Research Article - (2022) Volume 13, Issue 12
Abstract
A solid lipid nanoparticle provides the opportunity of developing new therapies due to their unique size-dependent characteristics. They are a small particle that ranges between 1-100 nm in size. Lipid nanoparticles have risen in popularity in this context due to their widespread acceptance as nontoxic, biocompatible, and simplified formulation. They are safe, efficacious, and scalable enough to be made on a large scale and advanced to clinical usage. Pharmaceutical uses of lipid nanocarriers include the transport and distribution of a wide range of therapeutic agents, from biotechnological products to tiny drug molecules. This review begins with a brief summary of the common starting materials used to make Solid Lipid Nanoparticles (SLNs), as well as their screening, SLNs formulation principles, and various methods used to formulate SLNs along with its diverse applications.
Keywords
Solid lipid nanoparticles, Lipids, Homogenization, Solvent injection applications
Abbreviations
NP: Nanoparticles; SLN: Solid Lipid Nanoparticles; NLC: Nanostructure Lipid Carrier; LNP: Lipid Nanoparticles; gm: Gram; nm: Nanometer
Introduction
Nanoparticles are colloidal particles very small i.e. 10-1000 nanometers in size. They are made of synthetic/natural polymers and are designed to improve drug delivery while lowering toxicity. They have evolved into a versatile alternative to liposomes as medication carriers over time. Nanoparticles (NP) are able to cross many anatomical barriers and releases their contents for the prolonged period, and their stability in the nanoscale size are all important factors in the effective use of NP for drug delivery. Solid lipid nanoparticles are at the vanguard of the rapidly emerging field of nanotechnology, with a wide range of potential uses in medication delivery, clinical treatment, research, and other fields (Mukherjee S, et al., 2009).
Lipid Nanoparticles (LNPs) (Figure 1) provides an opportunity of developing new therapies due to their unique size-dependent characteristics. Lipids have been proposed as an alternate carrier to avoid the constraints of polymeric NP, notably for lipophilic medicines. SLNs are a type of LNPs that is receiving a lot of interests from formulators all over the world. SLNs are appealing because of their potential to increase the performance of medicines, neutraceuticals, and other materials due to their small size, vast surface area, high drug loading, and phase interaction at interfaces (Argimón M, et al., 2017).
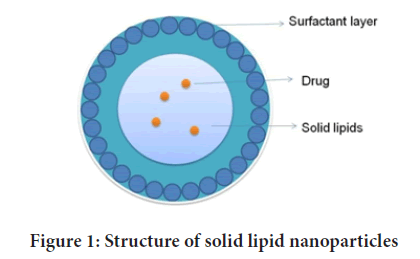
Figure 1: Structure of solid lipid nanoparticles
Materials and Methods
Starting materials used
In general terms, the ingredients used for preparation of Solid Lipid Nanoparticles (SLNs) and Nanostructure Lipid Carrier (NLC) include a solid lipid, liquid lipid, a surfactant and water, in addition to the active molecules to be incorporated. It is meant for the use of beginning materials that are usually regarded as safe.
Lipids: Lipids are the primary structural component of lipid nanoparticles and, as such, are the most important component of the matrix, defining the properties of these colloidal systems. The most common ingredients are free fatty acids, fatty alcohols, glycerol esters, and waxes. Phospholipids, glycolipids, and sphingolipids are also included. Furthermore, some of these lipids have a surfactant function that aids in particle formation. Several of the lipids commonly utilized in the synthesis of SLNs and NLCs are listed in Table 1.
| Category | Examples |
|---|---|
| Triglycerides | Tricaprin (Dynasan 110™), Trilaurin (Dynasan 112), Trimyristin (Dynasan 114), Tripalmitin (Dynasan 116), Tristearin (Dynasan 118) |
| Fatty acids | Stearic acid, Oleic acid, Palmitic acid, Behenic acid |
| Monoglycerides | Glyceryl monostearate (Imwitor 900, Geleol), Glyceryl behenate (Compritol 888 ATO), Glyceryl palmitostearate (Precirol® ATO 5) |
| Mixtures | Witepsol W35 (A mixture of 65%-80% of triglycerides, 10%-35% of diglycerides and 1%-5% of monoglycerides) |
| Witepsol H35 (triglycerides with portions of maximum 15% of diglycerides and maximum 1% of monoglycerides), Medium-chain triglycerides caprylic/capric (Miglyol®) | |
| Fatty alcohols | Stearyl alcohol, Cetyl alcohol, Lauryl alcohol |
| Waxes | Cetyl palmitate, Beeswax, Carnauba wax |
Table 1: Lipids that are commonly employed in the manufacture of SLNs and NLCs (Khatak S and Dureja H, 2017; Gordillo-Galeano A and Mora-Huertas CE, 2018)
Screening of lipids: The basic goal of lipid carrier-based formulations is to transport hydrophilic or lipophilic medicines to the desired region. Phospholipids are utilized to promote permeability by reducing the problem of the lipophilic moiety’s solubility. The permeability of hydrophilic drugs via biomembrane is limited, which can be enhanced by introducing the active moiety in the lipid core. Hence, both hydrophilic/lipophilic drugs can be encapsulated in the lipid carriers. Proteins and peptides are unstable in the GIT at various pH levels, which can be mitigated by using lipid carriers (Khatak S and Dureja H, 2017). Long-chain fatty acids crystallize at a slower rate than short-chain fatty acids. Because of their increased crystalline structure, waxy-lipid nanoparticles are more stable and display impressive drug ejection. By combining solid lipid with liquid lipid (oil) in the preparation of solid lipid dispersions, difficulties such lipid crystallinity and polymorphism can be avoided (Gordillo-Galeano A and Mora-Huertas CE, 2018).
Selection criteria of lipids: Following criteria is used for the selection of lipids for SLNs (Duan Y, et al., 2020)-
• Partition coefficient is the main criteria reported for the selection of lipid carriers.
• Compatibility between drug and lipid carrier(s).
• Melting point of the lipid carrier should be more than 45°C to minimize the stability problems.
• The HLB value of core materials should be less than 2. Since they are more lipophilic and have more chances to form solid matrices over the hydrophilic materials.
• Lipid carrier should have the property to stabilize the incorporated drug(s).
• Higher and lower crystalline lipid matrix will lead to drug expulsion and degradation.
• Thermodynamic stability and lipid packing density.
• The occlusive property for topical preparation depends on the level of the lipid crystallinity.
Stabilizing agents
Stabilizing agents, primarily surfactants (Table 2) are used in the preparation of SLNs and NLCs. Surfactants have the ability to reduce the interfacial energy between the lipid phase and the aqueous phase during particle preparation due to their tendency to accumulate in the binding interface, forming a layer around the particles that favors the physical stability of the dispersion during storage. Surfactants and co-surfactants that are routinely utilized in the Properties of stabilizing agents: The stability of lipid dispersion during storage can be reduced by using the right surfactant and co-surfactant. Non-ionic surfactants are recommended because they are less irritating and harmful. Following properties of surfactants are responsible for the improvement of the bioavailability of poorly soluble drugs (Mahajan PS, et al., 2015).
| Category | Examples |
|---|---|
| Nonionics | Polyoxyethylene (20) sorbitan monolaurate (Polysorbate 20, Tween 20), Polyoxyethylene (20) sorbitan monostearate (Polysorbate 60, Tween® 60) Polyoxyethylene (80) sorbitan monooleate (Polysorbate 80, Tween 80), Poloxamer188, Poloxamer 407, Poloxamer 182, Ethoxylated p-tert-octylphenol formaldehyde polymer (Tyloxapol) |
| Anionics | Sodium lauryl sulfate, Sodium cholate, Sodium glycolate |
| Cationics | 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), Cetrimonium bromide. |
| Amphoterics | Soybean lecithin (Lipoid S75, Lipoid S 100), Egg lecithin (Lipoid E) Phosphatidylcholine (Epikuron 170, Epikuron 200) |
| Co-surfactants | Polyvinyl alcohol (PVA), Butanol, Propylene glycol, Polyethylene glycol. |
Table 2: Surfactants typically utilized in SLNs and NLCs
• Non-ionic surfactants are more hydrophobic and have a higher potential to solubilize drugs that are difficult to dissolve.
• They do not irritate or harm biological membranes.
• Numerous non-ionic surfactants influence drug pharmacokinetics by influencing efflux pumps like P-glycoprotein and/or multi-drug resistance-associated proteins.
• Surfactants that are ionic, anionic, or amphoteric are less hazardous than cationic surfactants.
• Non-ionic surfactants prevent the degradation of lipid matrix in vitro.
• The density of polyethylene oxide chains on non-ionic surfactants can change the surface of a lipid nanoparticle, affecting the rate of breakdown in vivo.
Selection criteria of stabilizing agents: Following criteria is used for the selection of stabilizing agents for SLNs (Mahajan PS, et al., 2015; Sastri KT, et al., 2020; Garud A, et al., 2012).
• Invasive and non-invasive route of administration
• HLB value of the surfactant
• Consequences of lipid variation and particle size
• The role of gastrointestinal lipid instability and degradation
Other additives
Other additives such as glucose, fructose, and sorbitol are used as cryoprotectants in lyophilized formulations, chitosan has been reported as a coating material, and parabens or thiomersal are included as antimicrobial preservation agents for particle dispersions, in addition to the lipid components and stabilising agents used to prepare SLN and NLC. In addition, commercial preservatives composed from pentylene glycol, caprylyl glycol, phenoxyethanol, benzyl alcohol, tocopherol, and potassium sorbate, among other ingredients, were used (Montoto S, et al., 2020; Badilli U, et al., 2018).
Results and Discussion
Preparation of SLNs formulation
Principle: SLNs are made up of a phospholipid-coated solid hydrophobic core matrix containing the hydrophobic tails of the phospholipid part. SLNs also contain APIs such as drugs, genes, DNA, plasmids, and proteins, as well as solid lipid(s) and emulsifiers. The lipids used to make SLNs are surfactant stabilized, which means they are solid at physiological and room temperatures. Lipids are classified as fatty acids, fatty esters, fatty alcohols, triglycerides, and partial glycerides based on their structure. Ionic and nonionic polymers such as Pluronic such as F-68 and F-127 are used as emulsifiers, surfactants, and organic salts. Their physicochemical properties, on the other hand, have an impact on the behavior of the respective SLNs in both in vivo and in vitro release (Duong VA, et al., 2020; Singh R, 2019).
The interfacial tension and surface tension between two liquids determine the creation of colloidal nanoparticles. As a result, the adhesive forces between two liquids are the most important factor in the production of solid lipid nanoparticles. Because of the weaker adhesive forces compared to gas, the interfacial tension between two liquids is usually less than their surface tension. Surface free energy of interfacial tension is formed by molecules at the interface, which are agitated and form a spherical system to reduce surface free energy. The amount of work required to increase the surface area of the scattered particles is as follows (Müller RH, et al., 2000; Ganesan P and Narayanasamy D, 2017).
W=Υ × ΔA
Where, W=Work (in ergs)
Υ=Surface tension (in dynes/cm2)
ΔA=Increase in surface area (in cm2)
Surfactant selection is also based on Griffin’s HLB scale, which assigns a high value to a hydrophilic molecule and a low value to a hydrophobic molecule. Polyoxyethylene is the only hydrophilic component in non-ionic surfactants.
HLB=EO/5
Where, EO is the % by weight of ethylene oxide
In the case of polyhydric alcohol fatty acid esters, HLB=20(1-S/A)
Where,
S=Saponification number of the ester
A=Acid number of the acid
Solid lipids are employed as a substitute for the oil phase and melted and combined with the aqueous phase in SLNs, which are very similar to emulsions (Salah E, et al., 2020). This combination is agitated at a high speed, resulting in the creation of fine droplets of dispersed phase in the dispersion medium. The interfacial tension between the two liquids is decreased by introducing a surfactant as a third component, lowering the surface energy and forming stable SLNs. Solid lipids are employed as a substitute for the oil phase and melted and combined with the aqueous phase in SLNs, which are very similar to emulsions (de Oliveira IF, et al., 2020).
The word surfactant primarily refers to surface-active agent. They reduce the surface tension between two or more substances that are in the same or different physical states when they come into contact (Pardeike J, et al., 2009). Surfactants improve the stability and drug loading capability of SLNs. Surfactants such as CPC, Poloxamer 407, and Tween-80 are commonly utilized to improve the efficacy of SLNs during drug administration (Zhong Q and Zhang L, 2019).
Techniques of formulation development
There are various techniques of development of SLN formulation discussed below-
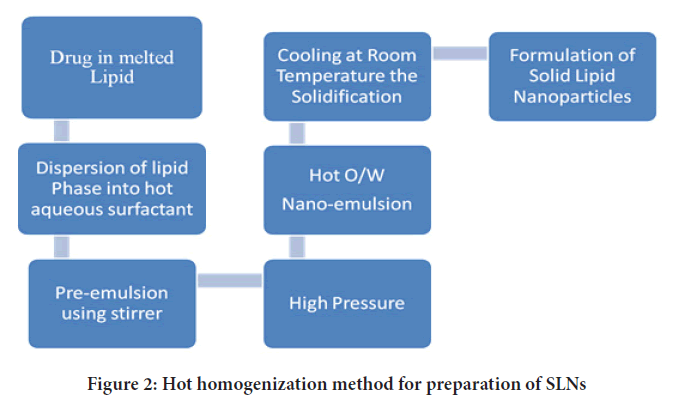
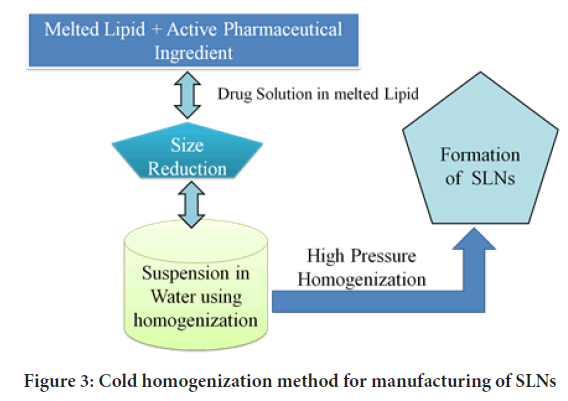
High pressure homogenization: High pressure homogenization (HPH) is a process in which a liquid or dispersion is pushed through a gap of a few micrometres at high pressure to produce submicron-sized particles. The particles are broken down by strong shear stress and cavitational forces, resulting in a reduction in particle size. HPH can be done at a high temperature or at a low temperature, which are referred to as High-hot pressure homogenization and high-cold pressure homogenization (Figures 2 and 3), respectively (Lin CH, et al., 2017). The lipid(s) and drug(s) are heated to about 5°-100°C over the melting point of the lipid in the first phase of both processes, so that the drug is dissolved or disseminated in the melted lipid. Lipid concentrations typically range from 5% to 20% w/v (Lingayat VJ, et al., 2017).
Figure 2: Hot homogenization method for preparation of SLNs
Figure 3: Cold homogenization method for manufacturing of SLNs
The amphiphile-containing aqueous phase is added to the lipid phase at the same temperature as the lipid melting, and the hot pre-emulsion is created using a high-speed stirring device in the second step of the HPH process. The lipid is driven through a confined space (few mm) at high pressure of about 100-1000 bar for 3 to 5 times, depending on the formulation and desired result. The API is disseminated or dissolved in the lipid melt before homogenization (Mehnert W and Mäder K, 2012). Furthermore, there are certain limitations to this methodology such as-
I. because of the deterioration of heat-sensitive medications, it cannot be utilized, and
II. When the number of rotations or the pressure of homogeneity is increased, the particle size usually increases as well.
Nevertheless, by preparing SLNs with cold-HPH, these limitations can be overcome (Parhi R and Suresh P, 2012). The second phase entails the production of a suspension of melting lipids and medicines, followed by rapid chilling in dry ice and liquid nitrogen, as previously mentioned. Milling converts the powder into microparticles in the third phase. The microparticles are then dispersed in a cold aqueous surfactant solution. Homogenization is commonly performed for 5 cycles at 500 bars in the final phase to develop SLNs (Paliwal R, et al., 2020; Sawant KK and Dodiya SS, 2008; Garse H, et al., 2015).
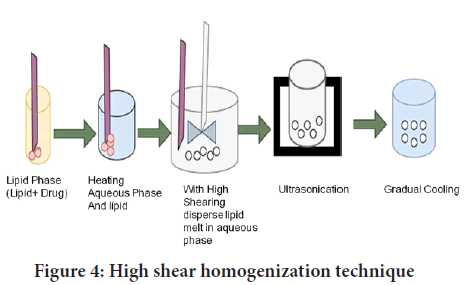
High shear homogenization and/or ultra-sonication: Ultrasonication and high shear homogenization are dispersion processes. Lipid nanoparticle dispersions are made via high shear homogenization followed by ultrasonication of melted lipid in a warm aqueous phase including surfactants. This approach entails heating a solid lipid to a temperature of about 5°C-10°C over its melting point. To form an emulsion, the lipid melt is dispersed in an aqueous surfactant solution at the same temperature with high-speed stirring. The emulsion’s droplet size is reduced after sonication. Lipid nanoparticle dispersion is achieved by gradually cooling the warm emulsion below the lipid utilizationtemperature. Ultracentrifugation can be used to obtain concentrated lipid nanoparticle dispersions (Figure 4). High-speed stirring and ultra-sonication have been widely used in combination to achieve SLNs dispersions with narrow particle distributions (Bi R, et al., 2009; Jawahar N, et al., 2013; Patel RR, et al., 2016).
Figure 4: High shear homogenization technique
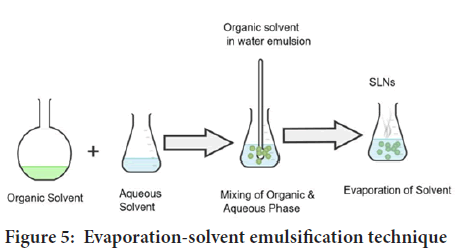
Evaporation-solvent emulsification: The lipophilic substance and the hydrophobic medication are dissolved in water-insoluble organic solvents such cyclohexane, toluene, and chloroform in this approach. The mixture is now emulsified in an aqueous phase using high-speed homogenization. The coarse emulsion is allowed to pass through a micro fluidizer very immediately. The organic solvent is evaporated in a rotary evaporator with mechanical agitation at room temperature and reduced pressure (Figure 5). Thermal stress is avoided in this method. As a result, the inclusion of extremely thermolabile compounds is now a possibility. The use of an organic solvent, which may react with drug molecules, is a distinct drawback (Bayón-Cordero L, et al., 2019; Delshadi R, et al., 2021).
Figure 5: Evaporation-solvent emulsification technique
Phase inversion method: In phase inversion methods, coarse emulsions are generated initially, then the composition or temperature is changed to cause phase inversion and the formation of fine oil droplets without the use of considerable mechanical energy (Nasiruddin M, et al., 2017). These methods can be used in settings where surfactant interfacial characteristics are a function of composition or temperature. Because the heating stage can be utilized to melt solid lipids, the Phase Inversion Temperature (PIT) approach is particularly interesting for forming SLNs.
Some surfactants’ interfacial characteristics, particularly nonionic surfactants, are temperature dependent. Preheating a heated emulsion induces the restructuring of oil/water/surfactant mixes to create SLNs or NLCs, and heating an emulsion with a constant composition above the PIT can induce complete or partial inversion between w/o and o/w emulsions (Palmer BC and de Louise LA, 2016).
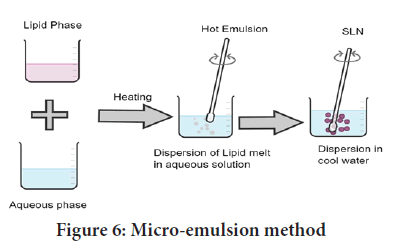
Method of using a micro-emulsion as a template: The lipid (fatty acid/ glyceride) is first melted, and the drug is then disseminated in the molten lipid. A mixture of water, surfactant, and co-surfactant is heated to a temperature that is at least as high as the lipid’s melting point (Pinto MF, et al., 2014). To make a translucent microemulsion, add this aqueous surfactant solution to the lipid melt while swirling gently. This microemulsion is then dispersed in water at a temperature of 2 to 10 degrees Celsius with gentle mechanical agitation. The typical hot microemulsion to cold water ratio is between 1:25 and 1:50. To improve the particle concentration, surplus water is removed using ultra-filtration or lyophilization (Figure 6) (Sonawane R, et al., 2014).
Figure 6: Micro-emulsion method
The microemulsion approach for large-scale manufacture of SLNs appears to be possible and is now being developed (Korkmaz E, et al., 2013). The microemulsion is manufactured in a large, temperature-controlled tank for the scale-up process, and then pumped from this tank into a cold water tank for the precipitation stage. For the scale-up procedure, the microemulsion is made in a large, temperature-controlled tank, and then pumped into a cold water tank for the precipitation stage. To preserve the same product attributes, these should alter as little as possible throughout scale up (Dhillon P, et al., 2019).
Solvent injection method: The solvent diffusion method’s core premise has been expanded to the production of SLNs using the solvent injection approach. An injection needle is used to quickly inject lipids dissolved in a water miscible solvent or a water miscible solvent mixture into an aqueous phase containing surfactants (Kong X, et al., 2016). Acetone, isopropanol, and methanol are normally employed solvents in this procedure. This method has a number of advantages over previous methods, including ease of use and a quick manufacturing process without the use of technically sophisticated equipment such as a high-pressure homogenizer (Badawi N, et al., 2020). The usage of organic solvents is a downside of the “solvent injection” method. Although some solvents are pharmaceutically acceptable, they cannot be utilized for particular routes such as parenteral, ocular, or intravenous since any residual solvent could injure the patient adversely (Pham DT, et al., 2019; Moglad EH, et al., 2020).
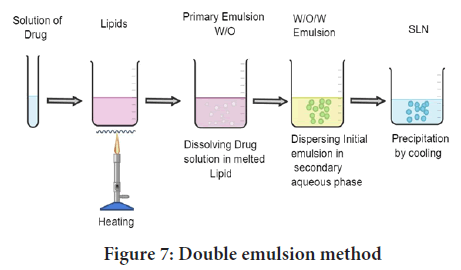
W/O/W double emulsion method: Two steps are involved in the manufacture of SLNs using the double emulsion approach. To make primary emulsion (w/o), drug (mostly hydrophilic drug) is dissolved in aqueous solvent (inner aqueous phase) and then dispersed in lipid-containing emulsifier/stabilizer (e.g. lecithin) (oil phase) (Puri A, et al., 2019). After adding an aqueous solution of a hydrophilic emulsifier (e.g. poloxamer, PVA) and stirring, a double emulsion (w/o/w) is generated, this is then isolated by filtration (Figure 7). The use of a double emulsion process eliminates the need to melt lipid for the synthesis of protein and peptide-loaded lipid nanoparticles, and there is the possibility of altering the surface of the nanoparticles to sterically stabilise them by incorporating a lipid-PEG derivative. The resistivity of these colloidal systems in the gastrointestinal fluids was greatly increased by sterical stabilization (Abdel-Salam FS, et al., 2016).
Figure 7: Double emulsion method
The polydispersity of the final double emulsion is exacerbated by each emulsification stage, which culminates in a highly polydisperse droplet distribution. As a result, any particles generated from such double emulsions are inherently uncontrollable in size and structure, limiting their applicability in applications requiring precise control and release of active ingredients (Carbone C, et al., 2020). This approach was used to prepare sodium cromoglycate containing SLN, but the resulting colloidal system produced average particles in the micrometre range. A unique reverse micelle-double emulsion process using sodium cholate-phosphatidylcholine based mixed micelles was used to make insulin-loaded SLN (Elnaggar YS, et al., 2011; Firdaus S, et al., 2021; El-Kamel AH, et al., 2007).
Applications of SLNs formulations
SLNs have a variety of potential uses, some of which are listed below-
SLN as drug carriers for topical drug delivery: SLNs are widely used for topical drug delivery. Systemic adverse effects such as skin shrinkage have been documented after using traditional prednicarbate cream (Begum M and Shaik NB, 2020). This may have been averted if the drug had been made into SLNs. Prednicarbate absorption was increased in the SLN formulation, and it was deposited in the epidermis with low quantity in the dermis (Odumosu P, et al., 2016). Triptolide is also delivered topically viaSLNs. Triptolide penetration into the skin was shown to be increased by SLN, as was anti-inflammatory activity. This method increased bioavailability at the site of action, lowering the required dose as well as dose-dependent adverse effects including stinging and irritation (Sarma A and Das MK, 2019). Tretinoin is also delivered topically using SLNs made from natural lipids (Gu Y, et al., 2018). When compared to methanolic tretinoin, tretinoin in SLNs improved photostability and prevented isomerisation, according to the findings (Liu X and Zhao Q, 2019). They claimed that it had better skin tolerability and a higher penetration profile than the commercial cream (Zamarioli CM, et al., 2015).
Anticancer drug delivery via SLNs: Multiple drugs both hydrophilic and lipophilic can be included into SLNs. The drug can be added in three ways, depending on the composition of the nanoparticles and the technique of synthesis-
a) It can be evenly disseminated in the lipid matrix
b) It can be included into the shell that surrounds the matrix in concern (or)
c) It can be dispersed throughout the outer shell (Gaba B, et al., 2015).
The drugs employed in antitumor chemotherapy are classified as alkylating agents, antimetabolites, natural products, or hormonal substances (Kumar V, 2022; El-Say KM and Hosny KM, 2018). Temozolomide is one of the alkylating agents that can be mentioned. Three nitrogen atoms close to a heterocyclic ring provide this chemical significant anticancer action (Pooja D, et al., 2015).
When compared to free temozolomide, SLN-administered temozolomide has shown to be more effective in the treatment of melanomas, delivering significantly more cytotoxicity in JR8, A2058 human, and B16-F10 murine melanoma cell lines (Krishnatreyya H, et al., 2019). To reduce toxicity while boosting pharmaceutical safety and absorption, mitoxantrone-loaded SLN local injections were devised. Doxorubicin (Dox) efficacy has been demonstrated to enhance when doxorubicin (Dox) is incorporated into SLNs (Üner M, et al., 2014). To make Dox-loaded solid lipid Nanoparticles, Dox was complexed with a soybean-oil-based anionic polymer and then dispersed in water with a lipid. The system’s effectiveness has increased, and cancer cells have decreased (Khalil RM, et al., 2014).
Antiviral medicines are delivered using SLNs: Microemulsions, nanoliposomes, nanoemulsions, solid lipid nanoparticles, biopolymer nanoparticles, and biopolymer nanogels are just a few of the nanoparticle delivery methods that can be made from pharmaceutical and/or food-grade materials (Kuo YC, et al., 2019). Top-down approaches, which use mechanical devices such as homogenizers, sonicators, or milling devices to break down bulk phases or large particles into small particles; bottom-up approaches, which use mechanical devices such as homogenizers, sonicators, or milling devices to break down bulk phases or large particles into small particles.
Bottom-up techniques rely on changes in solution or ambient circumstances to encourage the production of tiny particles from molecules such as crystallization, spontaneous emulsification, or anti-solvent precipitation (Parvez S, et al., 2020). These methods can be combined to make nanoparticles with new or better qualities in practise. For example, a nanoemulsion can be created by homogenization, and then the emulsifier-coated lipid nanoparticles can be electrostatically coated with nano-laminated polymer layers. Several earlier studies have shown that nanoparticles can encapsulate and transport antivirals (Gaur PK, et al., 2013).
Because of their tiny size and variable surface properties, nanoparticles can typically circumvent biological barriers when delivering antivirals to diseased areas (Mante PK, et al., 2021). Antivirals produced from nanoparticles, for example, can connect to viral receptors on the surface of host cells or be released inside the cell, disrupting the viral replication cycle (Chaudhari PM and Bind VM, 2019).
SLNs in the treatment of tuberculosis: The delayed, prolonged, and regulated release of nanobeads from a biodegradable particle is a noteworthy aspect of nanobead delivery. Various animal models have been used to create an antibiotic therapy based on polymer technology against M. tuberculosis, but none of them has lived up to expectations or accurately mimics all of the characteristics of human tuberculosis (Pham CV, et al., 2020). Drug delivery vehicles are made out of nanoparticles that are characterised as submicron (1 m) colloidal particles. Drugs can be covalently implanted on the particle surface or integrated into the particle matrix for therapeutic purposes (Londhe V and Save S, 2017). Polymers, which can be natural (e.g., gelatin and albumin), synthetic (e.g., polylactides and polyalkylcyanoacrylates), or solid lipids, are examples of biocompatible and biodegradable materials (Passos JS, et al., 2020).
Nanoparticles are taken up by cells more efficiently than bigger molecules, making them a promising mode of transport and delivery. The carriers have been modified to allow for regulated, gradual, and long-term drug release from the matrix.
The benefits of a nanoparticle-based medication delivery method for tuberculosis treatment are listed below-
a) Longer time period/higher consistency.
b) Multiple medications can be contained in the matrix due to its high carrier ability.
c) When compared to traditional medications, there are less negative effects.
d) Bioavailability has improved (slow, sustained, and controlled drug release).
e) The viability of alternative administration routes, such as oral delivery and inhalation
f) Side effects are minimal, and compliance is higher (Rapalli VK, et al., 2021)
SLNs in the treatment of psoriasis
Psoriasis is a chronic immune-mediated skin disease marked by keratinocyte hyperproliferation and increased dermal vascularity that affects 2%-3% of the global population (Arora R, et al., 2017). The aetiology of psoriasis is complex and multifaceted, and the antigenic trigger of disease onset is poorly understood, thus complicating antipsoriatic therapy. Topical application also decreases the drug’s systemic bioburden and, as a result, its harmful effects (Fang JY, et al., 2008). The first-line antipsoriatic therapy consists of a safe and well tolerated (75%-80%) topically applied medications. Betamethasone is an excellent antiproliferative drug among these dipropionate betamethasones. However, it is rigorously discouraged by patients due to the risk of skin atrophy and a psoriasis recurrence. Calcipotriol (CT), a vitamin D derivative, is another excellent antipsoriatic treatment option; however it can cause significant skin irritation. Combination therapy using two or more medications with different mechanisms of action may have synergistic effects (Fang JY, et al., 2008). Betamethasone dipropionate and calcipotriol loaded solid lipid nanoparticles topical gel was found to have stronger antipsoriatic efficacy in-vitro and in-vivo.
Conclusion
SLNs have piqued the interest of several researchers due to their superior properties and advantages over other traditional dosage forms, and other colloidal counterparts of SLN have proven to be a significant nanotechnology discovery due to their effective performance and as a safe vehicle for pharmaceuticals. SLN combines the benefits of polymeric nanoparticles and fat-based emulsions as a colloidal drug carrier. These systems offer a number of advantages, including the capacity to easily incorporate lipid- and water-soluble medicines, appropriate physical stability, and cheap cost and ease of fabrication. SLNs are qualified as competent nanocarriers in the expansion of targeted delivery systems for clinical trial investigations due to their inherent properties. SLNs, too favors continuous release in addition to excellent drug targeting.
References
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009; 71(4): 349.
[Crossref] [Google Scholar] [Pubmed]
- Argimón M, Romero M, Miranda P, Mombrú ÁW, Miraballes I, Zimet P, et al. Development and characterization of vitamin A-loaded solid lipid nanoparticles for topical application. J Braz Chem Soc. 2017; 28: 1177-1184.
- Khatak S, Dureja H. Structural composition of solid lipid nanoparticles for invasive and non-invasive drug delivery. J Nanomater. 2017; 2(3): 129-153.
- Gordillo-Galeano A, Mora-Huertas CE. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018; 133: 285-308.
[Crossref] [Google Scholar] [Pubmed]
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, et al. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020; 10(45): 26777-26791.
[Crossref] [Google Scholar] [Pubmed]
- Mahajan PS, Mahajan KB, Darekar AB. A review on Solid Lipid Nanoparticle (SLN): An advanced treatment modality. Int J Pharm Sci Res. 2015; 6(9): 3698.
- Sastri KT, Radha GV, Pidikiti S, Vajjhala P. Solid lipid nanoparticles: Preparation techniques, their characterization, and an update on recent studies. J Appl Pharm Sci. 2020; 10(6): 126-141.
- Garud A, Singh D, Garud N. Solid Lipid Nanoparticles (SLN): Method, characterization and applications. Int Curr Pharm J. 2012; 1(11): 384-393.
- Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020; 7: 587997.
[Crossref] [Google Scholar] [Pubmed]
- Badilli U, Gumustas M, Uslu B, Ozkan SA. Lipid-based nanoparticles for dermal drug delivery. Organic materials as smart nanocarriers for drug delivery. 2018; 369-413.
- Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020; 25(20): 4781.
[Crossref] [Google Scholar] [Pubmed]
- Singh R. Preparation of solid lipid nanoparticles through various methods using different precursors. J Drug Deliv Ther. 2019; 9(2): 415-419.
- Müller RH, Mäder K, Gohla S. Solid Lipid Nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur J Pharm Biopharm. 2000; 50(1): 161-177.
[Crossref] [Google Scholar] [Pubmed]
- Ganesan P, Narayanasamy D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 2017; 6: 37-56.
- Salah E, Abouelfetouh MM, Pan Y, Chen D, Xie S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf B Biointerfaces. 2020; 196: 111305.
[Crossref] [Google Scholar] [Pubmed]
- de Oliveira IF, Barbosa EJ, Peters MC, Henostroza MA, Yukuyama MN, dos Santos Neto E, et al. Cutting-edge advances in therapy for the posterior segment of the eye: Solid lipid nanoparticles and nanostructured lipid carriers. Int J Pharm. 2020; 589: 119831.
[Crossref] [Google Scholar] [Pubmed]
- Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009; 366(1-2): 170-184.
[Crossref] [Google Scholar] [Pubmed]
- Zhong Q, Zhang L. Nanoparticles fabricated from bulk solid lipids: Preparation, properties, and potential food applications. Adv Colloid Interface Sci. 2019; 273: 102033.
[Crossref] [Google Scholar] [Pubmed]
- Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017; 25(2): 219-234.
[Crossref] [Google Scholar] [Pubmed]
- Lingayat VJ, Zarekar NS, Shendge RS. Solid lipid nanoparticles: A review. Nanoscience and Nanotechnology Research. 2017; 4(2): 67-72.
- Mehnert W, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev. 2012; 64: 83-101.
[Crossref] [Google Scholar] [Pubmed]
- Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles-a review. Curr Drug Discov Technol. 2012; 9(1): 2-16.
[Crossref] [Google Scholar] [Pubmed]
- Paliwal R, Paliwal SR, Kenwat R, Kurmi BD, Sahu MK. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin Ther Pat. 2020; 30(3): 179-194.
[Crossref] [Google Scholar] [Pubmed]
- Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008; 2(2): 120-135.
[Crossref] [Google Scholar] [Pubmed]
- Garse H, Jagtap P, Kadam V. Solid lipid nanoparticles based gel for topical delivery of antifungal agent. Int J Pharm Sci Res. 2015; 6(8): 3571-3579.
- Bi R, Shao W, Wang Q, Zhang N. Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J Biomed Nanotechnol. 2009; 5(1): 84-92.
[Crossref] [Google Scholar] [Pubmed]
- Jawahar N, Meyyanathan SN, Reddy G, Sood S. Solid lipid nanoparticles for oral delivery of poorly soluble drugs. ChemInform. 2013; 44(27).
- Patel RR, Chaurasia S, Khan G, Chaubey P, Kumar N, Mishra B. Highly water-soluble mast cell stabiliser-encapsulated solid lipid nanoparticles with enhanced oral bioavailability. J Microencapsul. 2016; 33(3): 209-220.
[Crossref] [Google Scholar] [Pubmed]
- Bayón-Cordero L, Alkorta I, Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials. 2019; 9(3): 474.
[Crossref] [Google Scholar] [Pubmed]
- Delshadi R, Bahrami A, McClements DJ, Moore MD, Williams L. Development of nanoparticle-delivery systems for antiviral agents: A review. J Control Release. 2021; 331: 30-44.
[Crossref] [Google Scholar] [Pubmed]
- Nasiruddin M, Neyaz M, Das S. Nanotechnology-based approach in tuberculosis treatment. Tuberc Res Treat. 2017.
[Crossref] [Google Scholar] [Pubmed]
- Palmer BC, de Louise LA. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules. 2016; 21(12): 1719.
[Crossref] [Google Scholar] [Pubmed]
- Pinto MF, Moura CC, Nunes C, Segundo MA, Lima SA, Reis S. A new topical formulation for psoriasis: Development of methotrexate-loaded nanostructured lipid carriers. Int J Pharm. 2014; 477(1-2): 519-526.
[Crossref] [Google Scholar] [Pubmed]
- Sonawane R, Harde H, Katariya M, Agrawal S, Jain S. Solid lipid nanoparticles-loaded topical gel containing combination drugs: An approach to offset psoriasis. Expert Opin Drug Deliv. 2014; 11(12): 1833-1847.
[Crossref] [Google Scholar] [Pubmed]
- Korkmaz E, Gokce EH, Ozer O. Development and evaluation of coenzyme Q10 loaded solid lipid nanoparticle hydrogel for enhanced dermal delivery. Acta Pharm. 2013; 63(4): 517-529.
[Crossref] [Google Scholar] [Pubmed]
- Dhillon P, Mirza M, Anwer M, Alshetaili AS, Alshahrani SM, Iqbal Z. Development and optimization of erythromycin-loaded lipid-based gel by Taguchi design: In vitro characterization and antimicrobial evaluation. Braz J Pharm Sci. 2019; 55. [Crossref]
- Kong X, Zhao Y, Quan P, Fang L. Development of a topical ointment of betamethasone dipropionate loaded nanostructured lipid carrier. Asian J Pharm Sci. 2016; 11(2): 248-254.
- Badawi N, El-Say K, Attia D, El-Nabarawi M, Elmazar M, Teaima M. Development of pomegranate extract-loaded solid lipid nanoparticles: Quality by design approach to screen the variables affecting the quality attributes and characterization. ACS omega. 2020; 5(34): 21712-21721.
[Crossref] [Google Scholar] [Pubmed]
- Pham DT, Tran PH, Tran TT. Development of solid dispersion lipid nanoparticles for improving skin delivery. Saudi Pharm J. 2019; 27(7): 1019-1024.
[Crossref] [Google Scholar] [Pubmed]
- Moglad EH, Fatima F, Ahmed MM, Seshadri VD, Anwer MK, Aldawsari MF. Development of topical antibacterial gel loaded with cefadroxil solid lipid nanoparticles: In vivo wound healing activity and epithelialization study. Int J Pharmacol. 2020; 16(4): 298-309.
- Puri A, Bhattaccharjee SA, Zhang W, Clark M, Singh ON, Doncel GF, et al. Development of a transdermal delivery system for tenofovir alafenamide, a prodrug of tenofovir with potent antiviral activity against HIV and HBV. Pharmaceutics. 2019; 11(4): 173.
[Crossref] [Google Scholar] [Pubmed]
- Abdel-Salam FS, Elkheshen SA, Mahmoud AA, Ammar HO. Diflucortolone valerate loaded solid lipid nanoparticles as a semisolid topical delivery system. Bull Fac Pharm. 2016; 54(1): 1-7.
- Carbone C, Fuochi V, Zielińska A, Musumeci T, Souto EB, Bonaccorso A, et al. Dual-drugs delivery in solid lipid nanoparticles for the treatment of Candida albicans mycosis. Colloids Surf B Biointerfaces. 2020; 186: 110705.
[Crossref] [Google Scholar] [Pubmed]
- Elnaggar YS, El-Massik MA, Abdallah OY. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int J Nanomedicine. 2011; 6: 3195.
[Crossref] [Google Scholar] [Pubmed]
- Firdaus S, Hassan N, Mirza MA, Ara T, El-Serehy HA, Al-Misned FA, et al. FbD directed fabrication and investigation of luliconazole based SLN gel for the amelioration of Candidal vulvovaginitis: A 2 T (thermosensitive and transvaginal) approach. Saudi J Biol Sci. 2021; 28(1): 317-326.
[Crossref] [Google Scholar] [Pubmed]
- El-Kamel AH, Al-Fagih IM, Alsarra IA. Testosterone solid lipid microparticles for transdermal drug delivery. Formulation and physicochemical characterization. J Microencapsul. 2007; 24(5): 457-475.
[Crossref] [Google Scholar] [Pubmed]
- Begum M, Shaik NB. Formulation and evaluation of linezolid loaded solid lipid nanoparticles as topical gel. Int J Pharm Sci. 2020; 11: 4924-4933.
[Crossref] [Google Scholar] [Pubmed]
- Odumosu P, Lough J, Yakubu D, Thomas K, Williamson G, Haroune N. Anti-mycobacterial assessment and characterization of 5-O-caffeoylquinic acid methyl ester and rutin from Pavetta crassipes. J Appl Pharm Sci. 2016; 6(10): 001-007.
- Sarma A, Das MK. Formulation by Design (FbD) approach to develop tenofovir disoproxil fumarate loaded Nanostructured Lipid Carriers (NLCs) for the aptness of nose to brain delivery. J drug deliv ther. 2019; 9(2): 148-159.
- Gu Y, Yang M, Tang X, Wang T, Yang D, Zhai G, et al. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration enhancement and transport properties. J Nanobiotechnology. 2018; 16(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Liu X, Zhao Q. Long-term anesthetic analgesic effects: Comparison of tetracaine loaded polymeric nanoparticles, solid lipid nanoparticles, and nanostructured lipid carriers in vitro and in vivo. Biomed Pharmacother. 2019; 117: 109057.
[Crossref] [Google Scholar] [Pubmed]
- Zamarioli CM, Martins RM, Carvalho EC, Freitas LA. Nanoparticles containing curcuminoids (Curcuma longa): Development of topical delivery formulation. Rev bras farmacogn. 2015; 25: 53-60.
- Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharm. 2015; 53(2): 147-159.
- Kumar V. Optimization and evaluation of topical gel containing solid lipid nanoparticles loaded with luliconazole. Int J Pharm Res. 2022.
- El-Say KM, Hosny KM. Optimization of carvedilol solid lipid nanoparticles: An approach to control the release and enhance the oral bioavailability on rabbits. PLoS One. 2018; 13(8): e0203405.
[Crossref] [Google Scholar] [Pubmed]
- Pooja D, Tunki L, Kulhari H, Reddy BB, Sistla R. Characterization, biorecognitive activity and stability of WGA grafted lipid nanostructures for the controlled delivery of rifampicin. Chem Phys Lipids. 2015; 193: 11-17.
[Crossref] [Google Scholar] [Pubmed]
- Krishnatreyya H, Dey S, Pal P, Das PJ, Sharma VK, Mazumder B. Piroxicam loaded Solid Lipid Nanoparticles (SLNs): Potential for topical delivery. Ind J Pharm Ed Res. 2019; 53(2): 82-92.
- Üner M, Karaman EF, Aydoğmuş Z. Solid lipid nanoparticles and nanostructured lipid carriers of loratadine for topical application: Physicochemical stability and drug penetration through rat skin. Trop J Pharm Res. 2014; 13(5): 653-660.
- Khalil RM, Abd El Rahman AA, Kassem MA, El Ridi MS, Abou Samra MM, Awad GE, et al. Preparation and in vivo assessment of nystatin-loaded solid lipid nanoparticles for topical delivery against cutaneous candidiasis. Int J Pharm Pharm Sci. 2014; 8(7): 421-429.
- Kuo YC, Lee CH, Rajesh R. Iron oxide-entrapped solid lipid nanoparticles and poly (lactide-co-glycolide) nanoparticles with surfactant stabilization for antistatic application. J Mater Res Technol. 2019; 8(1): 887-895.
- Parvez S, Yadagiri G, Gedda MR, Singh A, Singh OP, Verma A, et al. Modified solid lipid nanoparticles encapsulated with amphotericin B and paromomycin: An effective oral combination against experimental murine visceral leishmaniasis. Sci Rep. 2020; 10(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Gaur PK, Mishra S, Purohit S. Solid lipid nanoparticles of guggul lipid as drug carrier for transdermal drug delivery. Biomed Res Int. 2013.
[Crossref] [Google Scholar] [Pubmed]
- Mante PK, Adomako NO, Antwi P, Kusi-Boadum NK, Osafo N. Solid-lipid nanoparticle formulation improves antiseizure action of cryptolepine. Biomed Pharmacother. 2021; 137: 111354.
[Crossref] [Google Scholar] [Pubmed]
- Chaudhari PM, Bind VM. Topical solid lipid nanoparticles based gel of lavender essential oil for anti‑inflammatory activity. Asian J Pharm Clin Res. 2019; 12(11): 175-182.
- Pham CV, van MC, Thi HP, Thanh CĐ, Ngoc BT, van BN, et al. Development of ibuprofen-loaded solid lipid nanoparticle-based hydrogels for enhanced in vitro dermal permeation and in vivo topical anti-inflammatory activity. J Drug Deliv Sci Technol. 2020; 57: 101758.
- Londhe V, Save S. Zaltoprofen loaded solid lipid nanoparticles for topical delivery: Formulation design, in vitro and ex vivo evaluation. MOJ Bioequiv Availab. 2017; 4(2): 00065.
- Passos JS, de Martino LC, Dartora VF, de Araujo GL, Ishida K, Lopes LB. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur J Pharm Sci. 2020; 149: 105296.
[Crossref] [Google Scholar] [Pubmed]
- Rapalli VK, Sharma S, Roy A, Alexander A, Singhvi G. Solid lipid nanocarriers embedded hydrogel for topical delivery of apremilast: In-vitro, ex-vivo, dermatopharmacokinetic and anti-psoriatic evaluation. J Drug Deliv Sci Technol. 2021; 63: 102442.
- Arora R, Katiyar SS, Kushwah V, Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin Drug Deliv. 2017; 14(2): 165-177.
[Crossref] [Google Scholar] [Pubmed]
- Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid Lipid Nanoparticles (SLN) versus Nanostructured Lipid Carriers (NLC). Eur J Pharm Biopharm. 2008; 70(2): 633-640.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Ashish B Wadekar1*, Jagdish V Manwar2, Ravindra L Bakal3 and Dipak D Kumbhar42Department of Pharmaceutical Chemistry, IBSS’s Dr. Rajendra Gode College of Pharmacy, Maharastra, India
3Department of Pharmaceutical Chemistry, IBSS’s Dr. Rajendra Gode Institute of Pharmacy, Maharastra, India
4Department of Pharmaceutics, KYDSCT’s College of Pharmacy, Sakegaon, Maharashtra, India
Citation: Wadekar AB: Mechanisms and Pharmaceutical Application of Solid Lipid Nanoparticles as Efficient Drug Delivery System
Received: 01-Nov-2022 Accepted: 25-Nov-2022 Published: 02-Dec-2022, DOI: 10.31858/0975-8453.13.12.862-869
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3