Review Article - (2022) Volume 13, Issue 4
Abstract
A variety of novel phenyl-pyrazoline-coumarin compounds were developed and synthesized to produce new anti-inflammatory medicines with improved pharmacological characteristics. All of the substances were tested for anti-inflammatory efficacy by measuring how well they inhibited LPS-induced Interleukin (IL)-6 productions. Compound 4m had the best anti-inflammatory action, decreasing IL-6, Tumor Necrosis Factor (TNF), and nitric oxide (NO) synthesis when induced by Li-popolysaccharide (LPS). Thr -kB ough the Nuclear Factor (NF-kB)/Mitogen-Activated Protein Kinase (MAPK) signaling pathway, title chemical 4m was found to dramatically decrease the expression of Nitric Oxide Synthase (iNOS), cox-2 (COX-2) and the production of IL-6, TNF-a , and NO. Carrageenan-induced paw edema was used to test compound 4m's anti-inflammatory efficacy the development of novel COX-2 inhibitors with high efficacy and an improved safety profile would be a big step forward towards anti-inflammatory drug research. The purpose of this study was to compare indomethacin and celecoxib in order to track and assess the anti-inflammatory and anti-microbial characteristics of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), as well as some of the expected side effects of pyrazoline derivative products. An in vivo Cyclooxygenase (COX) decrease experiment was used to assess the efficiency of new pyrazoline and pyrazoline compounds in suppressing ovine COX-1/COX-2 enzymes. Different heterocyclic' reactions and a number of outcomes were obtained using hydrazine hydrate in ethanol. 3,5-diaryl-2-pyrazoline derivatives are a class of 3,5-diaryl- 2-pyrazoline derivatives. A collection of NO-donating- 2-pyrazoline derivatives was generated by grafted a nitrate ester or an oxime group onto synthesized pyrazoline derivatives via various spacers. Proclivity to harm toxicity in the gastrointestinal tract the addition of the NO-donating ring to the mother pyrazoline compounds led in a non-significant loss in anti-inflammatory activity but a significant reduction in the gastrointestinal ulcers caused by the parent pyrazolines.
Keywords
Nitric oxide, Coumarin, Pyrazoline, Phenyl ring, Cyclooxygenase-2, Anti-inflammatory, Prostaglandin, Inflammation
Introduction
The reaction of different chalcones with hydrazine hydrate in ethanol yielded a series of 3,5-diaryl-2-pyrazoline derivatives. By grafting a nitrate ester or an oxime group onto produced pyrazoline derivatives via various spacers, a set of NO-donating- 2-pyrazoline derivatives was created. Coumarin and Pyrazoline with an oxime group or a nitrate ester group4 substituted at the 1 position of the pyrazoline have nitric oxide donor activity. This inspired us to begin working on the synthesis of derivatives in which the Coumarin ring and nitrate ester group are fixed substituents connected to the Pyrazoline ring at positions 3 and 1, respectively, while the other substituted pyrazoline's phenyl ring is at position (Shoman ME, et al., 2009) (Figure 1).
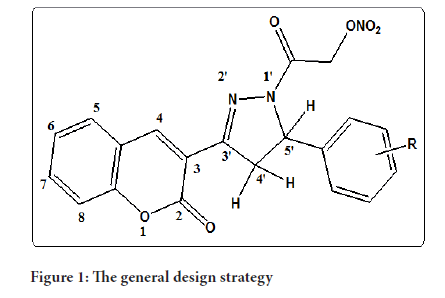
Figure 1:The general design strategy
NSAIDs (Nonsteroidal Anti-Inflammatory Drugs) are one of the most popular often prescribed the world's drugs. They are most widely used treatment for the inflammation and pain associated with arthritic conditions (Mohyel‐Din MM, et al., 2011). Illnesses of the cardiovascular and inflammatory systems, cancer, and ageing have all been linked to nitric oxide species. Efforts to mitigate the damage that these species do are gaining traction as a foundation for new treatment methods (Abdel-Aziz M, et al., 2009). When Cyclooxygenase is inhibited in thrombocytes, the generation Thromboxane A2 is a kind of thromboxane that is produced by the body is reduced. This phenomenon causes bleeding to take longer and platelet aggregation to be inhibited. Bronchoconstriction, which can result in asthmatic symptoms, is a serious adverse effect of NSAIDs. The bronchoconstriction impact of NSAIDs there looks to be caused by a decrease in bronchodilation Prostaglandin E2 (PGE2) and a shift in the biosynthetic enzymes from the Cyclooxygenase route to the Cyclooxygenase pathway on the one hand, and a change throughout the biosynthetic enzymes from the Cyclooxygenase route to the inhibition pathway on the other (Dannhardt G and Kiefer W, 2001).
Literature Review
Novel nitric oxide donating pyrazoline
Compounds containing pyrazoline moiety as a core structure exhibit a wide range of biological Activities. Nitric oxide has low solubility in water and is unstable in the presence of various oxidants. It makes it difficult to introduce as such into biological systems in a controlled or specific fashion. There for development of chemical agents that release NO is important. Pyrazoline com-pounds have been identified as potent COX inhibitors with good pharmacokinetic profiles. These reports prompted us to undertake the synthesis of some novel pyrazoline with NO donating properties. Combining of two bioactive molecules (Pyrazoline and NO) into one structure with enhanced biological activities (Goyal A and Jain S, 2011).
COX-Inhibiting Nitric Oxide Donors (CINODs)
The COX-Inhibiting Nitric Oxide Donors (CINODs) are a new class of agents designed for the treatment of pain and inflammation. CINODs have a multi-pathway mechanism of action that involves COX inhibition and nitric oxide donation. The anti-inflammatory and analgesic effects of COX inhibition are reinforced through inhibition of caspase-1 regulated cytokine production, while nitric oxide donation provides multiorgan protection. Whereas both conventional Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and COX-2-selective NSAIDs are associated with a variety of adverse effects on the renal system, such as hypertension and edema, CINODs may offer an improved renal safety profile. These agents are devoid of hypertensive effects in animal models and their mechanism of action suggests that they may not cause edema. CINODs also have other renal-sparing effects, being better tolerated than NSAIDs in models of kidney failure. CINODs have been shown to prevent platelet activation in vitro and exhibit anti thrombotic activity in vivo. In animal models of ischemia/reperfusion, CINODs treatment results in improved recovery of heart contractility and reduced left ventricular end-diastolic pressure, in contrast to the effects of aspirin. The combination of improved analgesia reduced gastrointestinal toxicity and cardio renal protection has been established in animal models, and early clinical results suggest a favorable gastrointestinal safety profile in humans. The potential for CINODs to provide cardio renal protection in humans is currently being investigated (Muscará MN and Wallace JL, 2006).
Pyrazoline-coumarins and nitric oxide and anti-inflammatory activity
To develop new anti-inflammatory agents with improved pharmaceutical profiles, a series of new phenyl-pyrazoline-coumarin derivatives were designed and synthesized. Compounds were determined by X-ray crystallography. All of the compounds have been screened for their anti-inflammatory activity characterized by evaluating their inhibition against LPS-induced IL-6 release. Among them, compound 4m showed the highest anti-inflammatory activity with inhibiting IL-6, TNF- α and Nitric Oxide (NO) production Lipopolysaccharide (LPS)-stimulated. The further study showed that title compound 4m could significantly suppress expressions of Nitric Oxide Synthase (iNOS), Cyclooxygenase-2 (COX- 2) and the productions of IL-6, TNF- α, NO through NF-kB/MAPK signaling pathway. The anti-inflammatory activity of compound 4m was determined by carrageenan induced paw edema. Furthermore in vivo evaluation results indicated that compound 4m could inhibit Acid Acid (AA)-induced rat ankle joints (Chen LZ, et al., 2017).
Role of nitric oxide in inflammatory diseases
Nitric oxide (NO) is a signaling molecule that plays a key role in the pathogenesis of inflammation. It gives an anti-inflammatory effect under normal physiological conditions. On the other hand, NO is considered as a pro-inflammatory mediator that induces inflammation due to over production in abnormal situations. NO is synthesized and released into the endothelial cells by the help of NOSs that convert arginine into citrulline producing NO in the process. Oxygen and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) are necessary co-factors in such conversion. NO is believed to induce vasodilation in cardiovascular system and furthermore, it involves in immune responses by cytokine-activated macrophages, which release NO in high concentrations. In addition, NO is a potent neurotransmitter at the neuron synapses and contributes to the regulation of apoptosis. NO is involved in the pathogenesis of inflammatory disorders of the joint, gut and lungs. Therefore, NO inhibitors represent important therapeutic advance in the management of inflammatory diseases. Selective NO biosynthesis inhibitors and synthetic arginine analogues are proved to be used for the treatment of NO-induced inflammation. Finally, the undesired effects of NO are due to its impaired production, including in short: Vasoconstriction, inflammation and tissue damage (Sharma JN, et al., 2007).
Undesired effects of NO
The undesired effects of NO are due to over or impaired production of such mediator and the affected endothelium becomes, as a result, damaged or dysfunctional. The following effects can result in vasoconstriction (e.g., coronary vasospasm, elevated systemic vascular resistance, hypertension), platelet aggregation and adhesion, which can lead to thrombosis, up regulation of leukocyte and endothelial adhesion molecules leading to enhanced inflammation, vascular stenosis or restenosis as occurs following balloon angioplasty and stent placement and increased inflammation and tissue damage mediated by Reactive Oxygen Species such as superoxide anion and hydroxyl radical (Sharma JN, et al., 2007).
Physiological role of nitric oxide
mtNOS-derived NO effectively controls mitochondrial respiration, O2 consumption, trans-membrane proton gradient and potential and Adenosine Triphosphate (ATP) synthesis Physiologic NO levels acutely and reversibly bind to and inhibit several ETC complexes, the most sensitive target being Complex IV very high NO levels, generated upon inflammatory iNOS induction, compete with O2, engendering NO-dependent hypoxia Nitrox promotes the generation of high levels of Reactive Oxygen Species (ROS)/Reactive Nitrogen Species (RNS) NO/RNS can then shut down mitochondrial respiration at multiple sites by irreversibly inhibiting Electron Transport Chain (ETC) complexes at the expense of Adenosine Triphosphate (ATP) production, with cytotoxic effect (Levine AB, et al., 2012).
The Cyclooygenase-2 system as a therapeutic target in inflammation
COX-2 is induced (and the kinetics are comparable) (NOS-2) By targeting the inflammatory location with It was also a source of nitric oxide donors discovered that substances containing nitric oxide funders have more efficacy than professionally prescribed drugs. NO NSAIDs, or COX-inhibiting vasodilator funders, Non-Steroidal Anti-Inflammatory Medicines (NSAIDs) are a new family of pharmaceuticals (NSAIDs) that are anticipated to be faster than smoking Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). The creation of nitric oxide-donating drugs is among the most important efforts for reducing NSAID adverse effects Analgesics (NSAIDs) capable of manufacturing a radical micro and neuroprotective agent NO has the capacity to improve the flow of blood via the mucous membranes which increases mucous membrane resistance to pressure ulcers; also it reduces lymphocyte adhesion to endothelial cells; it modulates mucus and bicarbonate secretion in the stomach; and it protects renal activity by enhancing renal blood flow. COX-2 was involved in numerous of processes physiological and pathological activities additionally to its role in inflammation areas. COX-2 is a type of enzyme that is produced in the body a high level in the developing kidneys and brain, where it aids in their development and function. COX-2 expression has also been discovered to be high in the kidneys during salt restriction, in the microglial of cognitive center inside the hippocampal formation in alzheimer's disease, and in gastrointestinal adenomas and colon cancers. The next sections go through some specifics (Dannhardt G and Kiefer W, 2001). During inflammation, PGEs generate plasma exudation, irritation, and discomfort in a spontaneous way with the participation of an enzyme and another component. Arthritis is a type of inflammatory in animals that results in the induction of COX-2, which is considered to be the cause of the increase in PG production. Human cartilage has been impacted by COX-2 induction in the treatment of rheumatoid arthritis and osteoarthritis. PGEs produce pain by sensitizing peripheral sensory nerve terminals near the site of inflammation. COX products are also assumed to be accountable for transmitting of medication through the spinal cord (Vishwakarma KR and Negi DS, 2020).
The Cyclooxygenase system in inflammation, pain
NSAIDs are useful in treating inflammatory pain, which is triggered by the discovery of painful stimuli (nociception) and exacerbated by processes that make the nociceptors system more reactive (Sensitization of peripheral and central nervous systems) 40 NSAIDs, unlike opioids, do not block central nociceptive pathways; instead, they impede the synthesis of Prostaglandin that are involved in the sensitivity of the nociceptors system. Consequently, NSAIDs' analgesic efficacy is limited on aggregate and highly reliant on the molecular pathways that cause pain (Grosser T, et al., 2017). Gastric toxicity is a side effect of NSAIDs (Non-Steroidal Anti-Inflammatory Medicines). Those are medications that are used to treat inflammation (NSAIDs) medicines (NSAIDs) such ibuprofen, diclofenac, indomethacin, and flurbiprofen. Gastrointestinal (GI) ulceration, bleeding, and nephrotoxicity have all been connected to long-term usage of these medicines. The local irritation because of the carboxylic acid moiety, which is found in the majority of NSAIDs (topical impact), as well as tissue damage proteinoids manufacturing, which is incompatible with biological function of neuroprotective prostaglandins are a type of Prostaglandin that is produced in the body maintenance of Health and equilibrium in the gastrointestinal tract are the two main causes of GI damage caused by NSAIDs. The pharmacological effect of NSAIDs is linked to the inhibition of Cyclooxygenases, which inhibit Prostaglandin formation from arachidonic acid (COXs). Because NSAIDs, such as aspirin, ibuprofen, can cause significant GI toxicity, synthetic techniques based on NSAID acid treatment have been developed with the goal of improving the safety profile of NSAIDs (Amir M and Kumar S, 2007).
Cyclooxygenase-2 inhibitors
Celecoxib: Rofecoxib 2, valdecoxib 3, and celecoxib are Selective cox-2 benzene sulfonamides that are they've been discovered to have less gastrointestinal side effects when taken to alleviate discomfort and inflammation. Due to the serious cardiovascular side effects of celecoxib 2 and valdecoxib 3, as well as a Food and Drug Administration (FDA) safety alert for celecoxib 4, new scaffold with COX2 inhibitory effect must be discovered and their anti-inflammatory properties evaluated. Several sulfone derivatives, such as methionine derivation, L-methionine sulfone 5, and austrasulfone speculative dihydroaustrasulfone ethanol 6, on the other hand, are excellent anti-inflammatory drugs. Furthermore, the anti-inflammatory action of PC-796 aryl-sulfones and 2-sulfonyl-O-aminoacetophenones was substantial (Abdel-Aziz HA, et al., 2014). Because coxibs block the carbonic anhydrase pathway, AA metabolism is diverted to the Lipoxygenase (LOX) pathway, which raises the likelihood of a cardiovascular thrombotic event. According to the results, the evolution of a new anti-inflammatory medication with dual COX-2LOX inhibitory efficacy will result in the introduction of an effective cardio-safe medicine that is not ulcerogenic (Abdelall EK and Kamel GM, 2016).
Rofecoxib: Merck and Co. stated on September 30 that rofecoxib (Vioxx) would be voluntarily removed off the market worldwide research found that people taking the drug for a long time have double the risk of heart disease as those taking a placebo. The approve (Adenomatous Polyp Preservation of Vioxx) 3-year clinical trial was assessing the efficacy of rivaroxaban in avoiding the reappearance of polyps in the colon in people with a family history of colon cancer colon adenocarcinoma. It was discontinued in early September (two months before it was planned to end). It found that even after 18 years of therapy, people using the relative risk of rofecoxib was major cardiovascular events, such as heart attacks, that was roughly double that of patients receiving placebo. For the first two years of the research, there was no evidence of an increased risk. According to acting US Food and Drug (FDA) Committee chair Dr. Lester M. Crawford, the danger of the chance of a heart attack due to rofecoxib is "extremely tiny," but "generally, patients taking the medicine seriously face twice the risk of cardiovascular disease compared to patients receiving a placebo, "according to the study. As according Crawford, the FDA would keep a close eye on other drugs in this class to see if they have similar side effects. Rofecoxib, a Non-Steroidal Anti-Inflammatory Medication (NSAID) that is a selective COX-2 inhibitor, was approved in Canada in the year 1999. It is now licensed for the treatment of rheumatoid arthritis, acute arthritis pain, and menstruation pain in both acute and chronic forms. Trials conducted by the business at the end of approval showed that rofecoxib had no higher risk of heart events when compared to Other NSAIDs or placebo (Sibbald B, 2004) (Figure 2).
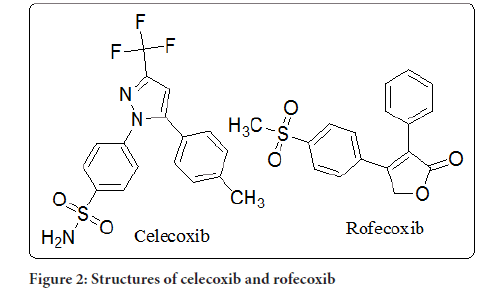
Figure 2:Structures of celecoxib and rofecoxib
Valdecoxib: This article discusses the clinical pharmacological, pharmacokinetic, negative consequences, possible medication interactions, contraindications, and cautions for the novel COX-2 selective inhibitor valdecoxib. The clinical effectiveness and tolerability findings studies are summarized. Valdecoxib is a COX-2-selective inhibitor, commonly referred to as celecoxib, COX-2-selective NSAID, COX-2-specific inhibitor, or COX-2-lsparing NSAIDs. COX-2-selective inhibitors bind both to COX-1 and COX-2 are two types of COX enzymes in a reversible way at first, but their long side chains form a tight complex with the COX-2 hydrophilic channel, because of the delayed dissociation, higher potency, and specificity of COX-2 inhibition. Valdecoxib does not suppress COX-1 at therapeutic dosages in humans. COX-1 is a human enzyme that is expressed in a variety of organs, including the intestinal (GI) tract, platelet, kidneys, and brain. It plays a role in various physiologic activities, including the production of eicosanoids, which protect the GI tract's mucosal integrity, regulate renal function, and stimulate platelet aggregation. Traditional NSAIDs suppress COX-1, which can have negative consequences for renal and platelet function (Chavez ML and Dekorte CJ, 2003). Following the identification of COX-2-specific inflammatory pathways, the COX-2 Selective inhibitor valdecoxib is developed as an incredibly effective but better tolerated option to nonselective NSAIDs. Osteoarthritis (OA) and Rheumatoid Arthritis (RA), two chronic conditions in which pain is a prominent component, and primary dysmenorrhea, an acute but recurrent pain disease, are now approved therapy indications. All of these illnesses put patients at risk of long-term NSAID use, as well as its side effects. Clinical trials and post-marketing surveillance in individuals with acute pain from different sources have given researchers a thorough understanding of valdecoxib's short and medium efficacy and tolerability (Fenton C, et al., 2004).
Diclofenac: Diclofenac belonging to a class of medications known as steroidal anti-inflammatory drugs (Non-steroidal anti) (NSAIDs) that suppress both COX-1 and COX-2 catalysts. NSAIDs block the emergence of proteinoids (Prostaglandin-E2 (PG-E2), PGD2, PGF2, repairs or replacement [PGI2], and Thromboxane A2 (TX A2)) via binding to COX isozymes. PGE2 is the most popular abundant proteinoid produced during inflammation, and NSAIDs' suppression of its synthesis is thought to be the fundamental mechanism for their powerful anti-inflammatory and analgesic properties. The inhibition of COX-2-dependent formation of PGE2 generated by monocytes following peptidoglycan (LPS) stimuli versus COX1-dependent creation of TXB2, the-anti hydrogenation component of TXA2 produced by NSAIDs was measured using the human whole urine in vitro assay formed by Adriana Partigiani and colleagues. Despite the fact that diclofenac is generally referred to be a classic NSAID in the research, these assays have shown that, unlike most traditional NSAIDs, it has a good specificity for COX-2 than for COX-1. The COX-2 selectivity of diclofenac is equivalent to that of celecoxib. COX-2 isoenzymes are more resistant to diclofenac than COX-1 isoenzymes. However, the estimated percentage inhibition for COX-1 and COX-2 of various COX inhibitors have been demonstrated to range between model, and selectivity is risk evaluation in some circumstances (Altman R, et al., 2015).
Prostaglandins produced by COX-2
COX-2 is typically overexpressed in colon PGE2 has been identified as the major proteinoids boosting cell growth and survival in cancer Patients carcinoma cells, as previously noted. COX has a role in the production of proteinoids like eicosanoids, prostacyclin, and thromboxane. COX transforms α-keto acid to Prostaglandin H2, which is the first and rate-limiting step in prostanoid synthesis. When it comes to colorectal cancer, PGE2 has pleiotropic effects; boosting growth, survival, vasculature, migration, and invasion are all processes that occur in the body (Greenhough A, et al., 2009).
Biosynthesis of Prostaglandin (PG)
When Acid Acid (AA) is a chemical compound that is used to make other chemicals 20-carbon unsaturated amino from the cell membrane, acid is released by phosphatases and processed by Prostaglandin G/H (PGG/H) synthase or Lipoxygenase (LOX) as well as their respective synthases, proteinoids are produced. They are widely produced-each cell type typically produces one or two major products-and operates as autocrine lipid mediators in the human body to maintain local homeostasis. The volume and character of PG production fluctuate drastically during an inflammatory reaction. In non-inflamed tissues, PG synthesis is minimal, but it rises quickly in chronic inflammatory before the recruiting of tumor the cells, and infiltrate of immune cells. The action of PGG/H synthases, also known as COXs, bifunctional enzymes that include both COX and oxidase activity and exist as different isoforms known to COX-1 and COX-2, for example, is required for PG synthesis (Figure 1). COX- 1 is the most popular common producer of proteinoids, which are used for housekeeping activities like homeostasis and cytoprotection in gastric epithelial cells 3 COX-2 is the most major source of eicosanoids synthesis in inflammation and proliferative illnesses like cancer, as it is activated by proinflammatory cytokines, Hormones and growth factors are two types of growth factors. 3 Both enzymes, however, have a part in the creation of cross and homeostatic prostacyclin, as well as the secretion of proteinoids during inflammation COX isoforms 1 and 2 create PGH2, which is the most widely used substrates for a number of protein phosphatase and synthesis enzymes that generate PGE2, PGI2, PGD2, PGF2, and Thromboxane A2 (TXA2). COX-1 primarily links with prostaglandins synthesis, PGF reductase, and the cytoplasm (c) PGE biosynthesis pathway (Prostaglandin E Synthase (PGES)) isozymes, although not exclusively. 4 COX-2 favors the Prostaglandin I Synthesis (PGIS) and infrastructure services (m) PGES isozymes, which are frequently induced with COX-2 by mediators and tumor promoters. The variable activation of these enzymes inside cells present at areas of inflammation determines the proteinoid synthesis profile. PGD2 is mostly produced by mast cells, whereas PGE2 and TXA2 are primarily produced by macrophages. On cellular activation, changes in the profile of eicosanoids production can also occur. Although macrophages create more TXA2 than PGE2 at rest, after bacteria Lipopolysaccharide (LPS) activation, the ratio shifts in favor of PGE2 (Ricciotti E and Fitzgerald GA, 2011).
Receptors for proteinoids
A proteinoid’s ability to impact immune cell function is determined by its ability to bind to Protein complex cell surface receptors. Separate receptors, including Prostaglandin F (FP), Prostaglandin I (IP), and Pt mediate the activities of PGF2, PGI2, and TXA2, respectively. PGD2 and PGE2On the other hand, if you're looking for a unique way to express yourself, activate numerous receptors. PGD2 works by interacting with two receptors the DP receptor and newly discovered CRTH2 channel (Chemo attractant Receptor homologous Th2-expressed molecule) Prostaglandin E (EP) 1-EP4 are the four subtypes each of which is made up of PGE2 receptors encoded by a different gene. Because a large number of receptors produced by distinct immune cell groups differ, the functionality these cells are is altered differentially during an immune reaction by prostacyclin existing in the immediate environment. The activation of eicosanoids receptors on lymphocytes is summarized, which is based both on pharmacological research and mRNA analysis (Tilley SL, et al., 2001).
Mechanism of action COX-2
We proposed in 1971 that aspirin-like medicines limit prostaglandin production, which would be the mechanism of action. Since then, the relationship between this broad set of inhibitors and the protein known as Cyclooxygenase have piqued researchers' curiosity (COX or PGH2 synthase). COX-1 and COX-2 are there are two kinds of COX that we now know about (see later). COX-1 enzyme screens in the previous two decades, various innovative drugs have been developed Picot who figured out the two structure of COX-1, which sheds fresh light on how COX inhibitors work. COX uses a Cyclooxygenase reaction to convert arachidonic to Prostaglandin G2 (PGG2), which it subsequently per oxidizes to PGH2. Three folding components make up this bifunctional enzyme: A vascular endothelial growth factor-like region, a membrane binding motif, and an enzymatic domain. The peroxidase and Cyclooxygenase action sites are close together but spatially separated. The membrane binding motif's shape clearly indicates that the enzyme incorporates into only one lipid bilayer leaflet, making it a monotopic membranes protein. Three of the structure's helices constitute the Cyclooxygenase channel's entrance, and their implantation into the membranes could enable arachidonic acid to reach the current location of the bilayer's interior (Vane JR, 1996).
Cyclooxygenase-2 available in market
Concerns about the efficacy of trying to take advantage (COX-2) inhibitor have been raised since the removal of rofecoxib. COX-2 Inhibitors might be a better option for some people than non-selective COX-2 inhibitors have a public health advantage over Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); nevertheless, their use in patients who are at a greater than normal risk of NSAID adverse effects is reliant on their use in patients who are at a higher than usual risk of NSAID side effects. We looked at trends in COX-2 medication use depending on the danger of NSAID-related side effects. Following the discontinuation of rivaroxaban in 2004 and general duty in 2005 resulting from excessive increase in risk of heart disease and stroke, celecoxib is now the sole COX-2 inhibitor sold in the United States. Celecoxib is more costly than nonselective NSAIDs, however it is also accessible in generic form. Since their debut, there has been a significant increase in the usage of COX-2 inhibitors, mainly among patients who are at low risk of NSAID-related side effects. These findings underscore the importance of difficulty of confining novel medicines to the contexts where they are test case and most effective (Dai C, et al., 2005).
Pathophysiologic roles for Cyclooxygenase-2
Cyclooxygenase-2 may potentially participate in the physiology of other regions of the GI tract. Endothelial cells express COX-2 in response to pathogenic microbe invasion, resulting in increased prostaglandin synthesis. This appears to protect the intestine by stimulating chloride and fluid flux, which flushes bacteria out. As a result, COX inhibitors prevent the fast release of fluid from the intestines, which is associated with the salmonella infection in rhesus monkeys. Antibodies against PGE2 also stop bacterially infected intestinal cells from producing chloride at a faster pace. The findings suggest that intestinal cells produce COX-2 as a result of pathogenic microorganism invasion. COX-2's specific role in intestinal health is unknown. Integrity in humans, although the exact mechanism of COX-2 inhibitors is unknown, they may impair intestinal repair or reduce resistance to invading bacteria (Lipsky PE, et al., 2000).
Conclusion
COX-2 inhibitors with pyrazoline and triazole ring in their structure as a follow-up to this work; more research is needed to synthesis a range of pyrazoline and triazole molecules with various spacer lengths and substitutions to assess their function in COX-2 inhibitory action and selectivity. Specific COX-2 inhibitors show promise in terms of gastrointestinal safety while preserving analgesic and anti-inflammatory activity, compared to earlier NSAIDs. Long-term safety and efficacy in clinical use will require more research. For the purpose of treatment of inflammatory joint problems and related chronic orofacial discomfort, diclofenac and rofecoxib are effective dental therapeutic drugs. Additionally, because of its faster onset, rofecoxib may be helpful in the treatment of severe postsurgical pain in some circumstances. Novel COX-2 inhibitors with a better safety profile and higher potency have been discovered. By targeting the inflammatory site with providers of nitrite COX-inhibiting vasodilation funders (CINODs), also known as NO-NSAIDs, it was also discovered that substances containing nitric oxide providers have more efficacy than professionally prescribed drugs the undesired effects of NO are due to over or impaired production of such mediator and the affected endothelium becomes, as a result, damaged or dysfunctional.
References
- Shoman ME, Abdel-Aziz M, Aly OM, Farag HH, Morsy MA. Synthesis and investigation of anti-inflammatory activity and gastric ulcerogenicity of novel nitric oxide-donating pyrazoline derivatives. Eur J Med Chem. 2009; 44(7): 3068-3076.
[Crossref] [Google Scholar] [Pubmed]
- Mohyel‐Din MM, Senbel AM, Bistawroos AA, El‐Mallah A, NourEl‐Din NA, Bekhit AA, et al. A novel COX‐2 inhibitor pyrazole derivative proven effective as an anti‐inflammatory and analgesic drug. Basic Clin Pharmacol. 2011; 108(4): 263-273.
[Crossref] [Google Scholar] [Pubmed]
- Abdel-Aziz M, Gamal-Eldeen AM. Synthesis and screening of anti-cancer, antioxidant, and anti-inflammatory activities of novel galloyl pyrazoline derivatives. Pharm Biol. 2009; 47(9): 854-863.
- Dannhardt G, Kiefer W. Cyclooxygenase inhibitors-current status and future prospects. Eur J Med Chem. 2001; 36(2): 109-126.
[Crossref] [Google Scholar] [Pubmed]
- Goyal A, Jain S. Synthesis of novel nitric oxide donating pyrazoles. Acta Pharm Sci. 2011; 53(2).
- Muscará MN, Wallace JL. COX-Inhibiting Nitric Oxide Donors (CINODs): Potential benefits on cardiovascular and renal function. Cardiovasc Hematol Agents Med Chem. 2006; 4(2): 155-164.
[Crossref] [Google Scholar] [Pubmed]
- Chen LZ, Sun WW, Bo L, Wang JQ, Xiu C, Tang WJ, et al. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur J Med Chem. 2017; 138: 170-181.
[Crossref] [Google Scholar] [Pubmed]
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007; 15(6): 252-259.
[Crossref] [Google Scholar] [Pubmed]
- Levine AB, Punihaole D, Levine TB. Characterization of the role of nitric oxide and its clinical applications. Cardiology. 2012; 122(1): 55-68.
[Crossref] [Google Scholar] [Pubmed]
- Vishwakarma KR, Negi DS. The development of COX-1 and COX-2 inhibitors: A review. Int J Pharm Sci Res. 2020; 11(8): 3544-3555.
- Grosser T, Theken KN, FitzGerald GA. Cyclooxygenase inhibition: Pain, inflammation, and the cardiovascular system. Clin Pharmacol Ther. 2017; 102(4): 611-622.
[Crossref] [Google Scholar] [Pubmed]
- Amir M, Kumar S. Synthesis and evaluation of anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm. 2007; 57(1): 31-45.
[Crossref] [Google Scholar] [Pubmed]
- Abdel-Aziz HA, Al-Rashood KA, ElTahir KE, Suddek GM. Synthesis of N-benzenesulfonamide-1H-pyrazoles bearing arylsulfonyl moiety: Novel celecoxib analogs as potent anti-inflammatory agents. Eur J Med Chem. 2014; 80: 416-422.
[Crossref] [Google Scholar] [Pubmed]
- Abdelall EK, Kamel GM. Synthesis of new thiazolo-celecoxib analogues as dual cyclooxygenase-2/15-lipoxygenase inhibitors: Determination of regio-specific different pyrazole cyclization by 2D NMR. Eur J Med Chem. 2016; 118: 250-258.
[Crossref] [Google Scholar] [Pubmed]
- Sibbald B. Rofecoxib (Vioxx) voluntarily withdrawn from market. Can Med Assoc J. 2004; 171(9): 1027-1028.
[Crossref] [Google Scholar] [Pubmed]
- Chavez ML, Dekorte CJ. Valdecoxib: A review. Clin Ther. 2003; 25(3): 817-851.
[Crossref] [Google Scholar] [Pubmed]
- Fenton C, Keating GM, Wagstaff AJ. Valdecoxib. Drugs. 2004; 64(11): 1231-1261.
[Crossref] [Google Scholar] [Pubmed]
- Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in Non-Steroidal Anti-Inflammatory Drug (NSAID) development: Evolution of diclofenac products using pharmaceutical technology. Drugs. 2015; 75(8): 859-877.
[Crossref] [Google Scholar] [Pubmed]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009; 30(3): 377-386.
[Crossref] [Google Scholar] [Pubmed]
- Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011; 31(5): 986-1000.
[Crossref] [Google Scholar] [Pubmed]
- Tilley SL, Coffman TM, Koller BH. Mixed messages: Modulation of inflammation and immune responses by Prostaglandins and Thromboxanes. J Clin Invest. 2001; 108(1): 15-23.
[Crossref] [Google Scholar] [Pubmed]
- Vane JR. Introduction: Mechanism of action of NSAIDs. Rheumatology. 1996; 35(1): 1-3.
[Crossref] [Google Scholar] [Pubmed]
- Dai C, Stafford RS, Alexander GC. National trends in Cyclooxygenase-2 inhibitor use since market release: Nonselective diffusion of a selectively cost-effective innovation. Arch Intern Med. 2005; 165(2): 171-177.
[Crossref] [Google Scholar] [Pubmed]
- Lipsky PE, Brooks P, Crofford LJ, DuBois R, Graham D, Simon LS, et al. Unresolved issues in the role of Cyclooxygenase-2 in normal physiologic processes and disease. Arch Intern Med. 2000; 160(7): 913-920.
[Crossref] [Google Scholar] [Pubmed]
Author Info
AB Harale*, SY Chaudhari and SN BaviskarCitation: Harale AB: Novel Nitric Oxide-Donating Pyrazoline-Coumarin as a Cyclooxygenase-2 Inhibitors for Anti-Inflammatory Activity
Received: 08-Mar-2022 Accepted: 29-Mar-2022 Published: 05-Apr-2022, DOI: 10.31858/0975-8453.13.4.251-255
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






