Research Article - (2023) Volume 14, Issue 5
Abstract
Taylorella equigenitalis is the causative agent of Contagious Equine Metritis (CEM) and is a severe epidemic that causes interim infertility in the horse nurture industry. Nowadays, the Polymerase Chain Reaction (PCR) technique is applied to detect these bacteria as one of the most sensitive and the fastest methods. In the current study, T. equigenitalis was followed by sampling from northwest of Iran in summer 2021 in clitoris sinusoidal-fossa and urinary tract in 210 mares and 30 stallions. Mares’ age range was 3 to 20 years old and stallions’ age range was 4 to 10 years old. Two swabs one for culture, and the other was used for PCR in equine clitoris sinusoidal and mares’ urinary tract sinus. They were transferred to the veterinary microbiology laboratory of the University of Tabriz. In received samples, investigation with bacterial culture method in chocolate agar medium and one-week presence in CO2, the examination was not successful; but we observed three positive samples from 240 cases (3 mares) with the genotypic method after DNA extraction and PCR in received samples for studied horses of Ardabil province and one sample (mare) was positive in West Azerbaijan. East Azerbaijan didn’t have a positive sample among 80 cases. This study shows that the rapid method for recognition of T. equigenitalis by using PCR is based on bacterial genome 16S rDNA part and it is a special technique with high-level features against culturing cases.
Keywords
Mares, Contagious equine metritis, T. equigenitalis, Polymerase chain reaction
Introduction
Taylorella equigenitalis is a gram-negative, microaerophilic, catalase-positive, oxidase-positive, but biochemically unreactive coccobacillus that grows slowly (3-7 days on specialized hemolyzed blood agar). This bacterium is the source of CEM and its only natural host is the horse. The disease caused by this bacterium is called equine infectious metritis (Timoney PJ, 1996). CEM has been replicated in donkeys but attempts to transmit the disease to cattle, swine, sheep, and cats have failed. Certain species of laboratory rodents are susceptible to experimental infection with T. equigenitalis following exposure by the intrauterine route (Timoney PJ and Powell DG, 1988). Since the initial reports, CEM has been recorded in various horse populations worldwide, including many European countries, Japan, Australia, and North and South America. In some of these countries, the disease has been successfully eradicated. Among the different horse breeds, the Thoroughbred breed is highly sensitive to this bacterium (Timoney PJ, 1996). IEM is a highly contagious, sexually transmitted disease first described in 1977 in Newmarket, England (Timoney PJ, 1996). T. equigenitalis is isolated from the clitoral sinus, urethral, mare pair, dorsal sheath, and prefrontal sinus of infected stallions. The carrier mare or stallion is the most important source of infection in outbreaks of CEM (Timoney PJ, 1996; Timoney PJ and Powell DG, 1988).
T. equigenitalis is transmitted through infected fomites or direct genital contact with carrier stallions or mares during mating (vaginal speculums, artificial vagina, or wash buckets). An important factor in the spread and persistence of T. equigenitalis in different horse populations worldwide is its vector in male and female horses (Timoney PJ, 1996; Timoney PJ and Powell DG, 1988).
Unlike mares, stallions exposed to T. equigenitalis do not develop clinical signs of disease. Vaginal discharge with variable degrees of vaginal and cervix irritation, temporary and transient infertility, and premature abortion (first 60 days) in mares are all clinical indicators of this condition (Quinn PJ, et al, 2002). The main disease reservoirs are stallions, who are asymptomatic and can carry the disease for weeks or even years without showing any symptoms. Re-exposure to T. equigenitalis results in minimal or no clinical evidence of CEM in mares that have previously been infected (Timoney PJ and Powell DG, 1988). The persistence of the organism in the reproductive system of chronically infected mares will not be an obstacle to maintaining a normal pregnancy in most cases. Abortion is a rare consequence of an infection in the pregnant mare (Nakashiro H, et al., 1981).
Unlike mares, stallions exposed to T. equigenitalis do not develop clinical signs of disease. Vaginal discharge with variable degrees of vaginal and cervix irritation, temporary and transient infertility, and premature abortion (first 60 days) in mares are all clinical indicators of this condition (Quinn PJ, et al, 2002). The main disease reservoirs are stallions, who are asymptomatic and can carry the disease for weeks or even years without showing any symptoms. Re-exposure to T. equigenitalis results in minimal or no clinical evidence of CEM in mares that have previously been infected (Timoney PJ and Powell DG, 1988). The persistence of the organism in the reproductive system of chronically infected mares will not be an obstacle to maintaining a normal pregnancy in most cases. Abortion is a rare consequence of an infection in the pregnant mare (Nakashiro H, et al., 1981).
Infectious metritis of horses has been reported mainly in Europe. Detecting and identifying this bacterium can be somewhat challenging due to the difficulty of growing it on the culture medium. T. equigenitalis is microaerophilic and grows slowly. When transferred to a laboratory in Amies Harcoull culture medium, it may lose viability relative to the matrix in the horse's genital membrane (Swerczek TW, 1978). Several days of culture (3-10 days) are required to produce visible colonies. This is problematic not only because commensals overgrow the T. equigenitalis (Bleumink-Pluym NM, et al., 1994), but also because bacteria with similar morphology and phenotypic characteristics may be present (Baron EJ, et al., 2013). While isolation of T. equigenitalis in culture has long been used as the only definitive tool for diagnosing CEM in mares or confirming vector status in stallions (Timoney PJ, 1996; Timoney PJ and Powell DG, 1988), a polymerase chain reaction has recently been developed to identify this organism. This method may provide an equally sensitive and faster tool for confirming infection (Bleumink-Pluym NM, et al., 1994). When it is extremely difficult to culture T. equigenitalis from locations contaminated with other gram-negative or gram-positive bacteria, polymerase chain reaction will be especially helpful. T. equigenitalis is a fastidious organism that requires specific conditions for growth in the laboratory (Timoney PJ, 1996). Therefore, all organism culture swabs should be placed in an antibiotic-free transport medium, e.g., Amie's medium, immediately after collection and preferably maintained under transport condition (Baron EJ, et al., 2013). In this paper, our method was compared with the methods of several papers including Arata AB, et al., 2001; Bleumink-Pluym NM, et al., 1994; Luddy S and Kutzler MA, 2010; Moore JE, et al., 2001; Ousey JC, et al., 2009, Zdovc I, et al., 2005; Anzai T, et al., 2005; Duquesne F, et al., 2007; Matsuda M and Moore JE, 2003; Parlevliet JM, et al., 1997; Premanandh J, et al., 2003; Tel OY, et al., 2010; Timoney PJ, 1996; Timoney PJ and Powell DG, 1988; Timoney PJ, et al., 1978.
Materials and Methods
Sampling survey and sample transporting
The present study was conducted in the summer of 2021 for horse torture centers in the northwest of Iran (Ardabil, East Azerbaijan, and West Azerbaijan provinces) (Figure 1).
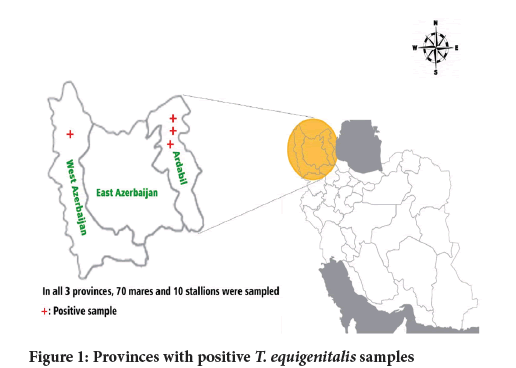
Figure 1: Provinces with positive T. equigenitalis samples
Sampling was done in the clitoris middle sinusoidal- cavity and urinary tract of 210 infertile mares, penile skin, and urinary tract for 30 stallions by using sterilized swabs. It should be noted that two sterilized swabs were received from each animal. One swab was prepared for culture on chocolate agar medium, and another swab was prepared for conducting PCR and tracking T. equigenitalis.
Swabs were placed on capped and sterilized Falcons containing 6 ml of AMIES transfer medium to culture bacteria. Also, the prepared swab was placed in a 2 ml Phosphate-Buffered Saline (PBS) microtube for direct PCR analysis. Received samples were recorded and coded separately based on province, horse features, sample codes such as age, number of parturitions, number of infertile estruses, club name, infertility time for mares, extension experience during season, disease experience, and club name in Stallions. The samples were transferred to the microbiology laboratory of the Tabriz University Faculty of Veterinary Medicine on ice in rubber dishes for 24 hours.
Mares and stallions received sample features
Two hundred forty samples, including 210 mares (7.4 ± 4.7 years old) and 30 stallions (6.9 ± 2.0 years old), were collected from different study areas (63 samples from Ardabil, 80 samples from Urmia, 14 samples from Ahar, 66 samples from Tabriz, 10 samples from Sarein and 7 samples from Nir cities). In mares, the average number of births and infertile estrus were 2.4 and 3.0, respectively.
Bacteriological examinations
Samples were streaked onto Columbia agar plates enriched with 10% horse blood. Each sample was tested on two agar plates, one containing 200 mg/L streptomycins and 100 mg/L cycloheximide and the other containing 1 g/L trimethoprim, 5 g/L clindamycin, and 5 mg/L amphotericin B. After incubating for five days at 37°C in 5% CO2, gram staining, catalase, and oxidase tests were performed on isolated colonies.
PCR
Bacterial DNA extraction was performed directly from the swabs using the FavorPrep tissue genomic DNA extraction Mini Kit according to the manufacturer’s instructions (Yekta Tajhiz Azma, Iran). To minimize false-positive results, genomic DNA was extracted from T. equigenitalis (Type strain NCTC 11225).
Alignment of 16S rDNA sequences of different bacterial species revealed regions that contained sequences specific to T. equigenitalis. Two primers with the following sequences were selected-Tay F (positions 43 to 67), CAGCATAAGGAGAGCTTGCTTTTCT; primer Tay R (positions 608 to 628), CTCGACAGTTAGTTAGAAATGCAGT. These sequences were synthesized by Sinacolon.
PCR amplification
To minimize contamination, all reactions were conducted in a PCR hood, separate from the room used to extract DNA, amplify and post-amplify. The initial optimization of PCR amplification settings involved adjusting the annealing temperature, primer concentration, and DNA template concentration independently. Following optimization, reaction mixes (25 µl) were set up as follows-12.5 µl 2 × master mix, 0.1 µM (10 pmol/ µl) of each primer (Tay F and Tay R), and 2 µl of DNA template. Thermal cycling conditions were 95°C for 5 minutes; 35 cycles at 95°C for 45 seconds and 58°C for one minute.
Results and Discussion
The results showed that out of 240 samples, four samples were positive and 236 samples were negative. Samples Taylorella isolates had no significant mean age, number of partitions, and number of infertile estruses (8.0 ± 4.5, 3.2 ± 2.0, and 3.0 ± 0.8), respectively.
Three samples from mares in Ardabil and one sample from the mare in Urmia had Taylorella equigenitalia infection. Samples from stallions did not have Taylorella isolate (Table 1). The results showed that there was no significant relationship between the city and the presence or absence of Taylorella isolate (Table 2). There was no significant relationship between the sex of animals and the presence or absence of Taylorella.
| City | Count and percentage | Outcome | Total | |
|---|---|---|---|---|
| - | + | |||
| Ardabil | Count | 60 | 3 | 63 |
| % within city | 95.2% | 4.8% | 100% | |
| Urmia | Count | 79 | 1 | 80 |
| % within city | 98.8% | 1.3% | 100% | |
| Ahar | Count | 14 | 0 | 14 |
| % within city | 100% | 0.0% | 100% | |
| Tabriz | Count | 66 | 0 | 66 |
| % within city | 100% | 0.0% | 100% | |
| Sarein | Count | 10 | 0 | 10 |
| % within city | 100% | 0.0% | 100% | |
| Nir | Count | 7 | 0 | 7 |
| % within city | 100% | 0.0% | 100% | |
| Total | Count | 236 | 4 | 240 |
| % within city | 98.3% | 1.7% | 100% | |
Table 1: Samples with Taylorella equigenitalia isolated from different cities.
| Statistical tests | Value | df | Asymptotic significance (2-sided) | Monte Carlo significance (2-sided) | Monte Carlo significance (2-sided) | |
|---|---|---|---|---|---|---|
| Significance | 99% Confidence Interval | |||||
| Upper bound | ||||||
| Pearson Chi-square | 5.412 | 5 | 0.368 | 0.321 | 0.309 | 0.333 |
| Likelihood ratio | 5.814 | 5 | 0.325 | 0.277 | 0.266 | 0.289 |
| Fisher's exact test | 4.741 | 0.451 | 0.438 | 0.463 | ||
| Note: No.of valid cases-240 | ||||||
Table 2: Relationship between city and presence or absence of Taylorella isolate
Gel electrophoresis (2% agarose), wells 1 to 6 of Taylorella isolates. Well 1 is the positive control, well 2 is the negative control, wells 3 to 6 are positive samples, and well M is the standard marker (kb 1) (Figure 2).
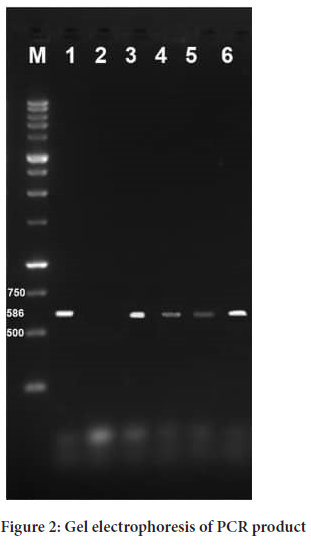
Figure 2: Provinces with positive T. equigenitalis samples
In mares, uterine health is directly correlated with nurturing and genesis, and its lack of health leads to pregnancy failure. T. equigenitalis causes severe cases of horse reproductive disease, such as CEM. Growth of the organism is often required for biochemical, morphological, and serological testing. Relying on these features may have hindered the understanding of true bacterial diversity and may not be applicable in many cases. With the increasing use of 16S rRNA gene sequences for phylogenetic, evolutionary, and diagnostic research, alternative techniques have become more common. All bacteria have 16S rRNA genes that accumulate mutations at a slow, steady rate over time, allowing them to be used as “molecular clocks” (Arata AB, et al., 2001).
The highly diverse 16S rRNA sequence provides unique signatures for each bacterium as well as critical information about their connections. Alternatively, because 16S rRNA molecules are structurally constrained, some conserved sequence sections may be found in all known bacteria (i.e., the eubacteria) and organisms. Species-specific primers may then be designed to identify these conserved bacterial 16S rRNA gene sequences and be used to amplify intervening, variable, or diagnostic segments (Swerczek TW, 1978; Alber J, et al., 2004). This method eliminates the need for bacterial culture and removes the necessity for prior phylogenetic knowledge. Traditional culture methods for the diagnosis of T. equigenitalis can be slow and insensitive, potentially leading to false-negative findings. T. equigenitalis is generally detected by culture after six days (Quinn PJ, et al., 2002). The inclusion of atypical colony morphology variations (Bleumink-Pluym NM, et al., 1994) and streptomycin sensitivity variants (Timoney PJ, et al., 1978) can further complicate phenotypic identification. For a variety of clinically significant infections, there has recently been a growing trend toward using PCR-based diagnostic methods. Although such a molecular approach has its drawbacks, the use of PCR-based detection assays has numerous advantages over those provided by the conventional methods, including increased speed and specificity of T. equigenitalis detection, as well as the ability to detect this organism in animals receiving antimicrobial chemotherapy. Findings from swabs obtained from mares and stallions in breeding programs rely heavily on disease prevention methods. The management of horses at stud may experience problems if inaccurate culture procedures are only relied upon, such as stallions refusing to accept mares unless they have a valid negative laboratory certificate (Timoney PJ, 1996).
Old methods of recognizing and separating bacteriologic culture were slow, expensive, and time-consuming. Also, they are not completely different in accurately distinguishing between some agents of the same phenotype (Luddy S and Kutzler MA, 2010). One of the main and fast methods for identifying bacterial agents in horse intercourse diseases is PCR, which is genomic-based and provides a faster and more sensitive diagnosis than previous bacterial culture methods. Fortunately, researchers have recently been able to diagnose the disease using PCR (Matsuda M and Moore JE, 2003).
The present study investigated the detection of T. equigenitalis in fertile and infertile mares in northwest Iran using culture and PCR methods. The results showed that the PCR method detected four positive cases.
Bleumink-Pluym NM, et al., 1994 used the PCR method to recognize T. equigenitalis among 64 received samples from the mare’s genital system for the first time. Parlevliet JM, et al., 1997 by investigating 107 mares in a random pattern without clinical symptoms, showed that there was no positive sample in the T. equigenitalis culture medium; but there were 54 positive cases (49%) in the PCR method. The mentioned study showed that PCR is a more accurate and sensitive method for bacteria recognition than the culture medium method.
Zdovc I, et al., 2005 in Slovenia showed that three samples of T. equigenitalis were positive in the PCR method among 245 mares under the experiment. All of the mare samples were negative in the culture medium.
Moore JE, et al., 2001 designed the PCR method with culture and sequencing by separating T. equigenitalis and Pseudomonas aeroginosa intercourse factors among horses. Sinus and clitoris swab samples in mares and urethral fossa and penile shaft in stallions were investigated. Samples were sequenced after culture and PCR reaction. They concluded that the PCR method in the culture medium was more efficient for the isolation and identification of horse breeding agents.
Subtracting and separating T. equigenitalis as the causative agent of CEM is a very difficult and complex procedure because of phenotype similarity in many organisms. Arata et al. studied T. equigenitalis subtraction and separation in similar organisms of horses and donkeys’ genital systems without clinical symptoms using a multiplex PCR method based on 16S rRNA coding gene reproduction. They found that PCR multiplex is a proper method for bacterial factor subtraction and separation against other similar cases (Arata AB, et al., 2001).
Premanandh J, et al., 2003 regarding some changes in Bleumink and Real-time Culture PCR methods in disease recognition showed that among 21 sampled mares in the Netherlands; 43 sampled mares in Dubai are important and only 10 cases in the Netherlands are positive according to the presence of T. equigenitalis. All Dubai mares are negative and their results are similar to the current study.
Duquesne F, et al., 2007 were successful in identifying T. equigenitalis and separating cases from T. asinigenitalis by direct PCR. They showed that the direct PCR method is more accurate and sensitive for the detection and isolation of T. equigenitalis without the need to grow the bacteria in the culture medium. From 1978 to 2010 in the United States, 86 horses were recognized as T. equigenitalis positive in the culture medium, which 50 samples in 1978, four samples in 1979, three samples in 2006, 28 samples in 2009, and one sample in 2010 confirmed.
Tel OY, et al., 2010 implemented T. equigenitalis recognition in Troboard horses of the Turkey Sanliurfa region. In the PCR method, five samples (27%) among 80 mares and eight samples among 90 mares (4.4%) were positive. Positive samples in the PCR method, are reported negative in the culture medium method.
Ousey JC, et al., 2009 investigated 2027 Troboard mares and mares with the RT-PCT method and bacteriological culture by investigating T. equigenitalis. Among 2027 mares regarding sinus and clitoris dominant, 26 cases were investigated with endometer in mares and 24 cases related to Utheral Fossa and sperm before vitality in swab pattern. After culture and RT-PCR reaction, six samples were recognized as positive among 2027 cases in the RT-PCR method and three cases were positive in the culture medium method. The sensitivity and specificity of the studied method with the RT-PCR method were 100%.
To increase the sensitivity and quality of the PCR method, as well as to obtain better and more accurate results, Dirsherl stated that it is better to perform the mentioned method on plates after incubation or directly on genital swab samples (Luddy S and Kutzler MA, 2010). Ibrahimi conducted a successful experiment in Iran by examining 57 mares for founding T. equigenitalis and 2 mares were investigated with the culture medium method in the horse nurturing club of ShareKurd city.
Researchers acted T. equigenitalis around Tehran horse nurturing centers which 9 mares and 3 mares were investigated by using the PCR method among a total of 80 samples. According to him, seven mare samples tested positive using direct PCR, and two were detected using PCR culture.
Conclusion
Based on the results of this study, T. equigenitalis is regarded as a severe threat in northwest Iran, and it is one of the main causes of infertility among horses in this region. It is concluded that the PCR method has high sensitivity and specificity in the detection of bacteria compared to the culture medium method. Considering the control measures for CEM instituted in most countries and the ever-increasing transportation of horses, both nationally and internationally, it would be beneficial to have an insight into the epidemiological status of this disease in Iran. Our findings highlight the need for the implementation of surveillance and control measures for CEM in Iran. Testing for disease prevalence should be made mandatory to reduce reproductive loss and stop the disease's propagation.
References
- Timoney PJ. Contagious equine metritis. Comp Immunol Microbiol Infect Dis. 1996; 19(3): 199-204.
[Crossref] [Google Scholar] [Pubmed]
- Timoney PJ, Powell DG. Contagious equine metritis-epidemiology and control. J Equine Vet Sci. 1988; 8(1): 42-46.
- Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC. Veterinary microbiology and microbial disease. Blackwell science. 2002.
- Nakashiro H, Naruse M, Sugimoto C, Isayama Y, Kuniyasu C. Isolation of Haemophilus equigenitalis from an aborted equine fetus. Natl Inst Anim Health Q. 1981; 21(4): 184-185.
[Google Scholar] [Pubmed]
- Timoney PJ, Ward J, McArdle JF. Daily variations in the shedding of the agent of contagious equine metritis 1977 by a carrier mare. Vet Rec. 1978; 103(10): 210-211.
[Crossref] [Google Scholar] [Pubmed]
- Swerczek TW. Inhibition of the CEM organism by the normal flora of the reproductive tract. Vet Rec. 1978; 103(6): 125.
[Crossref] [Google Scholar] [Pubmed]
- Bleumink-Pluym NM, Werdler ME, Houwers DJ, Parlevliet JM, Colenbrander B, van der Zeijst BA. Development and evaluation of PCR test for detection of Taylorella equigenitalis. J Clin Microbiol. 1994; 32(4): 893-896.
[Crossref] [Google Scholar] [Pubmed]
- Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson Jr RB, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)a. Clin Infect Dis. 2013; 57(4): e22-121.
[Crossref] [Google Scholar] [Pubmed]
- Arata AB, Cooke CL, Jang SS, Hirsh DC. Multiplex polymerase chain reaction for distinguishing Taylorella equigenitalis from Taylorella equigenitalis-like organisms. J Vet Diagn Invest. 2001; 13(3): 263-265.
[Crossref] [Google Scholar] [Pubmed]
- Luddy S, Kutzler MA. Contagious equine metritis within the United States: A review of the 2008 outbreak. J Equine Vet Sci. 2010; 30(8): 393-400.
- Moore JE, Buckley TC, Millar BC, Gibson P, Cannon G, Egan C, et al. Molecular surveillance of the incidence of Taylorella equigenitalis and Pseudomonas aeruginosa from horses in Ireland by sequence-specific PCR [SS-PCR]. Equine Vet J. 2001; 33(3): 319-322.
[Crossref] [Google Scholar] [Pubmed]
- Ousey JC, Palmer L, Cash RS, Grimes KJ, Fletcher AP, Barrelet A, et al. An investigation into the suitability of a commercial real-time PCR assay to screen for Taylorella equigenitalis in routine prebreeding equine genital swabs. Equine Vet J. 2009; 41(9): 878-882.
[Crossref] [Google Scholar] [Pubmed]
- Zdovc I, Ocepek M, Gruntar I, Pate M, Klobucar I, Krt B. Prevalence of Taylorella equigenitalis infection in stallions in Slovenia: Bacteriology compared with PCR examination. Equine Vet J. 2005; 37(3): 217-221.
[Crossref] [Google Scholar] [Pubmed]
- Anzai T, Kamada M, Niwa H, Eguchi M, Nishi H. Contagious equine metritis eradicated from Japan. J Vet Med Sci. 2012; 74(4): 519-522.
[Crossref] [Google Scholar] [Pubmed]
- Duquesne F, Pronost S, Laugier C, Petry S. Identification of Taylorella equigenitalis responsible for contagious equine metritis in equine genital swabs by direct polymerase chain reaction. Res Vet Sci. 2007; 82(1): 47-49.
[Crossref] [Google Scholar] [Pubmed]
- Matsuda M, Moore JE. Recent advances in molecular epidemiology and detection of Taylorella equigenitalis associated with Contagious Equine Metritis (CEM). Vet Microbiol. 2003; 97(1-2): 111-122.
[Crossref] [Google Scholar] [Pubmed]
- Parlevliet JM, Bleumink-Pluym NM, Houwers DJ, Remmen JL, Sluijter FJ, Colenbrander B. Epidemiologic aspects of Taylorella equigenitalis. Theriogenology. 1997; 47(6): 1169-1177.
[Crossref] [Google Scholar] [Pubmed]
- Premanandh J, George LV, Wernery U, Sasse J. Evaluation of a newly developed real-time PCR for the detection of Taylorella equigenitalis and discrimination from T. asinigenitalis. Vet Microbiol. 2003; 95(4): 229-237.
[Crossref] [Google Scholar] [Pubmed]
- Tel OY, Keskin O, Zonturlu AK, Dakman A, Yurdaydin N. Detection of Taylorella equigenitalis in horses by means of bacteriological culture and PCR in Sanliurfa region, Turkey. Kafkas Univ Vet Fak Derg. 2010; 16(4): 605-609.
- Alber J, El-Sayed A, Lämmler C, Hassan AA, Weiss R, Zschöck M. Multiplex polymerase chain reaction for identification and differentiation of Streptococcus equi subsp. zooepidemicus and Streptococcus equi subsp. equi. J Vet Med B Infect Dis Vet Public Health. 2004; 51(10): 455-458.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Aref Aalinasab1, Esmail Ayen1*, Katayun Nofouzi2 and Ali Soleimanzadeh Azad12Department of Veterinary Medicine, Tabriz University, Tabriz, Iran
Citation: Aalinasab A: Polymerase Chain Reaction (PCR) as a Rapid Examination Accompanied with Bacterial Culture to Recognition of Taylorella equigenitalis in Infertility Mares of the Northwest of Iran
Received: 11-Apr-2023 Accepted: 25-Apr-2023 Published: 02-May-2023, DOI: 10.31858/0975-8453.14.5.325-329
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






