Research Article - (2022) Volume 13, Issue 12
Abstract
In this study, the immune activity and mechanism of the antitumor activity of Brassica rapa L. Polysaccharides were investigated in vitro. BRNP inhibited the growth of human lung adenocarcinoma cell line A549 by changing the cell morphology, reducing the number of cells, promoting apoptosis, and increasing the content of Caspase-3. BRNP enhanced the secretion of immune factors IL-2, IL-6, IL-12, and TNF-α in the serum and spleen of A549 tumor-carrying mice and thus participated in immune regulation. HE staining showed that the pathological state of each dose of BRNP in the turnip group was reduced, and some cells were necrotic compared with the model group. Immunohistochemical results showed that apoptosis was promoted by down-regulating the expression of the Bcl-2 protein and up-regulating the expression of the Bax protein. The assay kit showed that the level of caspase-3 increased in mice, which may be the mechanism of promoting apoptosis.
Keywords
Brassica rapa L., Polysaccharide, A549 cells, Antitumor, Apoptosis
Abbreviations
BRNP: Brassica rapa L. Neutral Polysaccharides; A549: NonSmall-Cell Lung Cancer cell; β-actin: Beta-actin; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2 associated X protein; BRP: Brassica rapa L. Polysaccharides; Caspase-3: Cysteinyl aspartate specific proteinase-3; CCK-8: Cell Counting Kit-8; DMEM: Dulbeccopie’s Modified Eagle Medium; DMSO: Dimethyl Sulfoxide; DDP: Cisplatin; ELISA: Enzyme Linked Immunosorbent Assay; IL-2: Interleukin-2; IL-6: Interleukin-6; IL-12: Interleukin-12; NSCLC: Non-Small-Cell Lung Cancer; OD: Optical Density; p53: Tumor suppressor genes 53; PBS: Phosphate Buffered Saline; TNF-α: Tumour Necrosis Factor-alpha; λ: Wavelength; WB: Western Blot
Introduction
Lung cancer has become a public health concern worldwide (Sun G and Ni K, 2020). Patients with lung cancer account for 11.6% of the total number of patients with cancer, and it is the leading cause of cancer deaths (Siegel RL, et al., 2019; Bray F, et al., 2018). Primary bronchial lung cancer is called lung cancer and has two types, namely, non-small cell lung cancer and small cell lung cancer (Rudin CM, et al., 2021; Lin C, et al., 2021). Non-small cell lung cancer accounts for about 80%-85% of all lung cancer cases (Broderick SR, 2020). Lung cancer is one of the most common malignancies and has the highest morbidity and mortality among tumors (Hoy H, et al. 2019; Shankar A, et al., 2019). The increasing incidence of lung cancer has become a very serious social problem (Fan Y, et al., 2021).
Polysaccharides in natural extracts are generally non-cytotoxic and have a variety of biological activities; they can activate immune cells and promote the secretion of cytokines, thereby regulating the immune system (Shinchi H, et al., 2015; Gong Y, et al., 2015) and exerting anti-tumor (Ke M, et al., 2014; Sun L, et al., 2016), anti-oxidation, antiviral (Li HH, et al., 2021), and other functions. Natural polysaccharides are widely used in the clinical treatment of tumors due to their good efficacy, low toxicity, and slight side effects (Aso K, et al.,2013). At present, biological polysaccharides used in clinical treatment mainly include lentinan polysaccharide (Liu Y, et al., 2019; Jin X, et al., 2020).
Ganoderma lucidumpolysaccharide (Chan SW, et al., 2021; Zheng S, et al., 2020), Astragalus polysaccharide (Chen W, et al., 2018; Yang S, et al., 2020), and so on, which are used as immunomodulators and adjuvant drugs in chemotherapy (Ren Y, et al., 2019).
The polysaccharide content in the water extract of turnips from Aksu Keping County, which had the best quality in Xinjiang, was as high as 11.53%. The water extract of turnip also had strong ability to scavenge free radicals (Cao Q, et al., 2021). Two homogeneous polysaccharides, namely, two Brassica rapa L. Neutral Polysaccharide (BRNP) and two Brassica rapaL. Acid Polysaccharides (BRAP), were isolated from the crude polysaccharides of turnip for the first time by chromatography and spectroscopic techniques (Qiao LJ, et al. 2020; Li HH, et al., 2021; Hairenguli M, et al., 2020). The structure of these polysaccharides were identified, and in vivo and in vitro experiments were conducted to determine the efficacy and possible mechanism of the isolated BRNP on the lung cancer of A549 cells and tumor-bearing mice (Zhuoer C, et al., 2016; Reziyamu M, et al., 2018; Yao J, et al., 2014).
Experimental Materials
Cell lines
A-549 cells (Procell CL-0016) were kindly provided by Procell Life Science and Technology Corporation Limited.
Animals
BALB/c (Bagg and Albino) mice (6-8 weeks of age, male and female ratio of 50/50, weight of 22-30 g; License No: SCXK new 2018-0001) were purchased from the Animal Experimental Center of Xinjiang Medical University. Mice were housed in an environment with temperature of 22℃-25℃, relative humidity of 40%-60%, and 12 h light and 12 h dark cycle. The mice were given free diet and drinking water and were regularly fed. The bedding was replaced every 5 days, and the cage was kept clean and sanitary.
Materials
Turnip medicinal materials were purchased from Aksu Keping Shengquan Industrial Co., Ltd. in June 2019 and identified as the root of Brassica rapa L. by the Department of Raw Medicine/Natural Medicine, School of Pharmacy, Xinjiang Medical University. BRNP was obtained and purified by DEAE-650M ion-exchange chromatography column and HW-55F.
Main reagents
The following main reagents and materials were used: F-12K medium (Wuhan Procell Life Science and Technology Co., Ltd. PM150910-500), 0.25% trypsin (Gibco, Batch number: 25200056), fetal bovine serum (Gibco. Batch number: 2129075RP), Human Caspase-3 ELISA Kit 96T (Wuhan Elarite Biotechnology Co., Ltd. Batch number: E-EL-M0238c), CCK-8 Kit 100T (BioSharp. Batch number: BS350B), Cisplatin (Beijing Solaibao Technology Co., Ltd. Batch number: SC5170), 0.22 µm sterilizing head (Millipore, Batch number: SLGP033RB), rabbit polyclonal antibody against caspase-3 (Wuhan Elarite Biotechnology Co., Ltd. Batch number: E-AB-60017), rabbit polyclonal antibody against Bax (Wuhan Elarite Biotechnology Co., Ltd. Batch number: E-AB-33819), rabbit polyclonal antibody against Bcl-2 (Wuhan Elarite Biotechnology Co., Ltd. Batch number: E-AB-6-788), β-actin (Bioss, Batch number: bs-0061R), secondary anti-goat anti-rabbit (Bioss. Batch number: bs-40295G-HRP), cell lysate and protease inhibitor (Beijing Solaibao Technology Co., Ltd. Batch number: R0010), BCA Protein Quantitative Kit (Thermo. Batch number: 23235), SDS-PAGE Gel Rapid Preparation Kit (Beijing Botace Biotechnology Co., Ltd. Batch number: WB2102), ECL Luminescent Color Reactor (BioSharp, Batch number: BL520A), and PVDF membrane (Millipore. Batch number: ISEQ00010).
Instruments and equipments
The following instruments and equipment were used: constant-temperature incubator (Thermo, USA, Batch number: HERA cell 150), biosafety operating table (Thermo, USA, Batch number: SW-CJ-2F), inverted microscope (Thermo, USA, Batch number: IX71-12FL/PH), centrifuge (Eppendorf, Germany, Batch number: 5424R), electric thermostatic water bath (Shanghai Bo Industrial Co., Ltd. Batch number: HWS11), electrophoresis apparatus and electroconverter (Bio-Rad Company), gel imager (Bio-Rad), and shaker (Beijing Liuyi Biological Technology Co., Ltd. Batch number: WD-9405B)
In vitro experiment
Cell culture: A549 cells (Procell CL-0016) were kindly provided by Procell Life Science and Technology Co., Ltd. The cells were cultured in F-12k medium containing 10% FBS (Fetal Bovine Serum) and 1% P/S in an incubator at 37℃ with 5% CO2. The liquid was changed every day, and the cells were sub-cultured between days. Cells in the logarithmic growth phase were selected for experiment.
Calculation of cell inhibition rate and screening of BRNP concentration: CCK-8 method was used to digest A549 cells with trypsin. The cell suspension was prepared and diluted to 5 × 104 cells/mL. The diluted suspension was placed in each well of 96-well plates and cultured for 24 h until the cells adhered to the wall. Negative control group (no medication), Cisplatin group (5, 10, and 20 μg/mL), and different total concentrations of neutral polysaccharides (0, 100, 200, 400, and 600 μg/mL) were prepared. After treatment for 24 h, the medium was sucked out, and 100 μL of the complete medium containing 10% CCK-8 was added to each well for 1 h. OD value at 450 nm was detected by a microplate analyzer, and cell inhibition rate was calculated.
Observation of cell morphology: A549 cells were seeded into 96-well plates and cultured in respective media of the negative control group, Cisplatin group, medium-dose group, and half-dose combined group for 24 h. Cell morphological changes were observed under an inverted microscope and photographed.
Observation of cell apoptosis: Hoechst 33258 was used for testing. A549 cells were inoculated in six-well plates and cultured in the respective media of negative control group, Cisplatin group, medium-dose group, and halfdose combined group for 24 h. The cells were rinsed twice with PBS, fixed with fixed solution for 15 min, washed with PBS, and stained with Hoechst 33258 in a light shelter for 15 min. Results were observed by fluorescence microscope.
Detection of the expression of cell-related proteins by Western Blot (WB) assay: Separating glue was prepared according to the proportion of various components in the instructions of SDS-PAGE (Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis) preparation kit. The separating glue was quickly poured into the two glass plates of the vertical plate SDS-PAGE gel preparation device installed in advance according to the requirements of the equipment. Anhydrous ethanol was added in the plates to flatten the separating glue flat. The plates were placed at room temperature for 30 min to solidify. Anhydrous ethanol was removed, and concentrating glue was immediately poured. The matching comb tooth was placed in the plate and left at room temperature for 30 min. The electrophoresis tank was installed, and electrophoresis buffer was poured into it. The comb tooth was removed, and 20 μg of the protein solution of the sample was added. The glue was run with constant pressure, 80 V electrophoresis for 45 mins and 120 V electrophoresis for 1 h. The film was transferred in a constant current mode at 300 mA and 4°C for about 50 min. The PVDF (Polyvinylidene Difluoride) membrane was removed and immersed in skim milk sealant for 2 h. The target protein was cut according to the label, added with the diluted primary antibody, and incubated at 4°C overnight. The PVDF membrane was washed with TBST (Tris-Buffered Saline Tween-20) for three times and then added with diluted secondary antibody. The membrane was incubated at room temperature for 75 min. The PVDF membrane was washed with TBST on the shaker for three times. The membrane was removed and placed in the gel imaging system. The gray value of the preserved protein strips was analyzed by Image Lab professional software.
In vivo experiment
Establishment of a mouse model of lung cancer: The cells were changed the day before the experiment and collected the next day. The liquid was discarded, and the cells were washed 3 mL of PBS. The liquid was discarded, and the cells were digested with 1 mL of 0.25% trypsin in a constant-temperature box for 2 min. The cells were observed under microscope; cells that fell off were assumed to be circular. The cells were added with 3 mL of termination reagent to digest completely. The cells were placed in culture bottles with spear percussion. The cell suspension was transferred into a 15 mL centrifuge tube and centrifuged. The supernatant was discarded. The heavy-suspension cells were added with PBS and centrifuged again. The supernatant was discarded, and the cells were diluted with PBS to obtain cell density of 1 × 107/mL. A 1 mL sterile syringe pump was used for vaccination. After Trypan blue staining, the number of living cells was >95%. The right anterior axillary of mice was disinfected and 0.2 mL of tumor cells (about 1 × 107/mL) was inoculated subcutaneously. The model was successfully established when the average diameter of subcutaneous tumors in all mice reached 5 mm.
Grouping of experimental mice and medicine administration: The mice were divided into six groups, with 10 mice each group and a total of 60 mice: Blank control group (did not participate in modeling and injected with normal saline), model group (participated in modeling and injected with normal saline), Cisplatin group (intraperitoneal injection of 6 mg/kg-1), high-dose BRNP group (200 mg/kg-1/d), medium-dose BRNP group (100 mg/kg-1/d), low-dose BRNP group (50 mg/kg-1/d). The mice were treated for 10 d. The dose conversion relationship between human and mouse was investigated according to the “Pharmacological Research Methodologies of Chinese Materia Media” with reference to the clinical dosage. According to the body surface area, the dose conversion between mouse and human was 10 times, that is, 10 times of the clinical adult dosage. The preparation and dosage of Cisplatin solution were referred to the instructions and the above accounting methods. The experimental dose of Cisplatin in mice was found to be 6 mg/kg-1.
Evaluation of general state of mice: The body weight of mice was recorded before and every other day after the treatment. After 10 d of treatment, mice in all groups were dissected, and organs such as liver, spleen, lung, kidney, and thymus were taken and weighed. Tumor inhibition rate, organ index, and tumor mass were calculated. All the organs were divided into two parts. One part was fixed with 4% paraformaldehyde, embedded in conventional paraffin, sectioned, stained with HE (Hematoxylin and Eosin), observed under optical microscope, and photographed for blood clot. The other part was packed with EP (Eppendorf) tube and quickly frozen for subsequent detection of relevant indicators.
Determination of serum levels of IL-2, IL-6, IL-12, and TNF-α in mice with lung cancer: After 10 d of treatment, mice were fasted 12 h prior to death. About 0.8-1.2 mL of blood was collected from their eyes, placed at room temperature for 1 h, and centrifuged at 12000 r/min-1 for 15 min. Serum (supernatant) was taken and stored in a refrigerator at -80℃. The contents of IL-2, IL-6, IL-12, and TNF-α were determined by ELISA according to specific steps provided in the kit. Absorbance was determined by enzyme plate analyzer, and the content of immune factors in the serum was calculated based on a standard curve.
Tumor histological examination: Tumor tissue was removed from each group of nude mice and weighed. The tumor tissue was cut in the middle with a blade, and the necrotic part was peeled off. The tumor tissue samples were fixed with 4% paraformaldehyde, refrigerated at 4℃, and stored at 60℃. Under the environment by dehydration, transparent, dip wax and embedded wax blocks.
Detection of Bax and Bcl-2 protein expression by immunohistochemical method: After routine dewaxing and hydration of tissue sections, corresponding tests were performed. The protein expression of Bax and Bcl-2 was detected based on the instructions of the reagent box. The expression of Bcl-2 and Bax protein in tumor cells was determined by the intensity of intracellular brown color (Positive rate: Number of positive cells/total number of cells). The number of positive cells was determined.
Determination of Caspase-3 levels in tumor tissues: The tumor tissue was taken out from the -80℃ refrigerator and placed on ice. The tumor tissue was ground in a mortar and added with PBS at the ratio of 1:9 (1 g:9 mL) to form homogenate. The homogenate was repeatedly subjected to freeze–thawing to further lyse the tissue cells. The homogenate was centrifuged at 5000 r/min for 10 min, and the supernatant was collected for analysis.
Statistical methods
SPSS 25.0, Origin2015, and ImageJ were used for statistical processing of data. Data were represented as x ± s. One-way analysis of variance was used for comparison between groups, and differences at P<0.05 were considered statistically significant.
Results
Effects of BRNP and combination group on inhibition of human lung adenocarcinoma A549 cells
The inhibitory effect of BRNP on human lung adenocarcinoma A549 cells was enhanced with increasing dose in a concentration-dependent manner. The half-dose combined group had more significant inhibitory effect than single-dose groups (Table 1).
| Grouping | n | OD450 (24 h) | Inhibition ratio (%) |
|---|---|---|---|
| Blank group | 6 | 0.1600.01 | - |
| Positive control group | 6 | 1.0320.06 | - |
| 100 µg/mL BRNP | 6 | 0.7300.03 | 34.6 ± 1.31a |
| 200 µg/mL BRNP | 6 | 0.6930.00 | 39.4 ± 0.78a |
| 400 µg/mL BRNP | 6 | 0.5380.02 | 59.2 ± 0.93a |
| 600 µg/mL BRNP | 6 | 0.4990.03 | 64.2 ± 0.82a |
| 10 µg/mL Cisplatin+400 µg/mL BRNP | 6 | 0.4320.02 | 68.8 ± 0.57a |
Note: a: P<0.01 vs. negative control group, 24 h of administration; no significant differences were found among the treatment groups; OD: Optical Density
Table 1: Effects of BRNP (Brassica rapa L. Neutral Polysaccharides) and joint group on cell proliferation
A549 cell apoptosis detected by Hoechst 33258 staining
Compared with the negative control group, the fluorescence intensity of A549 lung cancer cells increased under the intervention of Cisplatin, turnip neutral polysaccharide, and dose-halved combined group (Figure 1). A large number of membrane vesicles on the cell membrane and obvious nuclear contraction were detected, indicating that turnip neutral polysaccharide promoted the morphology change in A549 lung cancer cells and their apoptosis.
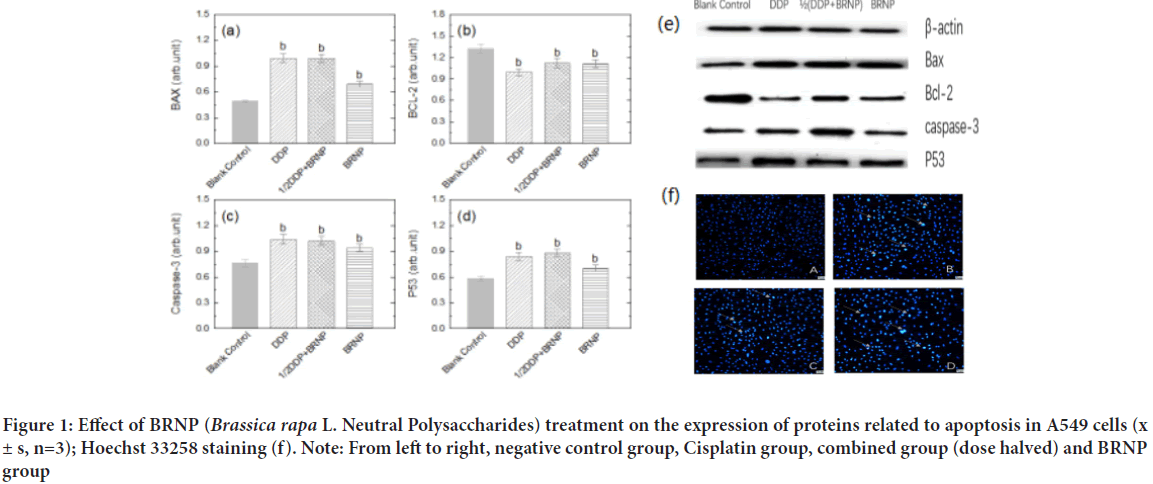
Figure 1: Effect of BRNP (Brassica rapa L. Neutral Polysaccharides) treatment on the expression of proteins related to apoptosis in A549 cells (x± s, n=3); Hoechst 33258 staining (f). Note: From left to right, negative control group, Cisplatin group, combined group (dose halved) and BRNP group
Changes in the expression of apoptosis-related proteins by BRNP detected by Western Blot assay
The expression levels of apoptosis-related proteins Bcl-2, Bax, caspase 3, and p53 in tumor cells were detected by Western Blot. The expression levels of Bax and caspase-3, which are pro-apoptotic proteins, and p53, a tumor-suppressor protein, significantly increased compared with those in the negative control group (P<0.05). The expression level of the anti-apoptotic protein Bcl-2 was significantly decreased compared with that in the negative control group (P<0.05). Hence, neutral polysaccharide BRNP and the combination group achieved the ultimate anti-tumor purpose by affecting the expression levels of apoptotic and tumor-suppressor proteins (Figure 1).
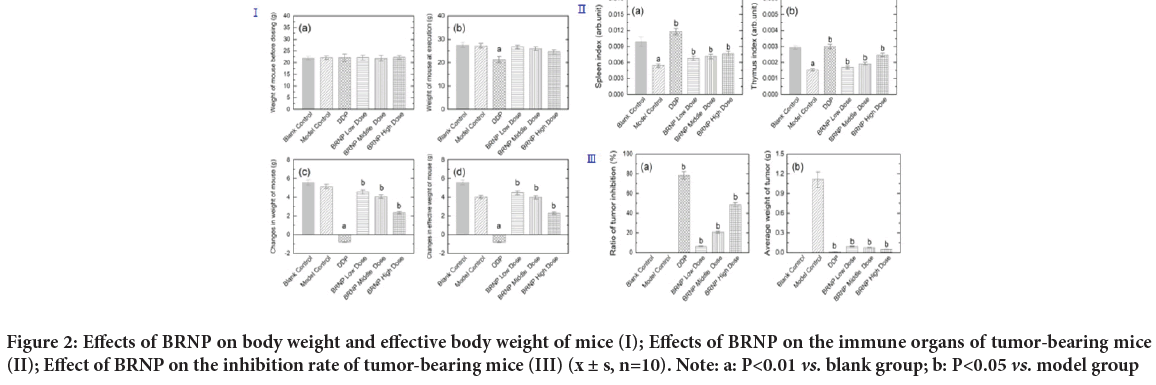
Effects of BRNP from turnip on the body weight and effective body weight of tumor-bearing mice
As shown in Figure 2Ia, 2Ib, no significant difference in the body weight of mice was found among the groups before administration (P>0.05). The mice were weighed before death. Compared with the model group, the body weight of mice in the positive control group was reduced. In Figure 2Ic, 2Id, the body weight and effective body weight of the positive control group were significantly decreased compared with those in the model group (P<0.01). The body weight and effective body weight of BRNP increased with increasing dose in the treatment groups compared with those in the positive control group.
Figure 2: Effects of BRNP on body weight and effective body weight of mice (I); Effects of BRNP on the immune organs of tumor-bearing mice (II); Effect of BRNP on the inhibition rate of tumor-bearing mice (III) (x ± s, n=10). Note: a: P<0.01 vs. blank group; b: P<0.05 vs. model group
Effect of BRNP on the immune organ index of tumor-bearing mice
The spleen and thymus indices of the model group decreased compared with those in the blank group (P<0.01). The indices of the positive control group and BRNP dosage groups increased with significant differences compared with those in the model group P<0.05 (Figure 2Ⅱ).
Effect of BRNP on tumor-bearing mice
The tumor inhibition rate in the positive control group (P<0.01) as well as in the medium and high-dose turnip groups was significantly different (P<0.01) from that in the model group. Although the low dose turnip polysaccharide group was effective in decreasing the tumor mass of tumor-bearing mice, the tumor inhibition rate was lower (Figure 2IⅡ).
Effects of BRNP on serum cytokines IL-2, IL-6, IL-12, and TNF-α in tumor-bearing mice
The contents of IL-2, IL-6, IL-12 and TNF-α in the serum of the BRNP dose groups increased compared with those in the model group. The contents of IL-2, IL-6, IL-12, and TNF-α in the medium and high-dose groups had statistical significant increase (P<0.01) and a certain relationship to dose. Compared with those in the blank group, the contents of IL-2, IL-6, IL-12 and TNF-α in the serum of the model group decreased (P<0.01). The contents of the four immune factors in the serum of the control group decreased, which may be caused by the side effect of Cisplatin in decreasing the immune function of the body (Table 2).
| Grouping | (mg/Kg-1) | IL-2 (Pg/mL-1) | IL-6 (Pg/mL-1) | IL-12 (Pg/mL-1) | TNF-α (Pg/mL-1) |
|---|---|---|---|---|---|
| Blank group | - | 34.35 ± 0.12b | 76.58 ± 0.13b | 121.06 ± 0.25b | 163.3 ± 0.17b |
| Model group | - | 17.60 ± 0.31a | 35.19 ± 0.23a | 46.18 ± 0.17a | 121.9 ± 0.19a |
| Positive control group | 6 | 15.63 ± 0.17a | 33.22 ± 0.11a | 23.51 ± 0.22ab | 120.0 ± 0.16a |
| Low-dose group | 50 | 18.59 ± 0.09a | 39.16 ± 0.15ab | 68.84 ± 0.13ab | 135.7 ± 0.07ab |
| Medium-dose group | 100 | 21.54 ± 0.13ab | 45.04 ± 0.03ab | 72.78 ± 0.04ab | 145.5 ± 0.31ab |
| High-dose group | 200 | 31.41 ± 0.08b | 47.02 ± 0.12ab | 90.52 ± 0.09ab | 149.5 ± 0.11ab |
Note: a: P<0.01 vs. blank group; b: P<0.01 vs. model group
Table 2: Effect of BRNP on serum cytokines from tumor-bearing mice (x ± s, n=10)
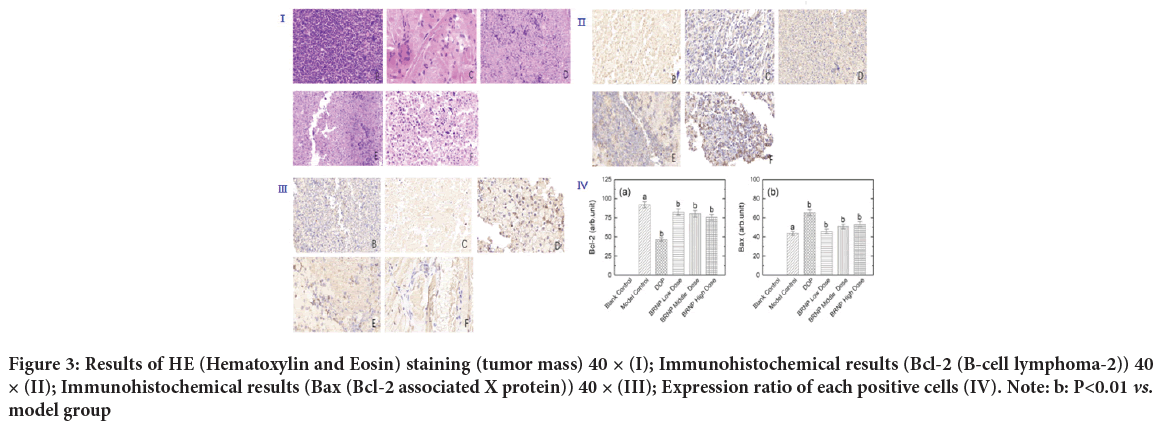
Tumor histological examination and expression of Bcl-2 in tumor tissuesCompared with the model group, the expression of the Bcl-2 protein in the tumor tissue of mice after BRNP treatment was significantly decreased, the ratio of negative cells decreased (P<0.05), and the positive intensity and score decreased (Figures 3I, 3Ⅱ ).
Figure 3: Results of HE (Hematoxylin and Eosin) staining (tumor mass) 40 × (I); Immunohistochemical results (Bcl-2 (B-cell lymphoma-2)) 40 × (II); Immunohistochemical results (Bax (Bcl-2 associated X protein)) 40 × (III); Expression ratio of each positive cells (IV). Note: b: P<0.01 vs. model group
Expression of Bax in tumor tissues
The expression of the Bax protein in different doses of BRNP and Cisplatin groups was significantly higher than that in the model group, and the ratio of positive cells increased. Compared with that in the model group, the ratio of Bcl-2-positive cells was significantly decreased in the Cisplatin control group (P<0.01), the positive expression of Bcl-2 was significantly decreased in medium and high-dose BRNP groups, and the ratio of Bcl-2-positive cells was significantly decreased (P<0.05). The percentage of Bcl-2-positive cells significantly increased in the Cisplatin control group (P<0.01), the positive expression of Bax was significantly increased in the medium and high dose BRNP groups, and the percentage of positive cells was significantly increased (P<0.05) compared with those in the model group(Figure 3ⅡI).
Caspase-3 levels in tumor tissues
Compared with those in the model group, the expression of the apoptotic protein caspase-3 in the tumor tissue increased in the positive control group and medium, high-dose BRNP groups. The result in the model group significantly differed from those in the positive control group and the medium and high-dose BRNP groups (P<0.01) (Figure 3IVand Table 3).
| Grouping | Dose (mg/Kg-1) | Caspase-3 (Pg/mL-1) |
|---|---|---|
| Model group | - | 2.642 ± 0.03 |
| Positive control group | 6 | 3.559 ± 0.10a |
| Low dose group | 50 | 3.154 ± 0.13 |
| Dose group | 100 | 7.705 ± 0.05a |
| High dose group | 200 | 14.240 ± 0.09a |
Note: a: P<0.01 vs. Model group
Table 3: Effects of BRNP on tumor cytokines in tumor-bearing mice (x ± s, n=10)
Discussion
Natural product provides a good auxiliary treatment for patients with advanced lung cancer due to its curative effect is distinct, low resistance, lower toxicity. Human body weight is positively correlated with the effect of tumor treatment, which can be used as an evaluation index of tumor treatment effect in clinic (Amano K, et al., 2020). With the deterioration of the disease, patients with malignant tumor are often accompanied by the decline of immune function. Organ index of immune organs is a preliminary indicator to measure the specific immune function and the non-specific immune function of the body (Sun L, et al., 2016; Sun G and Ni K, 2020). According to the weight changes of mice before and after administration, we found that mice in the positive control group has less diet and water companied by weight loss, which may be caused by the side effects of chemotherapy drugs. Compared with positive control group, the diet and weight of mice did not decrease significantly in the BRNP group, which indicated that the BRNP could against the toxicity of Cisplatin and improve the spleen index and thymus index of tumor-bearing mice.
The experiments showed that BRNP had inhibitory effect on tumor growth of BALB/c tumor-bearing mice. It enhanced the secretion of immune factors IL-2, IL-6, IL-12, and TNF-α in the serum and spleen of A549 tumor-bearing mice, thereby participating in immune regulation. BRNP is also toxic to the growth of human lung adenocarcinoma cell A549 in a concentration-dependent manner. The mechanism of its action may be that it changes the morphology of the cancer cells, reduce their number, and promote apoptosis, thereby increasing the content of caspase-3.
Conclusion
The expression of the anti-apoptotic target Bcl-2 was inhibited by activating the protein expression of pro-apoptotic Bax, Caspase-3, and p53 in cells. Finally, DNA formation in the nucleus was interfered to induce cell apoptosis. In this study, we investigated the toxicological mechanism of plant polysaccharide BRNP on lung adenocarcinoma cell line A549 and tumor-bearing mice from the perspective of apoptosis, and provided a toxicological basis for plant polysaccharide in the field of cancer treatment.
Declarations
Author’s contributions
KG and TH performed the experiments and contributed equally to this work. AA and QLJ conducted data analyses. TH, KJS and CJM designed the study and supervised the necessary work. QLJ wrote the manuscript. All authors have read and approved of the manuscript for submission.
Acknowledgements
We are extremely grateful to Taoredahong H for her guidance and support during this experiment. We also thank the editorial department and reviewers for their attention and kind suggestions.
Competing interest
We declare that the authors have no competing interests.
Ethic approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of Xinjiang medical university.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 81460615).
References
- Sun G, Ni K. The role of Cavin3 in the progression of lung cancer and its mechanism. Biomed Res Int. 2020; 6364801.
[Crossref] [Google Scholar] [Pubmed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69(1): 7-34.
[Crossref] [Google Scholar] [Pubmed]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394-424.
[Crossref] [Google Scholar] [Pubmed]
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021; 7(1): 1-20.
[Crossref] [Google Scholar] [Pubmed]
- Lin C, Zhang Y, Zhao Q, Sun P, Gao Z, Cui S. Analysis of the short-term effect of photodynamic therapy on primary bronchial lung cancer. Lasers Med Sci. 2021; 36(4): 753-761.
[Crossref] [Google Scholar] [Pubmed]
- Broderick SR. Adjuvant and neoadjuvant immunotherapy in non-small cell lung cancer. Thorac Surg Clin. 2020; 30(2): 215-220.
[Crossref] [Google Scholar] [Pubmed]
- Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin North Am. 2019; 31(3): 303-313.
[Crossref] [Google Scholar] [Pubmed]
- Shankar A, Saini D, Dubey A, Roy S, Bharati SJ, Singh N, et al. Feasibility of lung cancer screening in developing countries: Challenges, opportunities and way forward. Transl Lung Cancer Res. 2019; 8(1): 106.
[Crossref] [Google Scholar] [Pubmed]
- Fan Y, Su Z, Wei M, Liang H, Jiang Y, Li X, et al. Lung cancer risk following previous abnormal chest radiographs: A 27‐year follow‐up study of a Chinese lung screening cohort. Thorac Cancer. 2021; 12(24): 3387-3395.
[Crossref] [Google Scholar] [Pubmed]
- Shinchi H, Crain B, Yao S, Chan M, Zhang SS, Ahmadiiveli A, et al. Enhancement of the immunostimulatory activity of a TLR7 ligand by conjugation to polysaccharides. Bioconjug Chem. 2015; 26(8): 1713-1723.
[Crossref] [Google Scholar] [Pubmed]
- Gong Y, Wu J, Li ST. Immuno-enhancement effects of Lycium ruthenicum Murr. polysaccharide on cyclophosphamide-induced immunosuppression in mice. Int J Clin Exp Med. 2015; 8(11): 20631.
[Google Scholar] [Pubmed]
- Ke M, Wang H, Zhang M, Tian Y, Wang Y, Li B, et al. The anti-lung cancer activity of SEP is mediated by the activation and cytotoxicity of NK cells via TLR2/4 in vivo. Biochem Pharmacol. 2014; 89(1): 119-130.
[Crossref] [Google Scholar] [Pubmed]
- Sun L, Chu J, Sun Z, Chen L. Physicochemical properties, immunomodulation and antitumor activities of polysaccharide from Pavlova viridis. Life Sci. 2016; 144: 156-161.
[Crossref] [Google Scholar] [Pubmed]
- Li HH, Chen LC, Hailiqian T. Antioxidant effects of neutral polysaccharides from turnip on d-galactose induced aging mice. Food Sci Technol. 2021; 46(5): 168-173.
- Aso K, Goi T, Nakazawa T, Kimura Y, Hirono Y, Katayama K, et al. The expression of integrins is decreased in colon cancer cells treated with polysaccharide K. Int J Oncol. 2013; 42(4): 1175-1180.
[Crossref] [Google Scholar] [Pubmed]
- Liu Y, Zhao J, Zhao Y, Zong S, Tian Y, Chen S, et al. Therapeutic effects of lentinan on inflammatory bowel disease and colitis‐associated cancer. J Cell Mol Med. 2019; 23(2): 750-760.
[Crossref] [Google Scholar] [Pubmed]
- Jin X, Liu X, Ding J, Zhang L, Yang Y, Wang X, et al. Lentinan improved the efficacy of vaccine against Trichinella spiralis in an NLRP3 dependent manner. PLoS Negl Trop Dis. 2020; 14(9): 8632.
[Crossref] [Google Scholar] [Pubmed]
- Chan SW, Tomlinson B, Chan P, Lam CW. The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm Biol. 2021; 59(1): 1159-1169.
[Crossref] [Google Scholar] [Pubmed]
- Zheng S, Zhang W, Liu S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS One. 2020; 15(12): e0244749.
[Crossref] [Google Scholar] [Pubmed]
- Chen W, Ju J, Yang Y, Wang H, Chen W, Zhao X, et al. Astragalus polysaccharides protect cardiac stem and progenitor cells by the inhibition of oxidative stress-mediated apoptosis in diabetic hearts. Drug Des Devel Ther. 2018; 12: 943.
[Crossref] [Google Scholar] [Pubmed]
- Yang S, Sun S, Xu W, Yu B, Wang G, Wang H. Astragalus polysaccharide inhibits breast cancer cell migration and invasion by regulating epithelial‑mesenchymal transition via the Wnt/β‑catenin signaling pathway. Mol Med Rep. 2020; 21(4): 1819-1832.
[Crossref] [Google Scholar] [Pubmed]
- Ren Y, Bai Y, Zhang Z, Cai W, Flores ADR. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules. 2019; 24(17): 3122.
[Crossref] [Google Scholar] [Pubmed]
- Cao Q, Wang G, Peng Y. A critical review on phytochemical profile and biological effects of turnip (Brassica rapa L.). Front Nutr. 2021; 8: 721733.
[Crossref] [Google Scholar] [Pubmed]
- Qiao LJ, Cheng YF, Ayxiaguli B. Extraction, identification and content determination of refined polysaccharides from turnip. Journal of Food Safety and Quality Testing. 2020; 11(12): 4115-4120.
- Hairenguli M, Zulipiyan A, Hailiqian T. Preliminary study on the hypoglycemic effect of neutral polysaccharides from Turnip. Journal of Food Safety and Quality Testing. 2020; 11(2): 387-392.
- Zhuoer C, Duishanbieke G, Ying W, Baolin H, Taoerdahong H. Study on the anti-tumor effect of aqueous extract from Brassica rapa L. Northwest Pharmaceutical Journal. 2016; 31(3): 264-267.
- Reziyamu M, Li YT, Hailiqian T. Preliminary study on the immunomotor effect of Chiamagu polysaccharides on macrophage RAW264.7 in vitro. Natural products research and development. 2018; 30(1): 15-20.
- Yao J, Wu Y, Hailiqian T. Study on the antioxidant effect of polysaccharides from Chiama Ancient in vitro. West China Journal of Pharmaceutical Sciences. 2014; 29(5): 606-607.
- Amano K, Maeda I, Ishiki H, Miura T, Hatano Y, Oya K, et al. Significance of fluid retention, body mass index, and weight loss in patients with advanced cancer. JCSM Clinical Reports. 2020; 5(3): 69-78.
Author Info
Hailiqian Taoerdahong1*, Gulimila Kadeer1, Qiao Lijie2, Kang Jinsen3, Chang Junmin1 and Ajiranmu Abula12Packaging Materials and Excipients Testing Centre, Shanxi Food and Drug Inspection Institute, Shaanxi, China
3Department of Basic Medicine, College of Basic Medicine, Xinjiang Medical University, Xinjiang, China
Citation: Taoerdahong H: Mechanism of A549 Cell Apoptosis and Immune Regulating Effect of BRNP on Tumor-Bearing Mice
Received: 01-Nov-2022 Accepted: 25-Nov-2022 Published: 02-Dec-2022, DOI: 10.31858/0975-8453.13.12.840-846
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3