | ns |
| 4 | Kumar P, et al., 2021 | Hospital based retrospective trial | 2005-2010 | Hospital records | Medicine, Jinnah Sindh Medical University (Pakistan) | Hospital records | 4 Years |
| 5 | Hsu HL, et al., 2020 | Case control | 2000-2016 | Taiwan National Health Insurance Research Database | Wan Fang Hospital, Taipei Medical University (Taiwan) | Taiwan Cancer Registry database | 3.6 Years |
| 6 | Lin SY, et al., 2020 | Cohort | 2000-2012 | Longitudinal Health Insurance Database (LHID) | College of Medicine, China Medical University, Taichung (Taiwan | National Health Insurance Research Database (NHIRD) | ns |
| 7 | Wei J, et al., 2019 | Hospital retrospective clinical trial | 2016-2018 | Hospital records | Central South University (China) | Hospital records | 2 years |
| 8 | Hicks BM, et al., 2018 | Cohort | 1995-2015 | United Kingdom Clinical Practice Research Datalink | Queen’s University Belfast, Belfast (UK) | United Kingdom Clinical Practice Research Datalink | 10Years |
| 9 | Makar GA, et al., 2014 | Case control | 1987-2002 | EPIC’s General Practice Research Database | Perelman School of Medicine at the University of Pennsylvania (Pennsylvania) | EPIC’s General Practice Research Database | ns |
| 10 | Chiang YY, et al., 2014 | Cohort | 2000-2009 | Taiwan National Health Insurance Research Database (NHIRD) | China Medical University Hospital, Taichung (Taiwan) | Taiwan National Health Insurance Research Database (NHIRD) | 9 Years |
| 11 | Hallas J, et al., 2012 | Case control | 2000-2005 | Danish National Prescription Registry | University of Southern Denmark (Denmark) | Danish National Prescription Registry | 5 Years |
| 12 | Bhaskaran K, et al., 2012 | Cohort | 1995-2010 | The General Practice Research Database UK | London School of Hygiene and Tropical Medicine (UK) | The General Practice Research Database | 4.6 years |
| 13 | Azoulay L, et al., 2012 | Cohort | 1995-2010 | The General Practice Research Database UK | Centre for Clinical Epidemiology, Lady Davis Institute, Jewish General Hospital, Montreal, Quebec (Canada) | The General Practice Research Database | ns |
| 14 | Ganz PA, et al., 2011 | Cohort | 1997-2002 | Prescription database | Kaiser Permanente Northern California Cancer Registry (USA) | Questionnaires, medical records, death certificates | 8.2 Years |
| 15 | Keizman D, et al., 2011 | Cohort | 2004-2010 | Hospital records | John Hopkins Kimmel Cancer Center (USA) | Hospital records | ns |
| 16 | Melhem-Bertrandt A, et al., 2011 | Cohort | 1995-2007 | Hospital records | University of Texas MD Anderson Cancer Center (USA) | Hospital records | 5.3 Years |
| 17 | Chae YK, et al., 2011 | Cohort | 1999-2005 | Hospital records | Albert Einstein Medical Centre (USA) | Hospital records | 4.6 years |
| 18 | Pasternak B, et al., 2011 | Cohort | 1998-2006 | Hospital records | Statens Serum Institut, (Denmark) | Hospital records | 2.1 years |
| 19 | Nakai Y, et al., 2010 | Cohort | 2001-2009 | Hospital records | University of Tokyo Hospital (Japan) | Hospital records | 9.5 months |
| 20 | Wilop S, et al., 2009 | Cohort | 1996- 2007 | Hospital records | University Hospital Aachen (Germany) | Hospital records and state death register | 8.1 months |
| 21 | Yoshiji H, et al., 2009 | Hospital-based prospective trial | 2002-2005 | Hospital records | Nara Medical University Hospital (Japan) | Hospital test | 3 years |
| 22 | Ronquist G, et al., 2009 | Hospital-based prospective trial | 2002-2005 | Hospital records | Malar Hospital (Sweden) | Hospital test | 2.4 year |
| 23 | van der Knaap R, et al., 2008 | Cohort | 1991-2004 | The Rotterdam Study Records | Erasmus Medical Center, Rotterdam (Netherlands) | The Rotterdam Study Records | 9.6 years |
| 24 | Ontarget Investigators, 2008 | Randomized control trial | 2004-2004 | Hospital records | Multi centres | Hospital records | 4.7 years |
| 25 | Assimes TL, et al., 2008 | Case control | 1980-2003 | Saskatchewan Health databases | Stanford University School of Medicine (USA) | Saskatchewan Health databases. | 3.6 years |
| 26 | Heinzerling JH, et al., 2007 | Cohort | 1998-2002 | Hospital records | Dallas Veteran’s Affairs Medical Center (USA) | Hospital records | 3 Years |
| 27 | Buchler T, et al., 2005 | Cohort | 1995-2002 | Hospital records | Brno or Olomouc University Hospitals (Czech Rep.) | Hospital records | 4.1 years |
| 28 | Friis S, et al., 2001 | Cohort | 1989-1995 | Prescription Database of North Jutland County and the Danish Cancer Registry | Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen (Denmark) | Prescription Database of North Jutland County and the Danish Cancer Registry | 3.7 years |
| 29 | Rosenberg L, et al., 1998 | Case control | 1983-1996 | Hospital records | Hospitals in Baltimore, New York (USA) | Hospital records | 3.8 years |
| 30 | Lever AF, et al., 1998 | Cohort | 1980-1995 | Registrar General Scotland and the West of Scotland Cancer Registry | University of Glasgow, Western Infirmary (UK) | Registrar General Scotland and the West of Scotland Cancer Registry | 6.6 years |
| 31 | Jick H, et al., 1997 | Case control | 1990-1995 | General Practice Research Database (GPRD) | Boston University Medical Center (UK) | General Practice Research Database (GPRD) | 4 years |
| 32 | Pahor M, et al., 1996 | Cohort | 1988-1992 | Hospital records | Catholic University, Rome (Italy) | Hospital records | 2 years |
Note: ns: not stated
Table 1: Characteristics of studies included in the systematic review.
Study design
The research included 1,055,122 patients. There were differences in the types of cancers evaluated, and the average follow-up time ranged from 8.1 months to ten years. Fourteen researches looked at how patients used ACEIs or ARBs. Five studies focused solely on the usage of ACEIs. Four of the retrospective and prospective clinical investigations revealed the types of ACEIs and ARBs that were utilized (Table 1). The two prospective trials compared the effects of taking ACEIs against non-users. Yoshiji H, et al., 2009 allocated participants to one of three treatment groups: Combined treatment (preindopril: 4 mg/day and vitamin K 45 mg/day), single treatment (preindopril: 4 mg/day or vitamin K 45 mg/day), or control (no ACEI or vitamin K). The authors first compared the outcomes of patients in the two groups and Overall Survival was examined in several studies (Chae YK, et al., 2011; Ganz PA, et al., 2011; Keizman D, et al., 2011; Melhem-Bertrandt A, et al., 2011; Nakai Y, et al., 2010; Wilop S, et al., 2009; Heinzerling JH, et al., 2007; Buchler T, et al., 2005; Yoshiji H, et al., 2009). Four of the studies also assessed cancer specific mortality (Lee SH, et al., 2021; Kumar P, et al., 2021; Wei J, et al., 2019; Ganz PA, et al., 2011; Chiang YY, et al., 2014; Assimes TL, et al., 2008) and few research papers looked on progression free survival (Keizman D, et al., 2011; Nakai Y, et al., 2010; Buchler T, et al., 2005). Along with that, relapse/disease free survival was investigated in two studies (Chae YK, et al., 2011; Melhem-Bertrandt A, et al., 2011). Studies also looked into the results where possibility of tumor recurrence is present (Hicks BM, et al., 2018; Chae YK, et al., 2011; Ganz PA, et al., 2011; Yoshiji H, et al., 2009). The researchers looked at biochem-ical recurrence in prostate cancer patients (defined as persistent or rising blood Prostate-Specific Antigens (PSA) of 0.10 g/l or greater detected on at least two occasions) (Ronquist G, et al., 2009). A meta-analysis of the findings of this review was not possible due to a lack of relevant reports and significant heterogeneity among the studies in terms of malignancies studied and study outcomes.
Cofounding
In each of the retrospective cohort studies, there were inconsistencies among the parameters that were accounted (Table 2). Fourteen of the cohort studies were age and sex-adjusted (Bhaskaran K, et al., 2012; Friis S, et al., 2001; Hicks BM, et al., 2018; Anderson JL, et al., 2021; Lee SH, et al., 2021; Makar GA, et al., 2014; Chae YK, et al., 2011; Ganz PA, et al., 2011; Melhem-Bertrandt A, et al., 2011; Nakai Y, et al., 2010; Wilop S, et al., 2009; Heinzerling JH, et al., 2007; Buchler T, et al., 2005; Chiang YY, et al., 2014) and others studies adjusted for stage of cancer (Anderson JL, et al., 2021; Ganz PA, et al., 2011; Melhem-Bertrandt A, et al., 2011; Nakai Y, et al., 2010; Wilop S, et al., 2009; Heinzerling JH, et al., 2007; Buchler T, et al., 2005). While, six studies adjusted for Body Mass Index (BMI) and smoking status (Bhaskaran K, et al., 2012; Azoulay L, et al., 2012; Hicks BM, et al., 2018; Anderson JL, et al., 2021; Hsu HL, et al., 2020; Jick H, et al., 1997). Five studies are adjusted for diabetes diagnosis (Chae YK, et al., 2011; Ganz PA, et al., 2011; Melhem-Bertrandt A, et al., 2011; Heinzerling JH, et al., 2007; Buchler T, et al., 2005) and few other studies also adjusted for co-morbidities and concomitant medication (Azoulay L, et al., 2012; Hallas J, et al., 2012; Anderson JL, et al., 2021; Hsu HL, et al., 2020; Chiang YY, et al., 2014). Four studies were adjusted for performance status, a measure used to assess cancer patients’ functional status (Ganz PA, et al., 2011; Nakai Y, et al., 2010; Wilop S, et al., 2009; Buchler T, et al., 2005). No adjustments were required in the randomized controlled trials, because of the randomization method.
| Author (Year) | Exposure | Study outcome | Overall risk measurement | Adjustments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||||
| Anderson JL, et al., 2021 | ACEI/ARB | Overall Survival | HR=1.18, CI: 1.06, 1.31 | * | * | * | * | * | |||
| Lee SH, et al., 2021 | ACEI | Overall Survival | No significant difference | * | * | * | * | ||||
| Ganz PA, et al., 2011 | ACEI | Breast cancer-specific mortality | HR=1.23 (95% CI 0.82-1.83) | * | * | ||||||
| Keizman D, et al., 2011 | ACEI/ ARB | Overall Survival | HR=0.69 (p=0.21) | * | * | * | |||||
| Melhem-Bertrandt A, et al., 2011 | ACEI/ ARB | Overall Survival | HR=0.99 (95% CI 0.65-1.51) | * | * | * | |||||
| Chae YK, et al., 2011 | ACEI/ ARB | Overall Survival | 55.5 versus 55.0 months (p=0.47) | * | * | * | * | ||||
| Nakai Y, et al., 2010 | ACEI/ ARB | Overall Survival | HR=0.52 (95% CI 0.29-0.88) | * | * | * | * | ||||
| Wilop S, et al., 2009 | ACEI/ ARB | Overall Survival | HR=0.56 (95% CI 0.33-0.95) | * | * | * | * | * | * | ||
| Yoshiji H, et al., 2009 | ACEI | Cumulative survival rate | No significant difference | ||||||||
| Heinzerling JH, et al., 2007 | ACEI | Mortality | No significant difference | * | * | * | * | ||||
| Buchler T, et al., 2005 | ACEI | Overall Survival | HR=2.014 (95% CI 1.002-4.049) | * | * | * | * | * | |||
*Factors adjusted for: A: Age; B: Gender; C: Body mass index; D: Smoking status; E: Co-morbidities; F: Performance status; G: Diabetes; H: others
Table 2: Results from studies assessing cancer-specific and Overall Survival (OS) among cancer patients.
Reported results
Overall Survival: Table 2shows the Overall Survival risk estimations. Anderson JL, et al., 2021 observed the HR of lung cancer was modestly increased with ACEIs (unadjusted HR=1.11, CI: 1.02, 1.22; adjusted HR=1.18, CI: 1.06, 1.31). Lee SH, et al., 2021 observed no significant different in Overall Survival among lung cancer patients who treated with ACEIs. Overall mortality was significantly lower in pancreatic cancer pa-tients who took ACEIs or ARBs compared to non-users who were normotensive (HR=0.52, 95% CI 0.29-0.88). The researchers investigated survival outcomes between people taking different antihypertensive medicines and non-hypertensive patients found no statistically significant changes (HR=1.23, 95% CI 0.73-1.98). Wilop S, et al., 2009 observed patients with lung cancer who reported using ACEIs or ARBs had a statistically substantially longer median survival of 3.1 months when compared to those who did not, compared to non-users (HR=0.56, 95% CI 0.33-0.95). Patients with metastatic renal cell carcinoma who took ACEIs or ARBs had a 7-month survival advantage, but the difference was not statistically significant (HR=0.69, p=0.21) (Keizman D, et al., 2011). In a large cohort of breast cancer patients, there was no significant survival benefit for using ACEIs or ARBs (HR=0.99, 95% CI 0.65-1.51) (Melhem-Bertrandt A, et al., 2011). Furthermore, Chae YK, et al., 2011 found no difference in survival rates among breast cancer patients who used ACEIs or ARBs and those who did not (median survival: 55.5 vs. 55.0 months, p=0.47). A third study of 1,179 patients with early stage breast cancer found a non-significant increase in the risk of breast cancer-specific mortality (HR=1.27, 95% CI 0.74-2.19) among users of ACEIs (Ganz PA, et al., 2011). Buchler T, et al., 2005 also reported a worse Overall Survival in patients with multiple myeloma who used ACEIs (HR=2.014, 95% CI 1.002-4.049), compared to non-users. The authors of this study also found that ACEI users had a lower survival rate when compared to hypertensive patients taking other or no antihypertensive drugs (38.7 vs. 77.7 months, p=0.024). Yoshiji H, et al., 2009, reported there is no significant difference in Overall Survival between hepatocellular carcinoma patients treated with the ACEI captopril and those who were not using it. Furthermore, combination treatment with an ACE inhibitor and vitamin K had no effect on Overall Survival.
Overall associated risk of lung cancer with ACEIs/ARBs: Risk association of ACEIs/ARBs with lung cancer presented in Table 3. Anderson JL, et al., 2021 noted a small long-term increase in lung cancer risk with ACEIs compared with ARBs. A nationwide nested case-control study reported by Kristensen KB, et al., 2021, high cumulative ACEI doses was associated with modestly increased odds of lung cancer while use of lower doses showed neutral associations. Lee SH, et al., 2021 observed no incidence development of lung cancer with patients who are prescribed with ACEIs. A retrospective study conducted by Kumar P, etal., 2021 reported the incidence of lung cancer was relatively more among people using ACEIs than ARBs. Case control study concluded that ACEI and ARB at high cumulative doses might be associated with lung adenocarcinoma risk (Hsu HL, et al., 2020). Study in Taiwan reported ACEI users are at a higher risk of lung cancer than ARB users (Lin SY, et al., 2020). Wei J, et al., 2019 did not detect any incidence for lung cancer development associated with ACEIs/ ARBs. Another cohort study conducted by Hicks BM, etal., 2018 reported the use of ACEIs was associated with an increased risk of lung cancer. Five cohort studies reported the use of ACEIs/ARBs were not associated with an increased incidence for lung cancer (Bhaskaran K, et al., 2012; Azoulay L, et al., 2012; Ganz PA, et al., 2011; Keizman D, et al., 2011; Chiang YY, et al., 2014). Makar GA, et al., 2014 reported in a case study, long-term/high-dose usage of ACEIs/ARBs not a risk for lung cancer. Population based cohort study by Hallas J, et al., 2012 concluded that the ACEIs appears to be related to a small increase in cancer risk. The use of ACE-inhibitors/ARBs, statins, and the combination of both were all associated with a reduced risk of breast cancer recurrence (Chae YK, et al., 2011). In this large nationwide cohort, use of ARBs was not significantly associated with increased risk of overall incidence of lung cancer (Pasternak B, et al., 2011). van der Knaap R, et al., 2008 did not find any incidence for development of lung cancer among ACEIs users. Case study conducted by Assimes TL, et al., 2008 stated that long-term use of common antihypertensive drugs does not appear to promote or initiate cancer. Another cohort study did not confirm a protective effect of ACE inhibitors on the development of cancer (Friis S, et al., 2001). Lever AF, et al., 1998, reported that long term use of ACEIs may protect against lung cancer.
| Author (Year) | Study design | Exposure | Risk for lung cancer |
|---|---|---|---|
| Anderson JL, et al., 2021 | Cohort | ACEI/ARB | ✓ |
| Kristensen KB, et al., 2021 | Case control | ACEI | ✓ |
| Lee SH, et al., 2021 | Cohort | ACEI | ☒ |
| Kumar P, et al., 2021 | Hospital based retrospective trial | ACEI/ARB | ✓ |
| Hsu HL, et al., 2020 | Case control | ACEI/ARB | ✓ |
| Lin SY, et al., 2020 | Cohort | ACEI/ARB | ✓ |
| Wei J, et al., 2019 | Hospital based retrospective trial | ACEI/ARB | ☒ |
| Hicks BM, et al., 2018 | Cohort | ACEI | ✓ |
| Makar GA, et al., 2014 | Case control | ACEI/ARB | ☒ |
| Chiang YY, et al., 2014 | Cohort | ACEI/ARB | ☒ |
| Hallas J, et al., 2012 | Case control | ACE | ✓ |
| Bhaskaran K, et al., 2012 | Cohort | ACEI/ARB | ☒ |
| Azoulay L, et al., 2012 | Cohort | ARB | ☒ |
| Ganz PA, et al., 2011 | Cohort | ACEI/BB | ☒ |
| Keizman D, et al., 2011 | Cohort | ACEI | ☒ |
| Chae YK, et al., 2011 | Cohort | ACEI/ARB/Statins | ☒ |
| Pasternak B, et al., 2011 | Cohort | ARB | ☒ |
| Pasternak B, et al., 2011 | Cohort | ACEI | ☒ |
| Assimes TL, et al., 2008 | Case control | ACEI | ☒ |
✓Presence of risk for lung cancer
☒Absence of risk for lung cancer
Table 3: Overall associated risk of lung cancer with ACEIs/ARBs.
Discussion
The systematic review investigate the overall associated risk for lung cancer with the use of ACEIs/ARBs and the Overall Survival among cancer patients. A total of twenty cohort studies, seven case control, two hospital retrospective trial, two Hospital-based prospective trials and one randomized trial control were identified. The majority of the studies were conducted in hospitals and looked at various cancer sites. Most of the studies are small in size and had relatively short periods of follow-up. Two studies found that ACEI or ARB users with pancreatic or non-small cell lung cancer had a 44% and 48% lower risk of overall mortality, respectively (Nakai Y, et al., 2010; Wilop S, et al., 2009). Other studies found no evidence of an ACEI or ARB’s use having a substantial impact on overall mortality (Moher D, et al., 2009; Chae YK, et al., 2011; Ganz PA, et al., 2011; Keizman D, et al., 2011; Melhem-Bertrandt A, et al., 2011; Heinzerling JH, et al., 2007; Yoshiji H, et al., 2009). Those exposed to ACEIs or ARBs had a 46% lower risk of breast cancer recurrence, as well as an improvement in disease-free survival. Two studies found that people who used ACEIs or ARBs for renal cell carcinoma or pancreatic cancer had a better progression-free survival (Keizman D, et al., 2011; Nakai Y, et al., 2010). The overall associated risk for lung cancer with the use of ACEIs/ARBs were identified, six studies reported an increased risk for lung cancer with the long term use of ACEIs/ ARBs (Hicks BM, et al., 2018; Lin SY, et al., 2020; Anderson JL, et al., 2021; Hsu HL, et al., 2020; Kristensen KB, et al., 2021; Kumar P, et al., 2021). On the other hand, the other investigations concluded the use of ACEIs/ ARBs was not significantly associated with increased risk of overall incidence of lung cancer (Bhaskaran K, et al., 2012; Azoulay L, et al., 2012; Lee SH, et al., 2021; Makar GA, et al., 2014; Wei J, et al., 2019; Chae YK, et al., 2011; Ganz PA, et al., 2011; Keizman D, et al., 2011; Chiang YY, et al., 2014; van der Knaap R, et al., 2008; Assimes TL, et al., 2008; Pasternak B, et al., 2011). Nonetheless, well-designed observational studies with diverse ethnic populations are required to assess the long-term (over 10 years) association between ACEI use and lung cancer. Patients were selected from certain institutions in few studies, and some trials included only individuals who had received first-line chemotherapy (Keizman D, et al., 2011; Nakai Y, et al., 2010; Wilop S, et al., 2009), autologous peripheral blood stem cell transplantation (Buchler T, et al., 2005), or radical prostatectomy (Ronquist G, et al., 2009). The patients who are fit and healthy enough to undertake the therapy are selected, therefore they may not be representative of the broader case population. Patients who did not undergo treatment or died shortly after being diagnosed with cancer may potentially be underrepresented. Because most cohort studies used hospital medical records to determine drug use, there is a chance that misclassification of drug exposure occurred (Ontarget Investigators, 2008). Except for one cohort study, all of the studies adjusted for age; however, the studies differed in terms of other adjusted factors and most of the studies adjusted for gender, Body Mass Index and smoking status. Other studies also adjusted for important prognostic factors such as performance status, while six studies adjusted for diabetes diagnosis. For example, a higher performance status score has been linked to better survival outcomes in cancer patients (Blagden SP, et al., 2003). A recent meta-analysis found that cancer patients with pre-existing diabetes had a 41% higher risk of overall mortality than those without diabetes (Barone BB, et al., 2008). Confounding by indication is one of the most serious drawbacks in pharmacoepidemiological cohort and case-control studies, and it is likely to be substantial in relation to the results revealed in this review. For example, in the studies included in this analysis, links between ACEI or ARB use and lung cancer may be related to the reasons why patients are prescribed these medications rather than any possible treatment effects. Co-morbidities are more likely among ACEI or ARB users than in non-users, which could affect progression of the disease and mortality as well as cancer treatment availability. No inferences could be derived on the influence of drug dose, frequency, or duration on cancer progression or survival because the majority of studies defined usage of ACEI or ARB as ever versus never use only. The studies in this review included clinically relevant outcome measures such Overall Survival and overall lung cancer risk related with ACEI/ARB usage.
Conclusion
The results of this research do not allow for convincing conclusions about the influence of ACEIs or ARBs on lung cancer incidence or Overall Survival in patients with advanced cancer. There was some evidence that the use of these drugs may have increased or lowered the risk of lung cancer in general; however, bigger epidemiological studies with more participants are needed.
The review concluded that the usage of ACE inhibitors was not linked to a higher risk of lung cancer. Nonetheless, well-designed observational studies with diverse ethnic populations are required to assess the long-term (over ten-year) link between ACEI usage and lung cancer.
References
- Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: Cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ. 2012; 344.
[Crossref] [Google Scholar] [PubMed]
- Deshayes F, Nahmias C. Angiotensin receptors: A new role in cancer? Trends Endocrinol Metab. 2005; 16(7): 293-299.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Liang Z, Li J, Cai S. Angiotensin receptor blockers use and the risk of lung cancer: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2015; 16(4): 768-773.
[Crossref] [Google Scholar] [PubMed]
- Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci Transl Med. 2017; 9(410): eaan5616.
[Crossref] [Google Scholar] [PubMed]
- Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: A review. J Hum Hypertens. 2009; 23(10): 623-635.
[Crossref] [Google Scholar] [PubMed]
- Shen J, Huang YM, Wang M, Hong XZ, Song XN, Zou X, et al. Renin-angiotensin system blockade for the risk of cancer and death. J Renin Angiotensin Aldosterone Syst. 2016; 17(3): 1470320316656679.
[Crossref] [Google Scholar] [PubMed]
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: Population based cohort study. BMJ. 2018; 363.
- Rømer FK. Angiotensin-converting enzyme and its association with outcome in lung cancer. Br J Cancer. 1981; 43(2): 135-142.
[Crossref] [Google Scholar] [PubMed]
- Gallagher PE, Cook K, Soto-Pantoja D, Menon J, Tallant EA. Angiotensin peptides and lung cancer. Curr Cancer Drug Targets. 2011; 11(4): 394-404.
[Crossref] [Google Scholar] [PubMed]
- Meng L, Yang B, Qiu F, Jia Y, Sun S, Yang J, et al. Lung cancer adverse events reports for angiotensin-converting enzyme inhibitors: Data mining of the FDA adverse event reporting system database. Front Med. 2021; 8: 594043.
[Crossref] [Google Scholar] [PubMed]
- Prochazka J, Krepela E, Sedo A, Viklický J, Fiala P. Aminopeptidases and angiotensin I-converting enzyme activities in primary human lung tumors and lung parenchyma. Neoplasma. 1991; 38(5): 501-508.
[Google Scholar] [PubMed]
- Peddireddy V, Badabagni SP, Gundimeda SD, Mundluru HP. Association of eNOS and ACE gene polymorphisms and plasma nitric oxide with risk of non-small cell lung cancer in South India. Clin Respir J. 2018; 12(1): 207-217.
[Crossref] [Google Scholar] [PubMed]
- Lever AF, Hole DJ, Gillis CR, mcCallum IR, mcInnes GT, macKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998; 352(9123): 179-184.
[Crossref] [Google Scholar] [PubMed]
- Friis S, Sørensen HT, Mellemkjær L, McLaughlin JK, Nielsen GL, Blot WJ, et al. Angiotensin-converting enzyme inhibitors and the risk of cancer: A population-based cohort study in Denmark. Cancer. 2001; 92(9): 2462-2470.
[Crossref] [Google Scholar] [PubMed]
- Christian JB, Lapane KL, Hume AL, Eaton CB, Weinstock MA. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. J Natl Cancer Inst. 2008; 100(17): 1223-1232.
[Crossref] [Google Scholar] [PubMed]
- Lindholm LH, Anderson H, Ekbom T, Hansson L, Lanke J, Dahlöf B, et al. Relation between drug treatment and cancer in hypertensives in the Swedish trial in old patients with hypertension 2: A 5-year, prospective, randomised, controlled trial. Lancet. 2001; 358(9281): 539-544.
[Crossref] [Google Scholar] [PubMed]
- Pasternak B, Svanström H, Callréus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation. 2011; 123(16): 1729-1736.
[Crossref] [Google Scholar] [PubMed]
- Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PloS One. 2012; 7(12): e50893.
[Crossref] [Google Scholar] [PubMed]
- Hung JK, Zhou J, Lee S, Xia Y, Liu Y, Zhang Y, et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: A population-based cohort study. medRxiv. 2021.
- Hallas J, Christensen R, Andersen M, Friis S, Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: A population-based case-control study. Br J Clin Pharmacol. 2012; 74(1): 180-188.
[Crossref] [Google Scholar] [PubMed]
- Lin SY, Lin CL, Lin CC, Hsu WH, Lin CD, Wang IK, et al. Association between angiotensin-converting enzyme inhibitors and lung cancer-A nationwide, population-based, propensity score-matched cohort study. Cancers. 2020; 12(3): 747.
[Crossref] [Google Scholar] [PubMed]
- Coleman CI, Baker WL, Kluger J, White CM. Antihypertensive medication and their impact on cancer incidence: A mixed treatment comparison meta-analysis of randomized controlled trials. J Hypertens. 2008; 26(4): 622-629.
[Crossref] [Google Scholar] [PubMed]
- Anderson JL, Knowlton KU, Muhlestein JB, Bair TL, Le VT, Horne BD. Evaluation of treatment with angiotensin converting enzyme inhibitors and the risk of lung cancer: ERACER-An observational cohort study. J Cardiovasc Pharmacol Ther. 2021; 26(4): 321-327.
[Crossref] [Google Scholar] [PubMed]
- ARB Trialists Collaboration. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138 769 individuals. J Hypertens. 2011; 29(4): 623-635.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009; 151(4): 264-269.
[Crossref] [Google Scholar] [PubMed]
- Hsu HL, Lee CH, Chen CH, Zhan JF, Wu SY. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers might be associated with lung adenocarcinoma risk: A nationwide population-based nested case-control study. Am J Transl Res. 2020; 12(10): 6615.
[Google Scholar] [PubMed]
- Kristensen KB, Hicks B, Azoulay L, Pottegård A. Use of ACE (Angiotensin-Converting Enzyme) inhibitors and risk of lung cancer: A nationwide nested case-control study. Circ Cardiovasc Qual Outcomes. 2021; 14(1): e006687.
[Crossref] [Google Scholar] [PubMed]
- Lee SH, Chun KJ, Park J, Kim J, Sung JD, Park RW, et al. Angiotensin converting enzyme inhibitors and incidence of lung cancer in a population based cohort of common data model in Korea. Sci Rep. 2021; 11(1): 1-8.
[Crossref] [Google Scholar] [PubMed]
- Makar GA, Holmes JH, Yang YX. Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst. 2014; 106(2): djt374.
[Crossref] [Google Scholar] [PubMed]
- Kumar P, Kumar V, Murlidhar FN, Fatima A, Jahangir M, Khalid D, et al. Comparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for incidence of lung cancer: A retrospective study. Cureus. 2021; 13(5).
[Crossref] [Google Scholar] [PubMed]
- Wei J, Zhou Z, Xu Z, Zeng S, Chen X, Wang X, et al. Retrospective clinical study of renin-angiotensin system blockers in lung cancer patients with hypertension. PeerJ. 2019; 7: e8188.
[Crossref] [Google Scholar] [PubMed]
- Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 2011; 29(9): 585-593.
[Crossref] [Google Scholar] [PubMed]
- Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: Results from the LACE cohort. Breast Cancer Res Treat. 2011; 129(2): 549-556.
[Crossref] [Google Scholar] [PubMed]
- Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, et al. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: A retrospective examination. Eur J Cancer. 2011; 47(13): 1955-1961.
[Crossref] [Google Scholar] [PubMed]
- Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011; 29(19): 2645.
[Crossref] [Google Scholar] [PubMed]
- Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, et al. Inhibition of renin- gemcitabine. Br J Cancer. 2010; 103(11): 1644-1648.
[Crossref] [Google Scholar] [PubMed]
- Wilop S, von Hobe S, Crysandt M, Esser A, Osieka R, Jost E. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009; 135(10): 1429-1435.
[Crossref] [Google Scholar] [PubMed]
- Heinzerling JH, Anthony T, Livingston EH, Huerta S. Predictors of distant metastasis and mortality in patients with stage II colorectal cancer. Am Surg. 2007; 73(3): 230-238.
[Crossref] [Google Scholar] [PubMed]
- Buchler T, Krejci M, Svobodnik A, Adam Z, Minarik J, Bacovsky J, et al. Outcome of patients with multiple myeloma and hypertension treated with angiotensin-I-converting enzyme inhibitors during high-dose chemotherapy. Hematol J. 2005; 5(7): 559-564.
[Crossref] [Google Scholar] [PubMed]
- Chiang YY, Chen KB, Tsai TH, Tsai WC. Lowered cancer risk with ACE inhibitor’s/ARB’s: A population-based cohort study. J Clin Hypertens. 2014; 16(1): 27-33.
[Crossref] [Google Scholar] [PubMed]
- Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997; 349(9051): 525-528.
[Crossref] [Google Scholar] [PubMed]
- Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 2010; 11(7): 627-636.
[Crossref] [Google Scholar] [PubMed]
- van der Knaap R, Siemes C, Coebergh JW, van Duijn CM, Hofman A, Stricker BH. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: The Rotterdam Study. Cancer. 2008; 112(4): 748-757.
[Crossref] [Google Scholar] [PubMed]
- Rosenberg L, Rao RS, Palmer JR, Strom BL, Stolley PD, Zauber AG, et al. Calcium channel blockers and the risk of cancer. JAMA. 1998; 279(13): 1000-1004.
[Crossref] [Google Scholar] [PubMed]
- Assimes TL, Elstein E, Langleben A, Suissa S. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf. 2008; 17(11): 1039-1049.
[Crossref] [Google Scholar] [PubMed]
- Pahor M, Guralnik JM, Salive ME, Corti MC, Carbonin P, Havlik RJ. Do calcium channel blockers increase the risk of cancer? Am J Hypertens. 1996; 9(7): 695-699.
[Crossref] [Google Scholar] [PubMed]
- Yoshiji H, Noguchi R, Toyohara M, Ikenaka Y, Kitade M, Kaji K, et al. Combination of vitamin K2 and angiotensin-converting enzyme inhibitor ameliorates cumulative recurrence of hepatocellular carcinoma. J Hepatol. 2009; 51(2): 315-321.
[Crossref] [Google Scholar] [PubMed]
- Ronquist G, Frithz G, Wang YH, Lindeborg T. Captopril may reduce biochemical (prostate-specific antigen) failure following radical prostatectomy for clinically localized prostate cancer. Scand J Urol Nephrol. 2009; 43(1): 32-36.
[Crossref] [Google Scholar] [PubMed]
- Ontarget Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008; 358(15): 1547-1559.
[Crossref] [Google Scholar] [PubMed]
- Blagden SP, Charman SC, Sharples LD, Magee LR, Gilligan D. Performance status score: do patients and their oncologists agree? Br J Cancer. 2003; 89(6): 1022-1027.
[Crossref] [Google Scholar] [PubMed]
- Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008; 300(23): 2754-2764.
[Crossref] [Google Scholar] [PubMed]
Author Info
Omer Ibrahim A Omer1* and Abdelhafiz Mohammed Abdalla22Department of Pharmaceutical Technology, College of Pharmaceutical Science, Andhra University, Visakhapatnam, India
Citation: Omer OIA:Lung Cancer Increased Risk with Long Term Use of Angiotensin Converting Enzyme Inhibitors: A Systematic Review
Received: 02-Jan-2023 Accepted: 27-Jan-2023 Published: 03-Feb-2023, DOI: 10.31858/0975-8453.14.2.82-90
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






