Research Article - (2023) Volume 14, Issue 3
Abstract
Objective: Therefore, this study aimed to determine the physicochemical characterization of Demineralized Freeze Dried Dentine Matrix Nanoparticle (DFDDM-NP) for enhance bone repair.
Materials and Methods: Human tooth were minced into particles (Ø<1 mm), demineralized and freeze dried. Thorough physicochemical and biochemical characterizations of native and demineralized freeze dried materials were performed by Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Diffraction (XRD), Brunauer-Emmett-Teller (BET), Field Emission Scanning Electron Microscopy (FESEM), and Thermogravimetric analysis/Derivative Thermogravimetry (TGA/ DTG).
Results: For the physicochemical characterization, DFDDM-NPs appeared non porous structure, near spherical shapes of various size, and it appeared agglomerated nanoparticle of around 10-50 nm. DFDDM-NPS is type IV Isotherm and Hysteresis H3. The pore structure showed that 18 m2/g, pore diameter is about 5-3 nm. As 86%-99% DFDDM-NPs can be remaining and stable down to 1000°C.
Conclusion: DFDDM-NPs would allow the development of new bone graft. Further studies are recommended.
Keywords
Physicochemical Characteristics, Demineralized Freeze Dried Dentine Matrix (DFDDM), Nanoparticle, Bone Graft
Introduction
The need for off-the-shelf, readily available materials suitable for bone regeneration has inspired much research in the field of regenerative medicine. Nowadays, the commercial inorganic de-proteinized bovine substitute Bio-Oss® represents the gold standard for regenerative dentistry worldwide because of its osteo-conductive properties and bio functionality leading to effective and reliable bone regeneration. Despite the widespread use of such a gold standard graft material in alveolar bone augmentations, great interest has recently been focused on autologous bone-like materials as suitable substrates for bone regeneration of alveolar defects. However, although autogenous bone grafts are currently used in dentistry, the major drawbacks of bone-harvesting procedures, such as the limited availability of bone tissue and the need for second surgery, have hindered their efficacy in replacement therapy. These issues, together with the shortcomings ensuing from allogeneic transplantation, such as the transmission of possible infections and the lack of osteointegration with host tissues, have spurred on researchers and clinicians to look for novel graft solutions by means of challenging biomaterial techniques. More specifically, the use of tooth-derived materials has recently attracted much interest due to the inherent wide availability of teeth that are extracted every day and discarded as waste (Drosse I, et al., 2008; Sela JJ and Bab IA, 2012).
This research focuses on Demineralized Freeze Dried Dentin Matrix Nanoparticle (DFDDM-NPs), originating from extracted human teeth. Teeth and bone have similar histogenesis, similar components (both composed of an organic phase (90% type I collagen) and an intrafibrillar mineral phase (mostly apatite crystallites). The ratios of organic/inorganic components in human dentin and bone are both at approximately 2:1. Dentin was reported to have a bone-inducing function, and DFDDM was found to have better regenerative properties than Undemineralized Dentin Matrix (UDM). Human DFDDM is one of the most acid‐soluble scaffolds, containing a collagenous matrix and osteoinductive growth factors, in addition to a mineral phase, and is an ideal bone substitute. However, Demineralized Dentin Matrix showed lower rate of formation and deposition of hydroxyapatite crystals than deproteinazed dentin. Growth factors are present in DFDDM, while they are trapped and hidden in UDM. The demineralization process opens the dentin tubules and frees up the growth factors, which can stimulate tissue repair and regeneration. The new novel of nanoparticle for bone repair is developed. This present study was aimed to determined physicochemical characterization of demineralized freeze dried dentine matrix nanoparticle (Calori GM, et al., 2011; Karageorgiou V and Kaplan D, 2005; Gruskin E, et al., 2012).
Materials and Methods
Synthesis of Demineralized Freeze Dried Dentin Matrix Nanoparticles (DFDDM-NPs)
Tooth samples were extracted from otherwise healthy patients who have signed informed consent. Extracted teeth (vital and non vital) were debrided from its soft tissue, calculus, and restoration on the crown or root area. Sample was further powdered using percussion mill (Polymix1 PX-MFC 90 D, Kinematica AG, Swiss). Afterwards, dentine particle was demineralized using HCl 4 N for 8 hours. Then rinse with aquadest to remove HCl solution. Put the particle in deep freeze, then dried through a lyophilizer system (freeze drying).
Physicochemical characterization
Fourier Transform Infrared Spectroscopy (FTIR): The FT-IR spectra of the samples were recorded by Thermo Scientific, Nicolet iS10. The scanning range was 400-4000 cm-1 at a resolution of 4 cm-1. For the preparation of the pellets, KBr was used as a spectroscopic quality diluent, melted and then stored in an oven. The spectrum of the AEA was collected from the commercial ethanolic solution of the endocannabinoid using ZnSe windows. FTIR test results will be in the form of a graph.
X-Ray Diffraction (XRD): XRD was used to determine the mineral phases and crystallinity of the materials. X-rays were generated by a cathode ray tube, filtered to produce monochromatic radiation towards the sample, which in turn produces diffracted rays that are then detected, processed, and counted. Enough material from the sample was used to fill an empty sample holder. The wavelength of the X-ray was of the same order of magnitude as the bond distances between atoms in the crystal, producing a pattern unique to the atomic arrangement in the sample. The results obtained from each material were compared with standards to characterize its phase purity and crystallinity. A graph obtained was compared to JCPDS (Joint Committee on Powder Diffraction and Standards). The analysis was done on a Bruker D8 XRD machine.
Field Emission Scanning Electron Microscopy (FESEM): The SEM images were recorded to determine the morphology and size of the Nps‐AEA/ PCL (Anandamide/ε-Polycaprolactone) using a LEO1450DP microscope. The sample was directly observed on the aluminium foil in which it was collected, to which a gold cover was applied. The analysis of the images obtained was carried out using ImageJ version 1.x image processing software.
Brunauer-Emmett-Teller (BET): DFDDM-NPs inserted into the quartz rod, then placed in such a way on the station holder. Liquid nitrogen is then poured into a dewar (tube) for later analysis process to be run using Quantachrome NovaWin. After the analysis, data were obtained in the form of pore diameter, specific surface area, and pore volume of pumice. The weight of the sample used in this test is not more than 0.1 grams. In addition to the BET method, the diameter and volume of the pumice pore can also be determined using the BJH (Barrett-Joyner-Halenda) method.
Thermogravimetric Analysis/Derivative Thermogravimetry (TGA/DTG): The TG curves were recorded on a Shimadzu TGA‐51 instrument from room temperature to 300°C, with 50 ml min-1 of nitrogen flow and a heating rate of 5°C min-1. The samples were appropriately placed in platinum cells.
Results
Fourier Transform Infrared Spectroscopy (FTIR)
In fact, the spectrum of DFDDM-NPs exhibits all the most intense bands (at 400-4000 cm-1) and transmition at 70%-102%. As described at Figure 1, Demineralized Freeze Dried Bovine Bone Xenograft-Nanoparticles (DFDBBX-NPs) has 30 bands such at 3641.6; 3539.38; 3483.44; 3402.43; 3327.21; 3246.2;2929,87; 2879.72; 2515.18; 2129.41; 2083.12; 1998.25; 1869.02; 1791.87; 1681.93; 1620.21; 1417.68; 1149.57; 1091.71; 1033.85; 966.34; 916.19; 873.75; 800.46; 779.24; 711.73; 642.3; 570.93; 472.56; 443.63 cm-1.
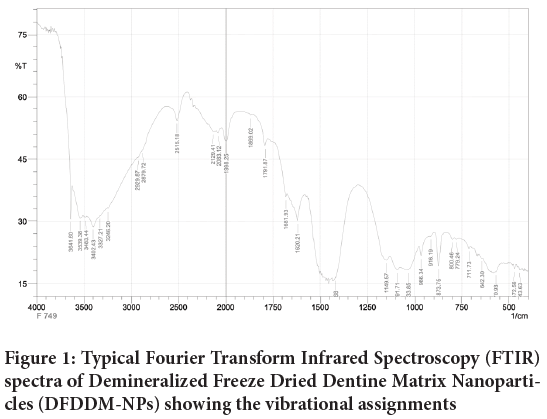
Figure 1: Typical Fourier Transform Infrared Spectroscopy (FTIR) spectra of Demineralized Freeze Dried Dentine Matrix Nanoparticles (DFDDM-NPs) showing the vibrational assignments
X-Ray Diffraction (XRD)
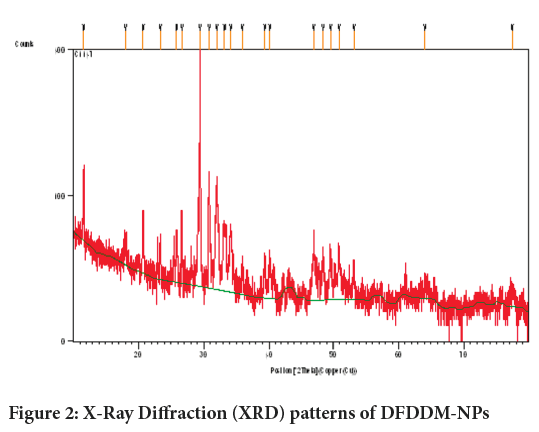
The XRD patterns of DFDDM-NPs are presented in Figure 2. The XRD diffractogram of the dentine contains a broad part between 10° and 40°, and some distinctive peaks at 2 =11.6333°, 17.9846°, 20.7172°, 23.3850°, 25.8127°, 26.6259°, 29.4173°, 30.83888° (Figure 2).
Figure 2: X-Ray Diffraction (XRD) patterns of DFDDM-NPs
Field Emission Scanning Electron Microscopy (FESEM)
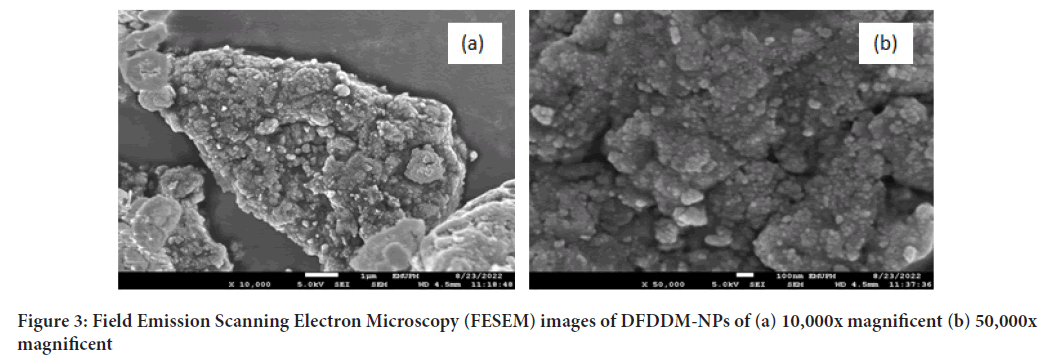
Based on Figure 3, it showed that there is non-porous structure, near spherical shapes of various sizes, and it appeared agglomerated nanoparticle of around 10-50 nm. The agglomeration is due to sample drying process.
Figure 3: Field Emission Scanning Electron Microscopy (FESEM) images of DFDDM-NPs of (a) 10,000x magnificent (b) 50,000x magnificent
Brunauer-Emmett-Teller (BET) and surface area
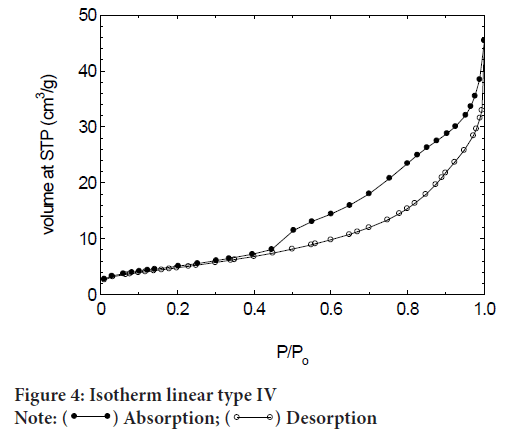
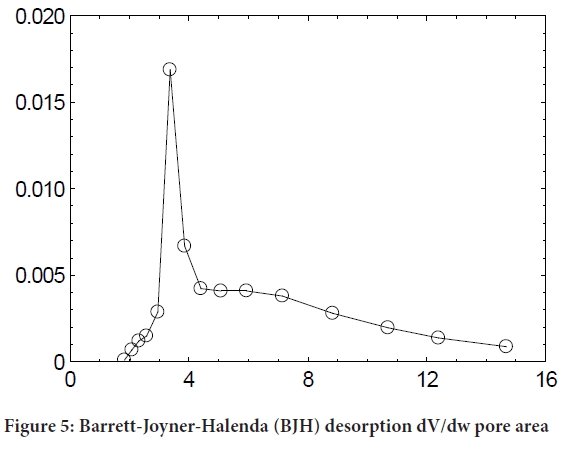
BET isotherm showed that DFDDM-NPs is Type IV isotherm (Figure 4). A type IV isotherm is observed for materials having mesopores of smaller widths and is common for mesoporous materials. From the size of pore diameters (Figure 5), it can be noticed that pore diameter is 5-3 nm, and pore volume is about 0.0444 cm3/g.
Figure 4: Isotherm linear type IV Note:  Absorption;
Absorption;  Desorption
Desorption
Figure 5: Barrett-Joyner-Halenda (BJH) desorption dV/dw pore area
Table 1showed the summary of surface area of DFDDM-NPs. DFDDM-NPS is type IV Isotherm and Hysteresis H3. The pore structure showed that 18 m2/g, pore diameter is about 5-3 nm.
| Sample | Type of isotherm | Lupac hysteresis classification | Surface area (m2/g) | BJH pore diameter (nm) | Pore volume (cm3/g) |
|---|---|---|---|---|---|
| DFDDM-NPs | IV | H3 | 18 | 5.3 | 0.0444 |
Note: DFDDM-NPs: Demineralized Freeze Dried Dentine Matrix Nanoparticles; BJH: Barrett-Joyner-Halenda
Table 1: Summary of pore structure
Thermogravimetric Analysis/Derivative Thermogravimetry (TGA/DTG)
The TGA curves obtained DFDDM-NPS under nitrogen atmosphere can be seen from Figure 6. For precise interpretation of differences in thermal degradation behaviour of all the samples, comparative analysis of thermal parameters derived from TG analysis is carried out.
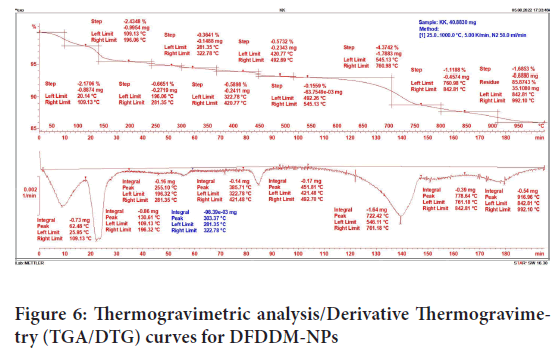
Figure 6: Thermogravimetric analysis/Derivative Thermogravimetry (TGA/DTG) curves for DFDDM-NPs
Table 2described about Tmax, Trange, and mass loss. At Tmax 62.48°C, it can be loss about 2.17%. It concluded that it adsorbed/absorbed water on the surface of the sample. Overall about 86%-99% DFDDM-NPs can be re maining. It is stable down up to 1000°C.
| No. | Tmax/oC | Trange/oC | Dm (%) |
|---|---|---|---|
| 1 | 62.48 | 25.95-109.13 | 2.17 |
| 2 | 130.61 | 109.13 -196.32 | 2.43 |
| 3 | 255.1 | 196.32 -281.35 | 0.67 |
| 4 | 303.37 | 281.35-322.78 | 0.36 |
| 5 | 385.71 | 322.78-421.45 | 0.59 |
| 6 | 451.81 | 421.45 -492.70 | 0.57 |
| 7 | 722.42 | 546.11-761.18 | 0.16 |
| 8 | 778.64 | 761.18-842.81 | 4.37 |
| 9 | 916.96 | 842.81-992.10 | 1.69 |
| Total | 13.01 | ||
Table 2: Tmax, Trange, and mass loss
Discussion
Autogenous bone graft has been used to repair bone defects because dentin and bone have the main osteoinductive characteristic, Demineralized Dentin Matrix (DDM) an organic material obtained from the dentine has the osteogenic ability because containing bioactive molecules of collagen type-I and its growth factors such as BMP-2 and fibroblast growth factors that contribute to osteoinduction and osteoconduction of human teeth as graft. Human teeth are rich sources of stem cells, matrix, trace metal ions, and growth factors. Although the structure of bone and dentin tissue is different, the ratio of the components is similar (70% minerals, 20% collagen, body fluids 10% of the weight). After demineralization, the main structure of the dentin matrix is collagen type-I (95%) and non-collagenous proteins such as growth. The growth factors were identified in human dentin including Insulin-like Growth Factor-I (IGF-I), skeletal growth factor/Insulin-like Growth Factor II (IGF-II), and transforming growth factor (Drosse I, et al., 2008; Sela JJ and Bab IA, 2012).
The ideal biomaterials for bone regeneration should not only be biocompatible and osteoconductive but also osteoinductive. They should be able to leverage the self-healing capabilities of the bone by (1) providing the main structural, compositional, and biochemical; (2) engaging the host’s immune system; (3) promoting recruitment, proliferation, and differentiation of progenitor cells. Recently, nanotechnology has become a domain with breakthrough potential to further propel the field of bone regeneration. Nanostructured biomaterials have proven superior at enhancing bone regeneration. These differences stem from an ability to be engineered to precisely mimic the composition and nano architecture of bone. So, nanostructured biomaterial should be investigated physicochemical characterization (Wang F, et al., 2019; Mauffrey C, et al., 2015; Biswas N, et al., 2009).
FTIR spectroscopy, including Fourier Transform Infrared Microspectroscopy (FTIRM) and FTIRI, providing information on all bone constituents at molecular level, represents an excellent tool to explore bone quality. The most frequently reported outcomes of FTIR related to bone material properties are the relative mineral and organic content, mineral maturity and crystallinity, carbonate substitution into the apatite lattice and collagen crosslinking (Sarmento B, et al., 2006; Danafar H, 2016). Because most of the bands of the bone spectrum are composed of several spectral components, deconvolution methods based on second derivative-spectroscopy and curve fitting have to be applied, being the results normally reported as a percentage of the area of specific underlying bands. Hence, FTIR data are most commonly used for comparison purposes (between regions of the same sample, among samples or throughout time) rather than in absolute terms. The proportion between the mineral and organic content in bone expresses its degree of mineralization and can be calculated from the ratio of the integrated area due to phosphate bands to that of a amide band (normally Amide I) (Mady FM and Shaker MA, 2017; Sathyamoorthy N, et al., 2017; Kalita S, et al., 2015).
The structural analysis by electronic microscopy allowed us to characterise size and shape, but also allowed to identify the presence of particle agglomerates in the FESEM image. These formations could be related to temporary blockages of the needle through which the polymer solution circulates and/or the inherent unctuous consistency of the endocannabinoid. Additionally, we consider as another frequent cause of the formation of agglomerates, the absence of stabilising agents such as surfactants or emulsifiers in the formulation of the polymer solution. However, and as verified in the physical and chemical tests, these agglomerates did not affect the results (El-Houssiny AS, et al., 2016; Bhattacharjya C, et al., 2016).
Conclusion
The use of biocompatible and biodegradable bone graft such as DFDDM-NPs would allow the development of new pharmaceutical formulations for the controlled/prolonged release of various types of active ingredients with attractive pharmacological properties such as DFDDM-NPs but dismissed for their physicochemical and pharmacokinetic limitations and disadvantages. Future in vitro and in vivo studies should be performed to demonstrate the usefulness of DFDDM-NPs for bone repair.
References
- Drosse I, Volkmer E, Capanna R, de Biase P, Mutschler W, Schieker M. Tissue engineering for bone defect healing: An update on a multi-component approach. Injury. 2008; 39: S9-20.
[Crossref] [Google Scholar] [Pubmed]
- Sela JJ, Bab IA. Physiology of bone healing: Part 1. Principles of bone regeneration. 2012: 10-48.
- Calori GM, Mazza E, Colombo M, Ripamonti C. The use of bone-graft substitutes in large bone defects: Any specific needs?. Injury. 2011; 42: S56-63.
[Crossref] [Google Scholar] [Pubmed]
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005; 26(27): 5474-5491.
[Crossref] [Google Scholar] [Pubmed]
- Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: History and use. Adv Drug Deliv Rev. 2012; 64(12): 1063-1077.
[Crossref] [Google Scholar] [Pubmed]
- Wang F, Xie C, Ren N, Bai S, Zhao Y. Human freeze-dried dentin matrix as a biologically active scaffold for tooth tissue engineering. J Endod. 2019; 45(11): 1321-1331.
[Crossref] [Google Scholar] [Pubmed]
- Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015; 23(3): 143-153.
[Crossref] [Google Scholar] [Pubmed]
- Biswas N, Thomas S, Sarkar A, Mukherjee T, Kapoor S. Adsorption of methimazole on silver nanoparticles: FTIR, Raman, and surface-enhanced Raman scattering study aided by density functional theory. J Phys Chem C. 2009; 113(17): 7091-7100.
- Sarmento B, Ferreira D, Veiga F, Ribeiro A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr Polym. 2006; 66(1): 1-7.
- Danafar H. MPEG-PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016; 3(1): 1142411.
- Mady FM, Shaker MA. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int J Nanomedicine. 2017; 12: 7405-7417.
[Crossref] [Google Scholar] [Pubmed]
- Sathyamoorthy N, Magharla D, Chintamaneni P, Vankayalu S. Optimization of paclitaxel loaded poly (ε-caprolactone) nanoparticles using Box Behnken design. Beni-Suef Univ J Basic Appl Sci. 2017; 6(4): 362-373.
[Crossref] [Google Scholar] [Pubmed]
- Kalita S, Devi B, Kandimalla R, Sharma KK, Sharma A, Kalita K, et al. Chloramphenicol encapsulated in poly-ε-caprolactone–pluronic composite: nanoparticles for treatment of MRSA-infected burn wounds. Int J Nanomedicine. 2015; 10: 2971.
[Crossref] [Google Scholar] [Pubmed]
- El-Houssiny AS, Ward AA, Mostafa DM, Abd-El-Messieh SL, Abdel-Nour KN, Darwish MM, et al. Drug-polymer interaction between glucosamine sulfate and alginate nanoparticles: FTIR, DSC and dielectric spectroscopy studies. Adv Nat Sci: Nanosci Nanotechnol. 2016; 7(2): 25014.
- Bhattacharjya C, Gadicherla S, Kamath AT, Smriti K, Pentapati KC. Tooth derived bone graft material. World J Dent. 2016; 7(1): 32-35.
Author Info
Ariyati Retno Pratiwi1,2*, Mohd Zobir Hussein3, Feni Istikharoh4, Zefri Zainal Abidin5, Irma Istiqamah6, Fadhillah Marzely6, Salwa Jasmine6 and Laura Naibaho62Department of Nanodentistry, Universitas Brawijaya, Jawa Timur, Indonesia
3Nanomaterials Synthesis and Characterication Laboratory, Institute of Nanoscience and Nanotechnology, Universiti Putra Malaysia, Selangor, Malaysia
4Department of Dental Material, Universitas Brawijaya, Jawa Timur, Indonesia
5Department of Dentistry, Kediri General and Teaching Hospital, Jawa Timur, Indonesia
6Department of Dentistry, Universitas Brawijaya, Jawa Timur, Indonesia
Citation: Pratiwi AR: Physicochemical Characterization of Demineralized Freeze Dried Dentine Matrix Nanoparticle as New Candidate Bone Graft
Received: 15-Feb-2023 Accepted: 10-Mar-2023 Published: 17-Mar-2023, DOI: 10.31858/0975-8453.14.3.159-163
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3