Review Article - (2021) Volume 12, Issue 12
Nanofibrils-Current and Future Aspects
Deepika B*, K. Jyothi Priya, M. Bhavana and U. Naga Mohana KumariAbstract
This review summarizes the preparation methods of cellulose nanofibrils (CNFs) and focuses on the mechanical treatment of cellulose, the surface modification of fibrillated fibers during pretreatment, the surface modification of nanocellulose and the modification of CNFs and their functional application. In the past five years, research on cellulose nanofibrils has progressed with developments in nanomaterial’s research technology. However, owing to its high energy consumption, high cost and challenging industrial production, the applications of nanocellulose remain limited. In addition, although nanofibrils exhibit strong biocompatibility and barrier and mechanical properties, their high hydrophilicity limits their practical application. Cellulose nanofibrils have mainly focused on the industrial production of CNFs, their pretreatment and functional modification and their compatibility with other biomass materials. In the future, with the rapid development of modern science and technology, the demand for biodegradable biomass materials will continue to increase. Furthermore, bio-based nanomaterials are expected to advance in the direction of functionalization and popularization.
Keywords
Nanofibrils, Cellulose nanofibrils, Nanomaterials
Introduction
Nanotechnology is the science concerned with the study of the phenomena and functions of matter within the dimensional range of 0.1-100 nm. A nano-meter abbreviated as nm, is a unit for length the measures on billionth of a meter (Wang W, et al., 2015) unusual and unique properties of nano-scale materials arise from their exhibited profile compared to macro materials. Novel properties exhibited by these materials investigated from their minute dimension which converted into unusual mechanical, thermal, biological, optical, magnetic and electrical properties (Zhang L, et al., 2015). Nano materials is currently under investigation in different fields such as self-assembly and thin films, quantum dots, nano-fibers, nanorods, nanotubes, nanowires, nanocrystals and nanofoams (de Castro DO, et al., 2015).
Nano-fiber technology incorporated in different application area such as batteries and fuel cells, capacitors, transistors and diodes, system for energy transfer, composites for aerospace structures, drug delivery and tissue engineering. Nano-fiber technology is a branch of nanotechnology whose primary objective is to create materials in the form of nanoscale fibere in order to achieve superior function. The unique combination of high specific surface area, flexibility and superior directional strength makes such fiber a preferred material for much application ranging from clothing to reinforcements for aerospace structure. The effect of fiber diameter on the performance and process ability of fibrous structures has long been recognized. Several scientists such as professor Parallel Reneker almost a decade ago had popularized the electro-spinning technology (Deepa B, et al., 2016). Although, the first patent on electro-spinning technique was published as early as in 1934 (Ramarajua B, et al., 2015), this technique has not been well established until recent times (Ma H, et al., 2015). Technically, electro-spinning is a process that uses a strong electric field to draw a polymer fluid into the filaments (Xia Y, et al., 2014).
Cellulose nanofibrils (CNFs), which have a diameter of 10-50 nm and a length of several micrometers (Wang W, et al., 2015; Wang Y, et al., 2014), have been prepared from lignocelluloses biomass. Due to their exceptional mechanical properties and high aspect ratio, they have attracted much attention for applications in films (Zhang L, et al., 2015), composite materials and electronics (Deepa B, et al., 2016). Although CNFs demonstrate excellent functionality, they have not yet been industrialized. The industrial production of CNFs has been mainly hindered by two factors. Firstly, CNFs are primarily manufactured by high-pressure homogenizers, high-energy ball mills (mechanical chemistry), micro fluidizers, and ultra-low temperature crushing and other methods. However, because of the relatively high lengths and diameters of CNFs, their specific surface area is large, the surface hydroxyl groups easily form hydrogen bonds and the fibers easily form hard agglomerations and are difficult to disperse. Simply relying on mechanical force to shear and separate the fibers generates a lot of energy consumption. In addition, the instantaneous high temperature generated during mechanical grinding affects the CNFs’ crystal structure, destroys their network structure and changes the gel behavior of the nanofibrils. Secondly, the fibrillation of cellulose must be completed in liquid and, after drying, irreversible hydrogen bonding occurs between the CNF fibers, that is, keratinization (Xia Y, et al., 2014).
Literature Review
Chemistry
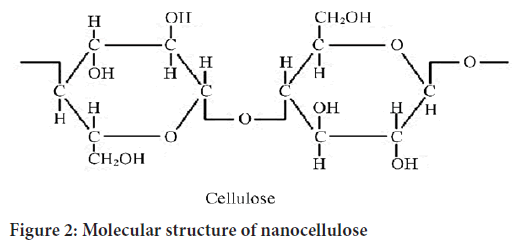
Its molecular structure is shown in Figure 1. The molecular backbone of cellulose is a linear rigid chain linked by β-D-glucopyranose (AGU) through 1,4-glycosidic bonds in a chair- shaped conformation (Deepa B, et al., 2016). The presence of tri-and hydroxyl groups, resulting from the strong hydrogen bonds composed of polyhydroxyl groups, indicate that the cellulose has a high cohesive microfiber network structure and the hydrogen bonding effect makes cellulose difficult to dissolve in common solvents (Wang W, et al., 2015; Wang S, et al., 2016; Ramarajua B, et al., 2015). In 1982, Turbak et al. used a high-pressure homogenizer to extract nanocellulose, namely cellulose nanofibrils (CNFs), from eucalyptus pulp (de Castro DO, et al., 2015; Ma H, et al., 2014). With advancing research, CNFs have exhibited high transparency, excellent mechanical properties and good biocompatibility. They have been gradually applied to antibacterial packaging, corrosion inhibitor carriers, gels, transparent conductive films, 3D printing, composite materials and drug delivery (Xia Y, et al., 2014). At the same time, compared with other nanocellulose fibers, CNFs have a simple preparation process. CNFs were developed earlier and are currently the main target for the industrial production of nanocellulose. This review focuses on nanocellulose fibrils (Figure 2).
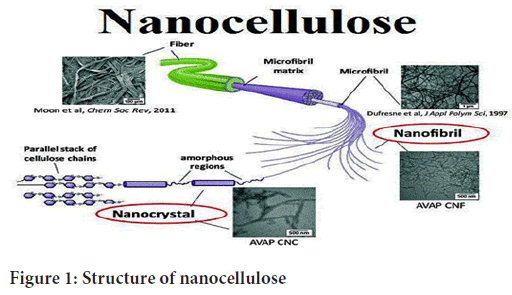
Figure 1: Structure of nanocellulose
Figure 2: Molecular structure of nanocellulose
Advantages of nanofibrils
• Low specific weight results in higher specific strength and stiffness than glass
• Renewable resources, production requires little energy, low CO2 emissions
• Friendly processing, no wear of tools, and no skin irritation
• Production with low investment at low cost good electrical resistance good thermal and acoustic insulating properties
• Biodegradable
Disadvantages of nanofibrils
• Lower strength, especially impact strength
• Variable quality, influenced by weather
• Poor moisture resistance, which causes swelling of fibers
Cellulose raw materials and CNFs
Plant cellulose mainly exists in the cell walls of plant fibers. According to the order of the cell wall formation, the chemical composition and structure varies and can be divided into the intercellular layer cell wall (M), primary wall (P) and the secondary wall (S). The intercellular layer is composed of linked cells and acts as a buffer between the cell structures. It does not contain cellulose and is instead mainly composed of lignin, hemicellulose and pectic substance formation (Gregorczyk K and Knez M, 2016). The primary wall is mainly secreted by the cell protoplast and is composed of cellulose, hemicellulose and pectic substances, demonstrating good elasticity and plasticity. The secondary wall is secreted by the protoplast in the primary wall via deposit growth. Cells stop growing when the secondary wall is formed. The secondary wall is approximately 5-10 m thick and generally consists of three layers: The outer layer of the secondary wall (S1), the middle layer of the secondary wall (S2) and the inner layer of the secondary wall (S3). The cellulose raw materials used to extract CNFs can come from wood, seed fibers (cotton fiber, cotton lint, kapok, etc.), plant bast fibers (hemp, flax, abaca, etc.) and various herbs (bagasse, straw, bamboo fiber) (Zhang L, et al., 2015). The average degree of polymerization of wood cellulose is approximately 10,000, that of cotton fiber is slightly higher at approximately 15,000 and that of herbal cellulose is slightly lower. In terms of fiber length, bast fiber has the longest fiber length, which can reach 120-180 mm and herbaceous straw has the shortest fiber length at approximately 1-2 mm. The fiber length for wood is approximately 3-5 mm. For the cellulose content, the cellulose content of the seed fibers, such as cotton, is the highest, usually above 95%, while wood fibers originally contain more impurities such as lignin and ash. The fiber content and fiber length of softwoods are higher than hardwoods. Generally, cellulose in raw plant materials mainly exists in mature plant cells (Zhang F, et al., 2015). Owing to the different physiological functions of plant cells, the thick-walled cells (fibers) of plants are most commonly used in production to reduce fiber fragments in CNFs products. For example, they can be used to remove parenchyma cells, stone cells, reticular wall cells and other structures. Generally, the higher the ratio of raw fiber materials used to prepare CNFs, the slenderer and uniform the single fibers, the lower the content of miscellaneous cells and the better the strength and fibrillation degree of the CNFs. Therefore, high-quality nanofibers are mostly made of pure cotton fibers (Zhang F, et al., 2015). The primary cell wall is approximately 30-1000 nm thick and contains cellulose microfibrils (MF) located crosswise. The secondary cell wall consists of three layers (S1, S2 and S3), which differ in microfibrils angle with respect to the fiber axis. In these layers the microfibrils are aligned parallel and are packed densely in a flat helix. The secondary wall contains most of the cellulose mass in the fiber and has a thickness varying from 100 nm (cotton) to 300 nm (spruce wood). The warty layer is a thin layer located in the inner surface of the cell wall mainly composed of lignin and hemicellulose. As described above, cellulose is present in the fibers in form of the microfibrils. For instance, in the spruce wood the microfibrils have a diameter of 10-20 nm. These microfibrils in turn are made of elementary fibrils, which are commonly considered as the smallest morphological units in the fiber (Zhao J, et al., 2015). However, recent studies (Khalil HPSA, et al., 2014) showed that it is possible to extract single cellulose polymer chains or 2 × 2 chain-packing structures during CNF production. The diameter of elementary fibrils typically varies in the range of 3-35 nm depending on cellulose source (Ching YC, et al., 2016), and is in the range of 2.2-3.6 nm for spruce wood (Wang Y, et al., 2014). Both cellulose microfibrils and elementary fibrils are referred to as cellulose nanofibrils. However, it is commonly desired to obtain individual elementary fibrils with a regular diameter, rather than their (or microfibril) bundles. Cellulose is a semicrystalline polymer comprising crystalline (ordered) and amorphous (disordered) regions within the microfibril, where the individual cellulose molecule is considered to pass through several crystalline and amorphous parts according to pulp aqueous suspension several times through a high-pressure homogenizer (Ayad MM, et al., 2014). During such treatment, strongly entangled networks of nanofibrils, having both crystalline and amorphous domains, are produced due to high shearing forces. They possess high aspect ratio and form gels in water with shear-thinning and thixotropic behavior (Zhao R, et al., 2014). Depending on the processing conditions, cellulose fibers can be disintegrated to flexible CNF with lateral dimensions starting from 5 nm, representing elementary fibrils, to tens of nanometers, which correspond to single microfibrils and their bundles. Typically, CNF have a diameter of 5-50 nm and a length of few micrometer (Agarwal S, et al., 2013).
Nanocrystalline cellulose usually refers to tiny fibers with a diameter of less than 100 nm, which is the smallest physical structural unit in cellulose. Nanocrystalline cellulose can be divided into three types according to the different raw materials and processing methods: CNFs, Crystalline Nano Cellulose (CNC) and Bacterial Nano Cellulose (BNC). CNFs are cellulose prepared by mechanical methods or mild pretreatments for cellulose fibrillation (Persano L, et al., 2015). CNC makes use of the strong physical and chemical properties resulting from the removal of the cellulose amorphous area. Generally, the product CI of CNC is high its diameter is 5-70 nm, its length is 100-250 nm and its aspect ratio is low (Jiang T, et al., 2014). BNC is a kind of high- purity ribbon, like nanocellulose, which is synthesized by bacteria and other microorganisms, with a diameter of 20-100 nm and a length of several microns. Generally, CNFs is a cellulose structure with a higher aspect ratio of 5-50 nm in diameter and several microns in length; moreover, owing to the effect of high shear force in the production process, the fibers are entangled with each other to form a microfiber network structure, which easily forms a gel in aqueous solution (Huang ZM, et al., 2004). Cellulose fiber is a kind of highly cohesive, multi-level structure, composed of a beta-1,4 glycosidic bond of a macromolecular, polysaccharide, rigid chain that, through intermolecular hydrogen bonding, intertwines to form a nanofiber by combining with a larger hemicellulose microfiber structure shows the fibrillation process from lignocellulose to cellulose nanofibrils and the crystal structure inside the cellulose nanofibrils. The primary cell wall is approximately 30- 1000 nm thick and contains cellulose microfibrils (MF) located crosswise (Zhang Y, et al., 2005). The secondary cell wall consists of three layers (S1, S2 and S3), which differ in microfibrils angle with respect to the fiber axis. In these layers the microfibrils are aligned parallel and are packed densely in a flat helix. The secondary wall contains most of the cellulose mass in the fiber and has a thickness varying from 100 nm (cotton) to 300 nm (spruce wood). The warty layer is a thin layer located in the inner surface of the cell wall mainly composed of lignin and hemicellulose (Li D and Xia Y, 2004). As described above, cellulose is present in the fibers in form of the microfibrils. For instance, in the spruce wood the microfibrils have a diameter of 10-20 nm. These microfibrils in turn are made of elementary fibrils, which are commonly considered as the smallest morphological units in the fiber (Greiner A, et al., 2007). However, recent studies (Murat S, et al., 2016) showed that it is possible to extract single cellulose polymer chains or 2 × 2 chain-packing structures during CNF production. The diameter of elementary fibrils typically varies in the range of 3-35 nm depending on cellulose source (Jiang G and Qin X, 2014), and is in the range of 2.2-3.6 nm for spruce wood (Koo BR, et al., 2014). Both cellulose microfibrils and elementary fibrils are referred to as cellulose nanofibrils. However, it is commonly desired to obtain individual elementary fibrils with a regular diameter, rather than their (or microfibril) bundles. Cellulose is a semicrystalline polymer comprising crystalline (ordered) and amorphous (disordered) regions within the microfibril, where the individual cellulose molecule is considered to pass through several crystalline and amorphous parts according to pulp aqueous suspension several times through a high-pressure homogenizer (Starr JD and Andrew JS, 2013). During such treatment, strongly entangled networks of nanofibrils, having both crystalline and amorphous domains, are produced due to high shearing forces. They possess high aspect ratio and form gels in water with shear-thinning and thixotropic behavior (Kayaci F, et al., 2014). Depending on the processing conditions, cellulose fibers can be disintegrated to flexible CNF with lateral dimensions starting from 5 nm, representing elementary fibrils, to tens of nanometers, which correspond to single microfibrils and their bundles. Typically, CNF have a diameter of 5-50 nm and a length of few micrometer.
Discussion
Structure and chemistry
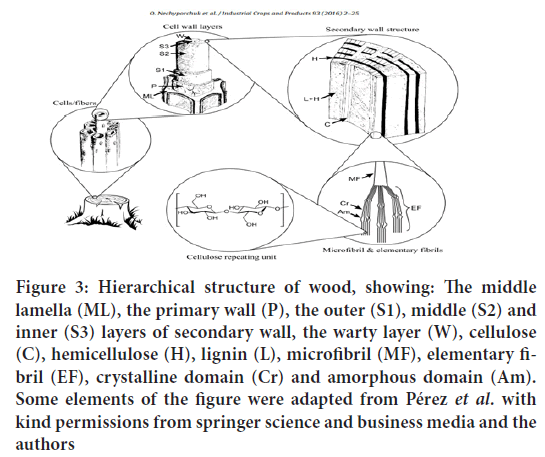
Cellulose can be found in some algae, fungi, tunicates etc. However, commercial cellulose production concentrates on harvested sources such as wood, annual plants or agricultural residues (Figure 3). Besides cellulose, they contain hemicelluloses, lignin, and a comparably small amount of extractives. In such products, cellulose is represented as a well-organized architecture of febrile elements composing cells. Cellulose is the primary structural component responsible for their mechanical strength. Each cell represents a fiber with a width of 10-50 μm (depending on the source), consisting of cell wall layers, which have the total thickness of 1-5 μm. These layers differ from each other with regard to their structure and chemical composition. The cell wall is composed of several layers the Middle Lamella (ML), the primary wall (P), the outer (S1), middle (S2) and inner (S3) layers of secondary wall and the warty layer (W). The middle lamella binds the neighboring cells because of the presence of high amount of lignin. The primary and secondary walls contains three main components: cellulose, hemicellulose, and a matrix, typically, composed of pectin in the primary wall and lignin in the secondary wall (Zhao J, et al., 2015).
Figure 3: Hierarchical structure of wood, showing: The middle lamella (ML), the primary wall (P), the outer (S1), middle (S2) and inner (S3) layers of secondary wall, the warty layer (W), cellulose (C), hemicellulose (H), lignin (L), microfibril (MF), elementary fibril (EF), crystalline domain (Cr) and amorphous domain (Am). Some elements of the figure were adapted from Pérez et al. with kind permissions from springer science and business media and the authors
The primary cell wall is approximately 30-1000 nm thick and contains cellulose microfibrils (MF) located crosswise. The secondary cell wall consists of three layers (S1, S2 and S3), which differ in microfibrils angle with respect to the fiber axis. In these layers the microfibrils are aligned parallel and are packed densely in a flat helix. The secondary wall contains most of the cellulose mass in the fiber and has a thickness varying from 100 nm (cotton) to 300 nm (spruce wood). The warty layer is a thin layer located in the inner surface of the cell wall mainly composed of lignin and hemicellulose (Tran C and Kalra V, 2013). As described above, cellulose is present in the fibers in form of the microfibrils. For instance, in the spruce wood the microfibrils have a diameter of 10-20 nm. These microfibrils in turn are made of elementary fibrils, which are commonly considered as the smallest morphological units in the fibre (Li F, et al., 2014). However, recent studies showed that it is possible to extract single cellulose polymer chains or 2 × 2 chain-packing structures during CNF production. The diameter of elementary fibrils typically varies in the range of 3-35 nm depending on cellulose source, and is in the range of 2.2-3.6 nm for spruce wood (Khalil HPSA, et al., 2014).
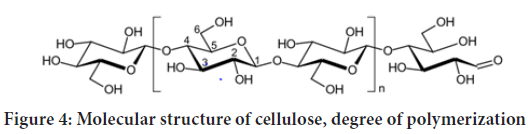
Both cellulose microfibrils and elementary fibrils are referred to as cellulose nanofibrils. However, it is commonly desired to obtain individual elementary fibrils with a regular diameter, rather than their (or microfibril) bundles. Cellulose is a semicrystalline polymer comprising crystalline (ordered) and amorphous (disordered) regions within the microfibril, where the individual cellulose molecule is considered to pass through several crystalline and amorphous parts according to fringe micelle theory. The native degree of crystallinity is usually in the range of 40%-70% and depends on the origin of cellulose and the isolation method. The building blocks of cellulose polymer chain are D-glucopyranose (glucose) molecules. When linked together by β-1,4-glucosidic bonds they turn into anhydroglucose units. Two anhydroglucose units compose anhydrocellobiose, which is the repeating unit of cellulose polymer. Nevertheless, cellulose degree of polymerization (DP) is usually expressed as a number of anhydroglucose units (Figure 4). Each anhydroglucose unit has six carbon atoms with three hydroxyl (viz., alcohol) groups (at C2, C3, and C6 atoms), giving a high degree of functionality to cellulose molecule.
Figure 4: Molecular structure of cellulose, degree of polymerization
There are three types of anhydroglucose units: (i) a reducing end with free hemiacetal or aldehyde group at C1 atom, (ii) a non-reducing end with free alcohol group at C4 atom, and (iii) internal rings. Due to the molecular structure, cellulose bears such properties as hydrophilicity, insolubility in water and most organic solvents, degradability and chirality. The DP of cellulose varies depending on the cellulose source (e.g., 10,000 in native wood; 20,000 in cotton; 44,000 in Valonia) and the isolation/purification method (e.g., 200-500 in regenerated cellulose, 1000 in bleached kraft pulps (Ching YC, et al., 2016).
Conclusion
This review discusses the most recent key developments of using CNFs in the fields of pharmaceutical science. The advantages of the CNs over other nanomaterials are remarkable, that they are friendly to environment and human beings; the vast abundance and recyclability make them easily available and unexpendable. In addition, the large amount of hydroxyl groups on the surface make the CNs modifiable and tunable, which are extremely important for the processing in the pharmaceutical science and pharmacology. The applications of CNs have been found in a variety of fields. As CNs experience an aggressive exploration, prospective and challenges co-exist, while we foresee another round of trends of nanomaterials going greener and more applicable.
References
- Wang W, Sabo RC, Mozuch MD, Kersten P, Zhu JY, Jin Y. Physical and mechanical perties of cellulose nanofibril films from bleached eucalyptus pulp by endoglucanase treatment and microfluidization. J Polym Environ. 2015; 23: 551-558.
- Zhang L, Liu Z, Cui G, Chen L. Biomass-derived materials for electrochemical energy storages. Prog Polym Sci. 2015; 43: 136-164.
- de Castro DO, Frollini E, Ruvolo-Filho A, Dufresne A. “Green polyethylene” and curaua cellulose nano-crystal based nanocomposites: Effect of vegetable oils as coupling agent and processing technique. J Polym Sci B Polym Phys. 2015; 53(14): 1010-1019.
- Deepa B, Abraham E, Pothan LA, Cordeiro N, Faria M, Thomas S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials. 2016; 9: 1-11.
- Wang S, Lu A, Zhang L. Recent advances in regenerated cellulose materials. Prog Polym Sci. 2016; 53: 169-206.
- Ramarajua B, Imae T, Destaye AG. Ag nanoparticle-immobilized cellulose nanofibril films for environmental conservation. Appl Catal A-Gen. 2015; 492: 184-189.
- Ma H, Burger C, Hsiao BS, Chu B. Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J Membr Sci. 2014; 454: 272-282.
- Xia Y, Wang Y, Fang HHP, Jin T, Zhong H, Zhang T. Thermophilic microbial cellulose decomposition and methanogenesis pathways recharacterized by metatranscriptomic and metagenomic analysis. Scientific Reports. 2014; 4(1): 1-9.
- Gregorczyk K, Knez M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Prog Mater Sci. 2016; 75: 1-37.
- Zhang F, Wu W, Sharma S, Tong G, Deng Y. Synthesis of cyclodextrin-functionalized cellulose nanofibril aerogel as a highly effective adsorbent for phenol pollutant removal. BioResources. 2015; 10(4): 7555-7568.
- Zhao J, Lu C, He X, Zhang X, Zhang W, Zhang X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl Mater Interfaces. 2015; 7: 2607-2615.
- Khalil HPSA, Davoudpour Y, Islam MN, Mustapha A, Sudesh K, Dungani R, et al. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydrate Polymers. 2014; 99: 649-665.
- Ching YC, Ali ME, Abdullah LC, Choo KW, Kuan YC, Julaihi SJ, et al. Rheological properties of cellulose nanocrystal-embedded polymer composites: A review. Cellulose. 2016; 23: 1011-1030.
- Wang Y, Zhang X, He X, Zhang W, Zhang X, Lu C. In situ synthesis of MnO2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohydr Polym. 2014; 110: 302-308.
- Ayad MM, Salahuddin NA, Minisy IM, Amer WA. Chitosan/polyaniline nanofibers coating on the quartz crystal microbalance electrode for gas sensing. Sens Actuators B. 2014; 202: 144-153.
- Zhao R, Li X, Sun B, Zhang Y, Zhang D, Tang Z, et al. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int J Biol Macromol. 2014; 68: 92-97.
- Agarwal S, Greiner A, Wendorff JH. Functional materials by electro spinning of polymers. Prog Polym Sci. 2013; 38: 963-991.
- Persano L, Camposeo A, Pisignano D. Active polymer nanofibers for photonics, electronics, energy generation and micromechanics. Prog Polym Sci. 2015; 43: 48-95.
- Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog Polym Sci. 2015; 46: 1-24.
- Huang ZM, Zhang YZ, Ramakrishna S, Lim CT. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer. 2004; 45(15): 5361-5368.
- Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electro spinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res Part B. 2005; 72B(1): 156-165.
- Li D, Xia Y. Electrospinning of nanofibers: Reinventing the wheel? Adv Mater. 2004; 16(14): 1151-1170.
- Greiner A, Wendorff JH. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew Chem Int Ed. 2007; 46(30): 5670-5703.
- Murat S, Zakir MOR, Ulviya B. Multifunctional electroactive electrospun nanofiber structures from water solution blends of PVA/ODA-MMT and poly (maleic acid-alt-acrylic acid): Effects of Ag, organoclay, structural rearrangement and NaOH doping factors. Adv Nat Sci Nanosci Nanotechnol. 2016; 7: 025009.
- Jiang G, Qin X. An improved free surface electrospinning for high throughput manufacturing of core-shell nanofibers. Mater Lett. 2014; 128: 259-262.
- Koo BR, Park IK, Ahn HJ. Fe-doped In2O3/-Fe2O3 core/shell nanofibers fabricated by using a co-electrospinning method and its magnetic properties. J Alloys Compd. 2014; 603: 52-56.
- Starr JD, Andrew JS. A route to synthesize multifunctional tri-phasic nanofibers. J Mater Chem C. 2013; 1: 2529-33.
- Kayaci F, Vempati S, Ozgit-Akgun C, Biyikli N, Uyar T. Enhanced photocatalytic activity of homoassembled ZnO nanostructures on electrospun polymeric nanofibers: A combination of atomic layer deposition and hydrothermal growth. Appl Catal B. 2014; 156: 173-183.
- Tran C, Kalra V. Co-continuous nanoscale assembly of Nafion-polyacrylonitrile blends within nanofiber: A facile route to fabrication of porous nanofiber. Soft Matter. 2013; 9(3): 846-852.
- Li F, Kang Z, Huang X, Zhang GJ. Fabrication of zirconium carbide nanofiber by electro spinning. Ceram Intern. 2014; 40(7): 10137-10141.
Author Info
Deepika B*, K. Jyothi Priya, M. Bhavana and U. Naga Mohana KumariReceived: 25-Jun-2021 Accepted: 09-Jul-2021 Published: 16-Jul-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3