Research Article - (2023) Volume 14, Issue 2
Abstract
Diospyros batokana (Ebenaceae) is used traditionally in Zambia to treat various ailments among degenerative diseases. This study aimed to screen the phytochemicals using chemical tests and LCMS/MS and evaluate the total phenolic and flavonoid contents and antioxidant activity of methanolic leaf extract of Diospyros batokana. Whereas maceration was used to extract phytocompounds for preliminary phytochemical screening, QuEChERS based method was used to extract polyphenolics in methanol for LCMS/MS screening. Total phenolic content was evaluated using the Folin-Ciocalteu method, at 765 nm. Total flavonoid content was estimated by aluminium chloride colorimetric assay at 510 nm. Antioxidant activity was determined using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging method, at 517 nm. Preliminary phytochemical screening revealed the presence of antioxidants; phenolic compounds and flavonoids, and various other important secondary metabolites such as alkaloids, terpenoids, and saponins. Total phenolic content was estimated at 50.3780 ± 0.1188 mg GAE/g, whereas total flavonoid content was estimated to be 105.5495 ± 0.4066 mg QE/g. LCMS/ MS polyphenolic screening revealed the presence of several phenolics and flavonoids such as gallic acid, quercetin, Kaempferol-3-O-glucoside, Quercetin 3’-O-glucoside, isoflavonoids, and flavones. The crude extracts displayed high DPPH free radical scavenging activity with a very low IC50 value of 13.08 μg/ mL. The high antioxidant capacity of the leaf extract of Diospyros batokana Ebenaceae might be the response for the use of the plant in traditional medicine in Zambia to manage and treat degenerative diseases.
Keywords
Phytochemical screening, LCMS/MS, Total phenolic content, Total flavonoid content, Antioxidant activity
Introduction
Although there are various medications for the treatment of degenerative diseases such as cancer, diabetes mellitus, cardiovascular disorders, and oxidative stress, among other ailments, these health conditions remain associated with high mortality globally (Furman D, et al., 2019). Apart from synthetic compounds, literature reports suggest that natural compounds can suppress or reverse the progression of these diseases (Li X, et al., 2019). Suffice to say that an estimated 75% to 80% of the world population use herbal medicines, usually in developing countries (Süntar I, 2020), for primary health care due to their easier availability, lesser side effects, and better acceptability with the human body. Globally, medicinal plants have been used to foster human health since antiquity. Even though accessibility to modern healthcare nowa- days is faster and much easier, people and communities choose to use medicinal plants to promote good health. For example, Jamu medicine, traditional Chinese medicine and Ayurvedic medicine (Krupa J, et al., 2019). Due to the availability of information concerning the safe use of phytochemicals and bioactive compounds as promising options for the management of degenerative diseases, there is a paradigm shift to embrace natural products as medicines (Krupa J, et al., 2019; Ahmed IA, et al., 2022). Many plant genera have been validated to possess phytocompounds eliciting pharmacological effects on humans and animals. A good example of a genus with essential pharmacological benefits is Diospyros of the Ebenaceae family (Kamler JF, et al., 2020).
Diospyrosspecies are important and conspicuous plant trees in their natural environments, particularly in Asia and Africa (Kamler JF, et al., 2020; Yao X, et al., 2022). More than 500 species of these trees have enormous economic and health values (Guan C, et al., 2020; Sulub-Tun RA, et al., 2020; Ramírez‐Briones E, et al., 2022). They are deciduous, evergreen shrubs and trees that thrive in warm regions of the world, such as parts of Africa, the United States, China, Italy, India, Turkey, and the middle east, with only a few found in temperate regions. In tropical regions, Diospyrosplants are ethnically used in traditional medicine, particularly leaves, fruits, barks, roots, and hard wood as a poultice, powder, and tonic to treat many degenerative diseases, and other illnesses such as asthma, lumbago, biliousness, atherosclerosis, haemorrhage, dermatitis, hypertension, insomnia as well (Rauf A, et al., 2017; Mishra Y, et al., 2021).
Diospyrosplant species are mainly rich in phenolic and flavonoid compounds (Esteban-Muñoz A, et al., 2020; Maulidiani M, et al., 2019; Matheus JR, et al., 2020). These compounds exhibit antioxidation properties. Antioxidants could be used to treat and manage pathophysiological health conditions involving free radicals, such as cardiovascular and neurodegenerative disease. The most prominent and established in vitro method for evaluating the antioxidant capability of phytochemicals such as phenolics and flavonoids is the DPPH radical scavenging assay.
Diospyros batokanahas neither been phytochemically screened nor phytochemically studied. This study carried out preliminary phytochemical screening of secondary metabolites in the methanolic leaf extract. Since the widely claimed pharmacological benefits of the medicinal plant in traditional Zambian medicine might be associated with some of its secondary metabolites, there is a need to find out the phytochemical profile of this plant for more nuanced prediction and easier verification of its medicinal worth. This would help in the identification and isolation of the therapeutic lead molecules in the plant for plausible synthesis of essential novel drugs in future studies. This study carried out LCMS/MS screening and evaluated the total phenolic content, total flavonoid content, and antioxidant activity of the leaves of Diospyros (D.) batokana (Ebenaceae).
Materials and Methods
Collection and authentication of plant material
The plant material was collected from the forests of Kalulushi District, Copperbelt Province of Zambia. The fresh leaves were collected and air dried in the shade. The plant was identified and authenticated as Diospyros batokanaEbenaceae by the forest department at the Kitwe District Herbarium of the Ministry of Environment and Natural Resources of the Republic of Zambia. Figure 1shows the plant laden with leaves in its natural environment.

Figure 1: Leaves of Diospyros batokana (Ebenaceae)
Drying and size reduction of Diospyros batokana (Ebenaceae) leaves
The leaves of D. batokana(Ebenaceae) were cleaned to remove the adhered foreign materials and then washed under tap water, subsequently washed with deionised water, air-dried in the shade, and homogenized to a fine powder.
Chemicals
Chemicals and reagents used for preliminary phytochemical screening, UHPLC screening, 1,1-Diphenyl-2-picrylhydrazyl (DPPH), AlCl3, folin-ciocalteu’s phenol reagent, sodium carbonate, and HPLC grade methanol were obtained from Merck (South Africa).
Sample preparation techniques
Extraction of secondary metabolites for preliminary phytochemical screening: Extraction of secondary metabolites was carried out using maceration. Briefly, 5 g of powdered dried leaves were soaked in 40 ml methanol (80%) and placed in 50 ml centrifuge tubes. The tubes were then placed for 10 hours in a water-bath shaker with a temperature of 40°C and a shaking vibration frequency of 150 rpm. After cooling, the extracts were filtered through a 0.45 μm filter. These extracts were used for preliminary phytochemical screening.
LCMS/MS sample preparation based on modification of QuEChERS procedure: The extraction of polyphenolic compounds was done following the method of QuEChERS (Guo J, et al., 2019). The QuEChERS technique consists of two steps; Extraction and clean-up. The technique depends on blending solvent with salts to detach the analyte from the sample material into acetonitrile. In the present study, the standard QuEChERS method was moderately modified. Instead of using acetonitrile, the solvent utilised was methanol. This is because methanol is a better solvent in terms of inter-molecular forces such as hydrogen bonding needed for effective extraction. Acetonitrile relies only on dipole-dipole interactions. Further, methanol was chosen as it is the solvent that was used for standards preparation during total phenolic and flavonoid content and DPPH scavenging assay. Briefly, 3.0 g of dried powdered D. batokana (Ebenaceae) leaves were weighed in a 50 mL polypropylene centrifuge tube. 20 mL HPLC grade methanol was added, and the contents were shaken by vortex for a minute. After that, 4.0 g MgSO4 and 1.0 g NaCl salts were added. The tube was vortexed for a minute and immediately centrifuged (4000 rpm) for 10 minutes. The 4.5 mL upper layer supernatant was collected in another centrifuge tube for the clean-up stage. Further, a 75 mg anhydrous MgSO4 and 150 mg PSA was added to the upper layer supernatant during the clean-up stage. Subsequently, the tube was vortexed for a minute and immediately centrifuged (4000 rpm) for 10 minutes. Finally, the purified extract was filtered through a 0.22 µm Tetrafluoroethylene (PTFE) syringe filter before LCMS/MS screening.
The adsorbents compounds, PSA removed unwanted substances including polar interferences, carbohydrates, organic acids, and pigments, and graphitized carbon black was used to remove pigments (chlorophylls). If these interferences are not removed from the plant extracts, they would interfere with the LCMS analysis. Therefore, it was necessary to use the QuEChERS method for sample preparation.
Extraction of total phenolic content: The extraction of total phenolics from D. batokana was carried out using a method from the literature (Pakade V, et al., 2013; Ralepele FM, et al., 2021) with modifications. The leaves were air dried away from sunlight and ground into powder using a mortar and pestle. The extraction was composed of 20 mL methanol (80%) and 3.0 g of leaf powder. The contents were sonicated for half an hour. Then the extract was centrifuged at 6000 rpm for 10 minutes, and the super natant was obtained. In order to attain maximum phenolic recovery, the residue was extracted again. The total phenolic content was determined using the Folin-Ciocalteu method with the aid of standard gallic acid (Ralepele FM, et al., 2021; Makkar HP, et al., 1997).
Extraction of Total Flavonoid Content (TFC): The extraction of total flavonoids was carried out using a modified method (Ralepele FM, et al., 2021; Makkar HP, et al., 1997). Essentially, 3.0 g of dry powdered leaf sample was extracted using reflux with 100 mL of methanol (80%) for 3 hours. The extract cooled naturally for 2 minutes and was centrifuged for 15 minutes at 4000 rpm, and a 0.45 µm filter was used to obtain the filtrate that was subsequently used to estimate the total flavonoid content.
Methods
Qualitative phytochemical screening: The phytochemical screening of the methanolic (80%) extract of the leaves of D. batokanawas carried out at the Environmental Analytical Chemistry Laboratory, School of Chemistry, University of the Witwatersrand, South Africa.
Phytochemical Screening of the methanolic leaf extract of D. batokanafor various phytochemical constituents were carried out using standard procedures from the literature, these being; test for alkaloids (Mukhtar Y, et al., 2022), anthraquinones and glycosides (Ebbo AA, et al., 2014), carbohydrates (Mukhtar Y, et al., 2022), flavonoid (Ebbo AA, et al., 2014), phenolic compounds and tannins (Ahmad T, et al., 2013), proteins (Ahmad T, et al., 2013), saponins (Ebbo AA, et al., 2014), steroids (Mukhtar Y, et al., 2022), terpenoids (Ebbo AA, et al., 2014), and volatile oils (Mukhtar Y, et al., 2022; Ebbo AA, et al., 2014).
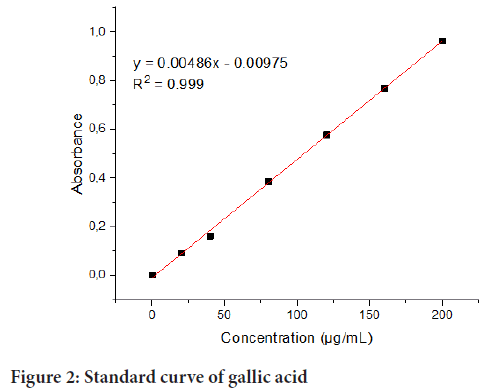
UHPLC-MS/MS Screening: Ultra-high-performance liquid chromatography screening was carried out using a previous method (Ralepele FM, et al., 2021) with modifications on a 3000 Series Rapid Resolution LC system (Ultimate 3000, Thermofisher South Africa), that is implanted with a vacuum degasser, an autosampler, a binary pump, and a diode array detector. Essentially, chromatographic separation was done using phenomenex C18, and formic acid and acetonitrile as mobile phases A and B, in that order. The flow rate was 0.50 mL/min and the gradient was scheduled as in the order thus: 0 min/5% B; 10 min/35% B; 15 min/95% B; 25 min/5% B. The injection volume in the UHPLC system was 10 µL. The UHPLC system was co-joined to a QTOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany). The mass spectrometer was equipped with Electro spray Ionization (ESI) that was operated in negative mode. Detection was executed in the mass range of 50-1300 m/z, utilizing a capillary voltage of +4000 V, and a dry gas temperature of 210°C, at a flow rate of 8.0 L/min. Further, 2.0 bar was the nebulizer pressure, and 1 Hz was the spectra rate.Total phenolic content: Polyphenols are essential phytocompounds possessing redox properties eliciting antioxidant activity (Mehdi MA, et al., 2019; Aryal S, et al., 2019). The hydroxyl groups on polyphenols are responsible for enabling free radical scavenging. Total phenolic content was measured in the methanolic (80%) extracts of D. batokana (Ebenaceae) leaves using the Folin-Ciocalteu reagent assay (Aryal S, et al., 2019; Pakade V, et al., 2013). Essentially, 200 μL of the extract was mixed with a freshly prepared solution of 750 μL ten times diluted Folin-Ciocalteu reagent and 2000 µL of 7.5% sodium carbonate solution. The final mixture was diluted by adding ultrapure water to a final volume of 10 mL. After that, the contents were incubated at room temperature and atmospheric pressure in the dark for 2 h before measuring the absorbances at 765 nm using a UV-visible spectrophotometer (Varian, Cary 50 Conc, Darmstadt, Germany). All the experiments were carried out in triplicate, and gallic acid was used as the calibration standard with standard concentrations of 0, 20, 40, 80, 120, 160, 200 µg/mL prepared in 80% methanol. Results were recorded as gallic acid equivalent and the total phenolic content expressed as mg Gallic Acid Equivalent (GAE) per gram. Therefore, the calibration curve of gallic acid was drawn (Figure 2).
Figure 2: Standard curve of gallic acid
Evaluation of total flavonoids
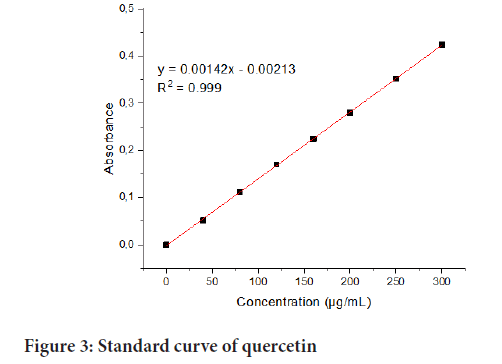
The evaluation of total flavonoids followed a previous method (Pakade V, et al., 2013) with some modifications. Essentially, 300 µL of the extract was put in a 10 mL volumetric flask, and 4000 µL of ultrapure water (Milli-Q) was added. After that, 300 µL of NaNO2 (5 g in 100 mL) was added, and the contents were shaken well. After 5 minutes, 3000 µL of AlCl3 solution (1:100, wt/vol) was also added. The contents were allowed to stand for 6 min, after which 2000 µL of 1.0 M sodium hydroxide solution was added. Finally, 400 µL of ultrapure water (Milli-Q) was added to bring the total volume of the reaction mixture to 10 mL. The contents were shaken well again, and the absorbance for triplicates was measured at 510 nm using a UV-visible spectrophotometer (Varian, Cary 50 Conc, Darmstadt, Germany). Quercetin was used as the standard for the calibration curve. The following calibration curve was used to calculate total flavonoid content in the methanolic (80%) extract of the sample (Figure 3).
Figure 3: Standard curve of quercetin
DPPH scavenging activity assay
A free radical 2,2 Diphenyl Picrylhydrazyl (DPPH) scavenging assay based on previous work (Brand-Williams W, et al., 1995) was followed to estimate the antioxidant activity of D. batokana leaves. Scavenging activity is the rate at which natural antioxidants attack free radicals and stabilise the reactive oxygen species (Rasheed A and Azeez RF, 2019). This procedure is used routinely in phytochemical studies because it is fast and accurate, and DPPH is able to interact with the whole sample extract. Briefly, the extract was composed of 3.0 g of dried D. batokanaleaves that were crushed and grinded into fine powder. To qualify and quantify the scavenging potential of DPPH on the D. batokanamethanolic (80%) leaf extract, a stock solution of DPPH was prepared by combining 50 mg of DPPH with 100 mL of 80% methanol. From the stock solution, a working solution was made by diluting the stock solution with methanol (80%) using a dilution ratio of 1:5 of stock solution to 80% methanol. For the extract, there were five quantities, 10, 20, 30, 40, and 50 µL. A reaction mixture was composed of the 10-50 µL extract, 700 µL of the working DPPH solution, and this was filled up with 290, 280, 270, 260 and 250 µL methanol (80%) to make up 1 mL. A blank was set up using the 80% methanol, and every time the control solution was prepared using the working DPPH solution, the absorbance of the control sample was measured. The mixture was incubated in the dark for 45 minutes. At a wavelength of 517 nm, a spectrophotometer (Spectroquant, Prove 100, Merck, Johannesburg-South Africa) was used to measure the absorbance. Inhibition(%) was quantified using the following equation-
% Scavenging/Inhibition=(Absample-Abblank)/Abcontrol × 100
Where Absample was absorbance of the sample, Abblank was absorbance of the solvent (methanol), and Abcontrol was the absorbance of DPPH working solution.
Results and Discussion
Phytochemical screening using chemical tests
The data presented in Table 1 shows screening results of methanolic leaf extract of D. batokana. Preliminary phytochemical screening of methanolic extracts of D. batokana leaves has shown the presence of alkaloids, anthraquinones, cardiac glycosides, flavonoids, phenolic compounds, tannins, saponins, steroids, terpenoids and volatile oils.
| S.no | Metabolites | Methanolic extract |
|---|---|---|
| 1 | Alkaloids | + |
| 2 | Anthocyanins | - |
| 3 | Anthraquinones | + |
| 4 | Carbohydrates | + |
| 6 | Flavonoids | + |
| 7 | Cardiac glycosides | + |
| 8 | Proteins | + |
| 9 | Saponins | + |
| 10 | Steroids | + |
| 11 | Tannins | + |
| 12 | Terpenoids | + |
| 13 | Phenolics | + |
| 15 | Volatile oils | + |
Note: +: Present and -: Absent
Table 1: Results of phytochemical screening of the leaf extract of Diospyros batokana (Ebenaceae)
LCMS/MS screening
LCMS/MS screening of D. batokana leaves was interesting for the plant’s phytocompounds, mainly polyphenols that may present a valuable influence on human health (Yan Z, et al., 2020). The polyphenolic LCMS/ MS screening of D. batokanaleaves was carried out in the negative mode, where a higher number of phenolic compounds were found with higher intensity. In total, 19 polyphenols were identified with the aid of literature (Maulidiani M, et al., 2019; Ressaissi A, et al., 2021), including gallic acid, quercetin, Kaempferol-3-O-glucoside, Quercetin 3’-O-glucoside, isoflavonoids, and flavones (Table 2). Qualitative polyphenol screening of D. batokana leaf extract shows that the leaves have a complex phenolic profile that may help to explain the beneficial effects of the traditional use of the plant as a medicinal herb.
| Peak no. | Retention time (RT) | [M-H]- (m/z) |
(Mass spectroscopy) MS2 | Tentative compound | Formula |
|---|---|---|---|---|---|
| 33 | 4.19 | 169.0109 | 79, 97, 125 (100) | Gallic acid | C7H6O5 |
| 36 | 4.23 | 301.0487 | 169 (100%) | Quercetin | C15H10O7 |
| 39 | 4.31 | 169.0113 | 79, 124, 125 (100%) | Gallic acid isomer | C7H6O5 |
| 41 | 4.42 | 447.1766 | 161, 401 (100%), 402 | Kaempferol 3‑(2″‑galloylglucoside) | C28H24O15 |
| 42 | 4.43 | 463.1616 | 418, 417 (100%) , 255, 179, 161, 119, 89 | Quercetin 3'‑O‑glucoside | C21H20O12 |
| 46 | 4.51 | 301.0491 | 168, 149, 125, 124, 123 | Quercetin isomer | C15H10O7 |
| 47 | 5.58 | 447.1761 | 161, 139, 119, 113, 101, 89, 71 | Kaempferol‑3‑O‑glucoside isomer | C21H20O11 |
| 59 | 4.92 | 431.1815 | 179 (100%), 119, 89 | 5,7,8-Trihydroxyflavone-7- galactoside (Flavones) | C21H20O10 |
| 61 | 4.93 | 457.1252 | 164, 163, 120, 119 | Daidzein-6’’-Oacetate (Isoflavonoids) | C23H22O10 |
| 65 | 4.98 | 431.1832 | 385 (100%), 179, 119, 89 | 5,7,8-Trihydroxyflavone-7- galactoside (Flavones) isomer | C21H20O10 |
| 66 | 5 | 431.1834 | 385 (100%), 223, 179, 161, 153, 119, 89 | 5,7,8-Trihydroxyflavone-7- galactoside (Flavones) isomer | C21H20O10 |
| 76 | 5.26 | 479.0707 | - | Ajugasterone C isomer | C27H44O7 |
| 84 | 5.49 | 463.0754 | 301, 300, 271, 255 | 6-Hydroxykaempferol-3- glucoside (Flavonols) | C21H20O12 |
| 88 | 5.56 | 447.1393 | 4.3, 4.2, 401 (100%), 269, 161,113, 101, 85, 71 | Kaempferol‑3‑O‑glucoside isomer | C21H20O11 |
| 99 | 6.32 | 301.029 | 227, 209, 183 (100%), 178, 150 | Quercetin | C15H10O7 |
| 103 | 6.53 | 329.2254 | 229, 211, 171, 139 | 1‑O‑Vanilloyl‑beta‑D‑glucose | C14H18O9 |
| 106 | 6.7 | 137.0202 | 108, 94, 93 (100%) | 2‑Methoxy‑1,4‑benzoquinone | C7H6O3 |
| 133 | 7.84 | 293.1681 | 236, 221 (100%), 220, 205 | Nordihydrocapsiate (Methoxyphenols) | C17H26O4 |
| 149 | 8.28 | 293.1693 | 236 (100%), 221 (94.1), 220, 205 | Nordihydrocapsiate (Methoxyphenols) isomer | C17H26O4 |
Table 2: Identification of compounds in D. batokana leaf based on their UHPLC MS/MS spectral characteristics in negative ion mode
Total phenolic content
Phenolic compounds are essential phytocompounds with a redox character offering antioxidant potential (Yildiz S, et al., 2021). The free radical scavenging ability of phenolic compounds results from the hydroxyl groups in these compounds. Consequently, the total phenolic content of the D. batokana methanolic (80%) leaf extract was determined using the Folin-Ciocalteu reagent as described earlier total phenolic content. The results presented in Table 3 were obtained using a calibration curve (y=0.00486x-0.00975, R2=0.999) of gallic acid (0-200 µg/mL) and expressed in mg of Gallic Acid Equivalent (GAE) per g of extract. The total content of phenolic compounds in 80% methanolic extract of D. batokanaleaves was determined to be 50.3780 ± 0.1188 mg GAE/g. The extraction technique and solvent type are some parameters responsible for dissolving analytes of interest from plant material (Krakowska-Sieprawska A, et al., 2020; Tzanova M, et al., 2020). Phytocompounds can either be polar or nonpolar. Phenolic compounds being organic and polar in nature, are obviously more soluble in polar organic solvents, and thus methanol was chosen as the extracting solvent in this study. It must be noted that the results for total phenolic content cannot show the content of compounds that are more specific in the extract obtained (Macheix JJ, et al., 2018; Hrnčič KM, et al., 2019; Rožanc J, et al., 2021). Consequently, total flavonoid content has to be determined as a presumption of a more specific chemical group of compounds.
| Replicate extracts | Absorbance (λ=765 nm) | Concentration | TPC (µg/mL) | STD |
|---|---|---|---|---|
| A | 0.611 | 127.7263 | 127.6578 | 0.118796 |
| B | 0.61 | 127.5206 | ||
| C | 0.611 | 127.7263 |
Table 3: Total Phenolic Content (TPC) of D. batokana (Ebenaceae) leaves (n=3)
Total Flavonoid Content (TFC)
The results of spectrophotometric estimation of the total flavonoid content in terms of standard quercetin are presented in Table 4. The determination method was based on the complexation reaction of flavonoids with aluminium chloride in a colorimetric method. It was found that the total flavonoid content was 105.5495 ± 0.4066 mg QE/g in the methanolic (80%) leaf extract (di Santo MC, et al., 2021; Gulcin İ, 2020). Quercetin Calibration standard solutions were prepared in triplicate and studied under ambient conditions. The calibration curve was found to be linear in the run 0.0300 µg/mL standard quercetin. The regression equation (y=0.00142x-0.00213) and R2=0.999 showed a good linearity reciprocation for the spectrophotometric method followed for estimating total flavonoid content. The chemical structure of flavonoids is founded on a fifteen-carbon skeleton with two benzene rings linked through a heterocyclic pyrene ring. The biological activities of these phytocompounds rely on certain elements, including their general class structure, substituents, conjugation, and extent of both polymerisation and hydroxylation (Savych A and Mazur O, 2021). In general, functional group hydroxyls in flavonoids bring about their antioxidant influence by chelating metal ions and scavenging free radicals. It has to be noted that antioxidant potential of flavonoids in the management, treatment, control and prevention of degenerative diseases is crucial in suppressing Reactive Oxygen Species (ROS) formation either by inhibiting enzymes or by chelating the trace elements involved in the formation of free radicals (Dzoyem JP, et al., 2020); reactive oxygen species quenching; inhibiting the enzymes that facilitate the formation of ROS such as mitochondrial succinoxidase, glutathione S-transferase, xanthine oxidase, nitric oxide synthase, Nicotinamide Adenine Dinucleotide Phosphate (NADH) oxidase, microsomal monooxygenase and so on (Savych A and Mazur O, 2021). Consequently, the high flavonoid content in the D. batokana leaves indicates these herbal drugs capacity to arrest the development of oxidative stress, the major pathogenic mechanism of degenerative diseases.
| Replicate extracts | Absorbance (λ=510 nm) | Concentration | TFC (µg/mL) | STD |
|---|---|---|---|---|
| A | 0.378 | 267.6072 | 267.4624 | 0.406585 |
| B | 0.378 | 267.6972 | ||
| C | 0.377 | 266.993 |
Table 4: Total Flavonoid Content (TFC) of D. batokana (Ebenaceae) leaves (n=3)
Antioxidant activity
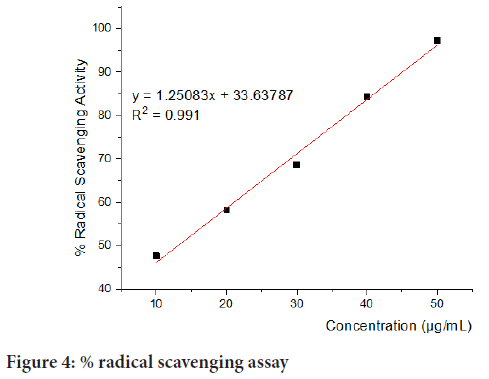
The radical scavenging activity of the D. batokanaleaf extracts was determined by using the DPPH assay. The potentiality of D. batokanaleaves to act as a reductant in turning the deep purple colour of DPPH to its reduced yellow form DPPH-H was examined. The radical scavenging activity was found to be 47.67, 58.14, 68.60, 84.22 and 97.18% at concentrations of 10, 20, 30, 40, and 50 μg/ml respectively (Figure 4). On the other hand, IC50 was found to be 13.08 µg/mL. The IC50 indicates the scavenging power of the leaf extract. On the one hand, a lower IC50 value, as found in this study, indicates a high antioxidant activity, as the extract scavenges or quenches the free radicals at a lower concentration, and on the other hand, a high IC50 value is indicative of lower antioxidant potential.
Figure 4: % radical scavenging assay
Medicinal plants are a rising and possible source of traditional medicine for managing, controlling, and treating both human and animal maladies (Dzoyem JP, et al., 2020). Various plant secondary metabolites have been found to exhibit medicinal characteristics and are extensively used in traditional medicine in Zambia in particular and Africa in general (McGaw LJ, et al., 2022). Preliminary phytochemical screening is carried out to reveal the presence of important phytocomponents in plant extracts, which assists in the discovery of novel drugs with beneficial pharmacological effects and finding out the ingenuity of the drug (Bharathi PS, et al., 2010). Preliminary phytochemical screening results revealed the presence of alkaloids, glycosides, flavonoids, phenolic compounds, tannins, saponins, steroids, terpenoids and volatile oils in the methanolic (80%) extract of D. batokana leaves. This is the first preliminary phytochemical screening results on the plant.
The occurrence of these phytocompounds is indicative of the multi-application of D. batokana plant in Zambian traditional medicine. For example, the analgesic properties of the plant could be attributed to the presence of alkaloids. Alkaloids like codeine and morphine have been found to possess analgesic properties (Bribi N, 2018). Further, the presence of anthraquinones makes the plant a good candidate for its use in cancer treatment. Anthraquinones possess anticancer, anti-arthritic and laxative activities (Cheemalamarri C, et al., 2022). In addition, the plant possesses phenolic compounds, which have been associated with antioxidative properties due to the presence of carboxylate and hydroxyl groups (Olszowy M, 2019). This might be why the plant under study is traditionally used to manage degenerative diseases such as diabetes in Zambia. Therefore, the leaf extract was investigated for antioxidant activity in this study.
The occurrence of cardiac glycosides in D. batokana may be behind its effectiveness in treating hypertension as traditional medicine. Cardiac glycosides are great diuretics and further impact the heart favourably with unmediated activity on the heart, aiding its sturdiness and rate of shortening as it contracts (Mukhtar Y, et al., 2022; Das BB, et al., 2021). In this vein, the plant might be a viable alternative for the management and control of heart diseases, and therefore, its lead cardiac glycosides responsible for this activity should be structurally elucidated.
The presence of flavonoids in the leaf of D. batokana indicates that the plant might possess antibacterial, antioxidant, antifungal, antiviral and antidiabetic potentialities because such pharmacological activities are associated with flavonoids (Alam F, et al., 2022; Rasouli H, et al., 2019; Ullah A, et al., 2020). Further, the plant was found to contain saponins in its leaves. These metabolites are associated with lowering cholesterolemia and assist the body in absorbing calcium (Mukhtar Y, et al., 2022). Thus, D. batokana is a promising medicinal plant for the prevention and treatment of arthrosclerosis which is a health challenge usually precipitated by too much cholesterol.
The presence of tannins might explain several medicinal properties of D. batokana. Firstly, tannins are useful for treating skin inflammation and promote wound healing by precipitating proteins present on the wound, hence creating a protecting film on the wound, and in this way help in arresting bleeding (Ren YY, et al., 2021). Secondly, tannins have also been used in treating diarrhoea (Girard M and Bee G, 2020; Russo M, et al., 2018) and traditionally for protecting the inflamed outer layer of the mouth and treating wounds and catarrh (Fraga-Corral M, et al., 2021). Furthermore, steroids were also tested to be present in the leaves of D. batokana, therefore validating the plant’s application to act against diarrheal in traditional herbal medicine (Mekonnen B, et al., 2018).
The health benefits of polyphenolic compounds have been alluded to and are well documented, including their potential antioxidant effects (Carrara M, et al., 2021). The results of total phenolic and flavonoid content are presented in Tables 3 and 4. The content of these polyphenols is commensurate with the LCMS/MS screening results, which revealed several phenolics and flavonoids, including gallic acid, quercetin, Kaempferol 3 O glucoside, Quercetin 3’ O glucoside, isoflavonoids, and flavones. Further, the enhanced polyphenolic content is in tandem with the enhanced antioxidative capacity of the extract, as revealed by DPPH radical scavenging activity results. The low IC50 value of 13.08 µg/mL indicates higher radical quenching ability and consequently better and desirable antioxidant potential. The results suggest that using D. batokanaleaves can improve human health and potentially reduce the damaging action of free radicals that enhance the progression of degenerative diseases related to oxidative stress, such as cancer and diabetes mellitus.
Conclusion
Preliminary phytochemical screening of D. batokanaleaves revealed the presence of important secondary metabolites alkaloids, glycosides, flavonoids, phenolic compounds, tannins, saponins, steroids, terpenoids and volatile oils. These phytocompounds are responsible for the observed therapeutic effects of the medicinal plant in traditional Zambian medicine. Further, the presence of significant amounts of phenolic compounds and flavonoids was in tandem with the tested high antioxidant activity of the plant. This is because polyphenols are major contributors to the antioxidant potential. Thus, D. batokanaleaves are a potential source of natural antioxidants.
Acknowledgement
Authors wholeheartedly acknowledges the support rendered by the Ministry of Education of the Republic of Zambia, Copperbelt University-Africa Centre of Excellence in Sustainable Mining (CBU-ACESM) and the Mukuba University, without which this study would not have been achievable.
Author Contributions
This study was carried out as a part of the doctoral dissertation work by Bitwell Chibuye and supervised by Professor Indra Sen Singh, Professor Kenneth Kakoma Maseka and Professor Luke Chimuka, which focused on phytochemical studies, LCMSMS screening and metal analysis of some medicinal plants traditionally used in the mining towns in Zambia.
References
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat med. 2019; 25(12): 1822-1832.
[Crossref] [Google scholar] [Pubmed]
- Li X, Sun R, Liu R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol Res. 2019; 144: 210-226.
[Crossref] [Google scholar] [Pubmed]
- Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020; 19(5): 1199-1209.
[Google scholar] [Pubmed]
- Krupa J, Sureshkumar J, Silambarasan R, Priyadarshini K, Ayyanar M. Integration of traditional herbal medicines among the indigenous communities in Thiruvarur District of Tamil Nadu, India. J Ayurveda Integr Med. 2019; 10(1): 32-37.
[Crossref] [Google scholar] [Pubmed]
- Ahmed IA, Mikail MA, Zamakshshari NH, Mustafa MR, Hashim NM, Othman R. Trends and challenges in phytotherapy and phytocosmetics for skin aging. Saudi J Biol Sci. 2022; 103363.
[Crossref] [Google scholar] [Pubmed]
- Kamler JF, Klare U, Macdonald DW. Seed dispersal potential of jackals and foxes in semi-arid habitats of South Africa. J Arid Environ. 2020; 183: 104284.
- Yao X, Zhang F, Corlett RT. Utilization of the hollies (Ilex L. spp.): A review. Forests. 2022; 13(1): 94.
- Guan C, Chachar S, Zhang P, Hu C, Wang R, Yang Y. Inter-and intra-specific genetic diversity in diospyros using SCoT and IRAP markers. Hortic plant J. 2020; 6(2): 71-80.
- Sulub-Tun RA, Rodríguez-García CM, Peraza-Echeverría L, Torres-Tapia LW, Peraza-Sánchez SR, Pérez-Brito D, et al. Antifungal activity of wild and nursery Diospyros cuneata, a native species of dune scrub. S Afr J Bot. 2020; 131: 484-493.
- Ramírez-Briones E, Rodríguez Macías R, Casarrubias Castillo K, Del Río RE, Martínez-Gallardo N, Tiessen A, et al. Fruits of wild and semi-domesticated Diospyros tree species have contrasting phenological, metabolic, and antioxidant activity profiles. J Sci Food Agric. 2019; 99(13): 6020-6031.
[Crossref] [Google scholar] [Pubmed]
- Rauf A, Uddin G, Patel S, Khan A, Halim SA, Bawazeer S, et al. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed Pharmacother. 2017; 91: 714-730.
[Crossref] [Google scholar] [Pubmed]
- Mishra Y, Sharma L, Dhiman M, Sharma MM. Endophytic fungal diversity of selected medicinal plants and their bio-potential applications. InFungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology. 2021; 227-283. Academic Press.
- Esteban-Muñoz A, Sánchez-Hernández S, Samaniego-Sánchez C, Giménez-Martínez R, Olalla-Herrera M. Differences in the phenolic profile by uplc coupled to high resolution mass spectrometry and antioxidant capacity of two Diospyros kaki varieties. Antioxidants. 2020; 10(1): 31.
[Crossref] [Google scholar] [Pubmed]
- Maulidiani M, Abdul-Hamid NA, Abas F, Park YS, Park YK, Kim YM, et al. Detection of bioactive compounds in persimmon (Diospyros kaki) using UPLC-ESI-Orbitrap-MS/MS and fluorescence analyses. Microchem J. 2019; 149: 103978.
- Matheus JR, Andrade CJ, Miyahira RF, Fai AE. Persimmon (Diospyros kaki L.): Chemical properties, bioactive compounds and potential use in the development of new products: A review. Food Rev Int. 2020: 1-8.
- Guo J, Tong M, Tang J, Bian H, Wan X, He L, et al. Analysis of multiple pesticide residues in polyphenol-rich agricultural products by UPLC-MS/MS using a modified QuEChERS extraction and dilution method. Food chem. 2019; 274: 452-459.
[Crossref] [Google scholar] [Pubmed]
- Pakade V, Cukrowska E, Chimuka L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. S Afr J Sci. 2013; 109(3): 1-5.
- Ralepele FM, Chimuka L, Nuapia Y, Risenga I. UPLC-DAD-QTOF-MS/MS analysis of targeted poly-phenolic compounds from Moringa oleifera leaves as function of seasonal responses. S Afr J Bot. 2021; 143: 107-115.
- Makkar HP, Becker K, Abel HJ, Pawelzik E. Nutrient contents, rumen protein degradability and antinutritional factors in some colour-and white-flowering cultivars of Vicia faba beans. J Sci Food Agric. 1997; 75(4): 511-520.
- Mukhtar Y, Aliyu BS, Zakari SM, Aliko AA, Habib AA, Zubairu SM, et al. Phytochemical, pharmacognostic and acute toxicity study of Diospyros mespiliformis (african ebony) stem bark. Biosci J. 2022; 10(1): 28-40.
- Ebbo AA, Mammam M, Suleiman MM, Ahmed A, Bello A. Preliminary phytochemical screening of Diospyros mespiliformis. Anat Physiol. 2014; 4: 2161.
- Ahmad T, Singh SB, Pandey S. Phytochemical screening and physicochemical parameters of crude drugs: A brief review. Int J Pharm Res Rev. 2013; 2(12): 53-60.
- Mehdi MA, Alarabi FY, Farooqui MA, Pradhan VI. Phytochemical screening and antiamebic studies of Tamarindus indica of leaves extract. Asian J Pharm Clin Res. 2019; 12(2): 507-512.
- Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019; 8(4): 96.
[Crossref ] [Google scholar] [Pubmed ]
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995; 28(1): 25-30.
[Crossref ] [Google scholar]
- Rasheed A, Azeez RF. A review on natural antioxidants. Tradit Complement Med. 2019.
- Yan Z, Zhong Y, Duan Y, Chen Q, Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr. 2020; 6(2): 115-123.
[Crossref] [Google scholar] [Pubmed]
- Maulidiani M, Abdul-Hamid NA, Abas F, Park YS, Park YK, Kim YM, et al. Detection of bioactive compounds in persimmon (Diospyros kaki) using UPLC-ESI-Orbitrap-MS/MS and fluorescence analyses. Microchem J. 2019; 149: 103978.
- Ressaissi A, Mannai A, Ben-Attia M, Serralheiro ML, El-Bok S. Diospyros kaki leaves decoction phytochemical characterization and bioactivities evaluation: LC-MS-QTOF identification, antioxidant activity, enzyme inhibition and cytotoxicity toward HepG2 and Mcf-7 Cell Lines. Research square. 2021.
- Yildiz S, Turan S, Kiralan M, Ramadan MF. Antioxidant properties of thymol, carvacrol, and thymoquinone and its efficiencies on the stabilization of refined and stripped corn oils. J Food Meas Charact. 2021; 15(1): 621-632.
- Krakowska-Sieprawska A, Rafinska K, Walczak-Skierska J, Buszewski B. The influence of plant material enzymatic hydrolysis and extraction conditions on the polyphenolic profiles and antioxidant activity of extracts: A green and efficient approach. Molecules. 2020; 25(9): 2074.
[Crossref] [Google scholar] [Pubmed]
- Tzanova M, Atanasov V, Yaneva Z, Ivanova D, Dinev T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes. 2020; 8(10): 1222.
- Macheix JJ, Fleuriet A, Billot J. Fruit phenolics. CRC press. 2018.
- Hrncic KM, Španinger E, Košir IJ, Knez Ž, Bren U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients. 2019; 11(2): 257.
[Crossref] [Google scholar] [Pubmed]
- Rožanc J, Kotnik P, Milojevic M, Gradišnik L, Hrncic KM, Knez Ž, et al. Different Cannabis sativa extraction methods result in different biological activities against a colon cancer cell line and healthy colon cells. Plants. 2021; 10(3): 566.
[Crossref] [Google scholar] [Pubmed]
- di Santo MC, D’Antoni CL, Rubio AP, Alaimo A, Pérez OE. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications: A review. Biomed Pharmacother. 2021; 142: 111970.
[Crossref] [Google scholar] [Pubmed]
- Gulcin I. Antioxidants and antioxidant methods: An updated overview. Arch Toxicol. 2020; 94(3): 651-715.
[Crossref] [Google scholar] [Pubmed]
- Savych A, Mazur O. Antioxidant activity in vitro of antidiabetic herbal mixtures. PharmacologyOnLine. 2021; 2: 17-24.
- Dzoyem JP, Tchuenteu RT, Mbarawa K, Keza A, Roland A, Njouendou AJ, et al. Ethnoveterinary medicine and medicinal plants used in the treatment of livestock diseases in Cameroon. InEthnoveterinary medicine. Springer, Cham. 2020.
- McGaw LJ, Omokhua-Uyi AG, Finnie JF, van Staden J. Invasive alien plants and weeds in South Africa: A review of their applications in traditional medicine and potential pharmaceutical properties. J Ethnopharmacol. 2022; 283: 114564.
[Crossref] [Google scholar] [Pubmed]
- Bharathi PS, Thippeswamy G, Sheela M, Lucas JR Jt, Steven AR. Targeting tumor angiogenesis for preclinical validation of antiangiogenic compounds from medicinal plants. 2010.
- Bribi N. Pharmacological activity of alkaloids: A review. Asian J Botany. 2018; 1(1): 1-6.
- Cheemalamarri C, Batchu UR, Thallamapuram NP, Katragadda SB, Shetty RP. A review on hydroxy anthraquinones from bacteria: Crosstalk’s of structures and biological activities. Nat Prod Res. 2022; 1-20.
[Crossref] [Google scholar] [Pubmed]
- Olszowy M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol Biochem. 2019; 144: 135-143.
[Crossref] [Google scholar] [Pubmed]
- Das BB, Moskowitz WB, Butler J. Current and future drug and device therapies for pediatric heart failure patients: Potential lessons from adult trials. Children. 2021; 8(5): 322.
[Crossref] [Google scholar] [Pubmed]
- Alam F, Mohammadin K, Shafique Z, Amjad ST, Asad MH. Citrus flavonoids as potential therapeutic agents: A review. Phytother Res. 2022; 36(4): 1417-1441.
[Crossref] [Google scholar] [Pubmed]
- Rasouli H, Hosseini-Ghazvini SM, Khodarahmi R. Therapeutic potentials of the most studied flavonoids: Highlighting antibacterial and antidiabetic functionalities. Stud Nat Prod Chem. 2019; 60: 85-122.
- Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020; 25(22): 5243.
[Crossref] [Google scholar] [Pubmed]
- Mukhtar Y, Aliyu BS, Zakari SM, Aliko AA, Habib AA, Zubairu SM, et al. Phytochemical, pharmacognostic and acute toxicity study of Diospyros mespiliformis (african ebony) stem bark. Biosci J. 2022; 10(1): 28-40.
- Ren YY, Zhang XR, Li TN, Zeng YJ, Wang J, Huang QW. Galla chinensis, a traditional chinese medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J Ethnopharmacol. 2021; 278: 114247.
[Crossref] [Google scholar] [Pubmed]
- Girard M, Bee G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal. 2020; 14(1): 95-107.
[Crossref] [Google scholar] [Pubmed]
- Russo M, Coppola V, Giannetti E, Buonavolontà R, Piscitelli A, Staiano A. Oral administration of tannins and flavonoids in children with acute diarrhea: A pilot, randomized, control-case study. Ital J Pediatr. 2018; 44(1): 1-6.
[Crossref] [Google scholar] [Pubmed]
- Fraga-Corral M, Otero P, Cassani L, Echave J, Garcia-Oliveira P, Carpena M, et al. Traditional applications of tannin rich extracts supported by scientific data: Chemical composition, bioavailability and bioaccessibility. Foods. 2021; 10(2): 251.
[Crossref] [Google scholar] [Pubmed]
- Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrheal activity of 80% methanolic leaf extract of Justicia schimperiana. Evid Based Complement Alternat Med. 2018.
[Crossref] [Google scholar] [Pubmed]
- Carrara M, Kelly MT, Roso F, Larroque M, Margout D. Potential of olive oil mill wastewater as a source of polyphenols for the treatment of skin disorders: A review. J Agric Food Chem. 2021; 69(26): 7268-7284.
[Crossref] [Google scholar] [Pubmed]
Author Info
Bitwell Chibuye1,2,3*, Indra Sen Singh2, Luke Chimuka3 and Kakoma Maseka22Department of Chemistry, School of Mathematics and Natural Sciences, The Copperbelt University, Kitwe, Zambia
3Molecular Sciences Institute, School of Chemistry, University of Witwatersrand, Johannesburg, South Africa
Citation: Chibuye B: Phytochemical and LCMS/MS Screening, Total Phenolic and Flavonoid Content and Antioxidant Activity of the Leaves of Diospyros batokana (Ebenaceae)
Received: 20-Jan-2023 Accepted: 03-Feb-2023 Published: 10-Feb-2023, DOI: 10.31858/0975-8453.14.2.105-112
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3