Research Article - (2022) Volume 13, Issue 2
Abstract
The article addresses the role of legal framing of the pharmaceutical industry in maintaining public health in Algeria. It discusses the legal regulation of this important type of industry at the national level. It reviews central government institutions that control pharmaceutical industrial activity by public institutions or national or foreign private companies and it shows the key roles played by the National Agency for Pharmaceutical Industry, the Ministry of Pharmaceutical Industry and the National Health Security Agency.
Keywords
Pharmaceutical industry, Public health, Legal framework
Introduction
Public health is defined as the science and art of disease prevention and life prolongation, and the improvement of individuals' mental and physical vitality through concerted collective action. Public Health policies aim to clean the environment, fight disease, educate the public hygiene about rules of organize medical and nursing services for diagnosis, early treatment, disease prevention, and implement specific social measures to ensure a healthy life for every member of the community (Winslow CE, 1920).
The relationship between the pharmaceutical industry and public health is demonstrated by the fact that the pharmaceutical industry contributes to the achievement of public health goals. However, this relationship is not always beneficial, and Bloor and Maynard have questioned whether legislation on the pharmaceutical industry protects health or protects wealth (Maynard A and Bloor K, 2015).
The pharmaceutical industry has public health benefits but can become an enemy if the law does not define an exact relationship to protect society from scientifically harmful or unjustifiable medicines (Abraham J, 2002). We must point out that pharmaceutical companies have contradictory obligations. This is because for they are obliged to make profit on the one hand, and to protect society from the rise of medicines on the other. Therefore, governments and non-governmental organizations play an important role in balancing these two contradictory commitments (Dukes MG, 2002).
Moreover, the pharmaceutical industry has a positive impact on the availability of treatment and medicine, especially in developing and poor countries, but industrial companies tend to be more expensive. It is not always necessary to find a balance between social benefits and legal protection for companies. Pharmaceutical companies need to be protected, and intellectual property rights rules are not an obstacle to ensuring access Poor communities of essential medicines (Henry D and Lexchin J, 2002). Therefore, international law tends to strike a balance between intellectual property rights and human rights norms relating to the right to health and access to medicines (Nomani MZ, et al., 2020). For example, the Convention on the Trade-Related aspects of Intellectual Property Rights imposes on countries the need to enact legislation to protect intellectual property, which many experts see as an obstacle to the availability of medicines in poor countries. But the 2001 Doha declaration to the Convention's member states eased restrictions for the benefit of poor countries.
The pharmaceutical industry also contributes to ensuring the right to combat pain as a human right, because article 11 of the 1966 International Covenant on Economic, Social, and Cultural Rights affirms that health is a human right. The Committee on Economic, Social, and Cultural Rights, the monitoring body of the Convention, decided that states should provide and facilitate access to adequate quantities of public health, health care, goods, services, and programs (Lohman D, et al., 2010).Furthermore, the local pharmaceutical industries helps countries to control epidemics and pandemics because it provides countries with medicines and medical supplies quickly and without the complications of importing. This makes it easier for countries to deal with health emergencies quickly and effectively. Algeria is aware of this, so Algeria is working to develop the local pharmaceutical industry.
The Corona pandemic also had a profound impact on the medical and pharmaceutical industries. Because of it, governments now have to change their legislation to allow more effective treatment of health emergencies (Ayati N, et al., 2020).
Our study concerns the legal framing of the pharmaceutical industry in Algeria to maintain public health and guarantee the right to medicine. It is important to note that in Algeria there are two types of pharmaceutical industrial enterprises, the first type being public institutions such as Saidal (Boukhari M, 2012). Type two, consists of private companies run by foreigners or Algerians. All institutions active in Algeria must be governed by the national law on activity.
There have been no previous studies published in English about this subject. There is an article by Laouisset DE entitled “Algeria Import Constitution: The case of the pharmaceutical industry”, but it concerns the import activity and is not related to the local industry (Laouisset DE, 2021). In Arabic, there is a study entitled “The role of industrial policies in the development of the pharmaceutical industry in Algeria”. The authors of the book, Ben Abdelrazzaq, and Khanshur, have a study that focuses on the economic aspects of Algerian national policy in the field of the medical industry and does not discuss the legal aspects.
The topic is particularly important because there is a direct relationship between the pharmaceutical industry and economic development, (Lall S and Bibile S, 1977). Development of a national pharmaceutical industry not only helps to break free from dependence on imports. It also contributes to job creation (Mooney KG, 2001). Moreover, patents on the pharmaceutical industry contribute to the development of economic development and constitute an important source of wealth (Kirim AS, 1985).
The need for a national pharmaceutical industry has also increased since the emergence of the Corona pandemic, where the need for drugs to mitigate symptoms of the disease and for supplies to reduce the spread of the disease as well as its vaccine has increased (Barshikar R, 2020).
Through this study, we are trying to evaluate the success of the Algerian legislature in developing a legal framework for the pharmaceutical industry that protects public health (Benabderazek K, 2018).
Methods
The study is based on Algeria's national legal framework for the pharmaceutical industry, namely, legislation on health law and regulations on pharmaceutical activity. The study focuses on legal texts and uses the method of comparison whenever necessary.
Results and Discussion
Overview of drug registration in Algeria
Executive Decree No. 92/284 of 6 July 1992 regulated the procedure for registering medicines and pharmaceutical products used in human medicine and subjected them to strict procedures.
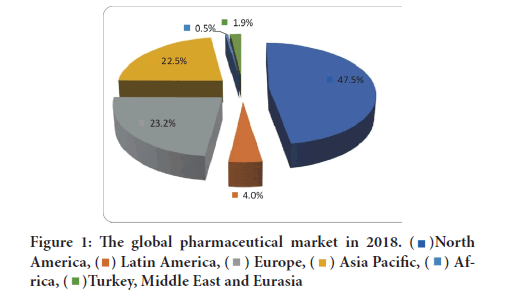
The article 23 of the above-mentioned decree states that it is possible to refuse to register a drug if it is proven to be harmful or if its use does not achieve the expected therapeutic effect, or if it does not include a qualitative and qualitative structure or its contents, or if its production does not allow for quality control (Figure 1).
Figure 1:The global pharmaceutical market in 2018. 


We must point out that pharmaceutical activity is not exclusive to public enterprises but is also open to private companies, but that the practice of pharmaceutical products is subject to the licensing system 92/285 of 6 July 1992, which contains to the conditions to be met by the institutions involved in the production or distribution of pharmaceutical products.Because of the seriousness of public information and publicity relating to pharmaceutical products, the Algerian public authorities have established legal regulations for even this type of information and advertising, following Executive Decree No. 92/286 of 6 June 1992.
According to the rules of this decree, pharmaceutical companies cannot make publicity without obtaining a visa from public authorities. The decree prohibits publicity to the public for any prescription drug, or for treatments for specific diseases such as cancer, tuberculosis, AIDS (Acquired Immune Deficiency Syndrome), diabetes, infertility, blindness, or narcotic drugs.
Role of the National Pharmaceutical Control Laboratory in maintaining public health
The National Laboratory for the Control of Pharmaceutical Products is one of the oldest regulatory institutions in the pharmaceutical industry, established by Decree 93/140 of 14 June 1993.
It is a public institution of an administrative nature with a legal personality, whose functions are focused on monitoring the quality and quality of pharmaceuticals. It records medicines and studies the harmlessness, effectiveness, and quality of marketed medicines.
Role of the National Pharmaceutical Agency in maintaining public health
It is important to point out that the National Pharmaceutical Agency is a privately run public institution with moral, personal and financial independence (article 2 of Decree 1999/190). It's not a pharmaceutical company, it's a pharmaceutical industry control. The agency consists of a board of directors and a scientific board and is managed by a director.
We note that issues relating to the agency's objectives are decided by the Governing Council following the legislation and organization in force, the agency's annual and multi-year projects, plans and program of work, and the agency's estimated budget. The agency's accounts, internal organization, and rules of procedure are also regulated. The Council also examines draft transactions, contracts, agreements, and conventions, appoints a governor, and establishes regional attachments (article 10 of Decree 1999/190).
It should be noted that the board of directors of the agency consists of representatives of ministries of the state, such as the interior, as well as representatives of the Ministries of Health, Labor, Social Security, and Higher Education. Three persons are appointed by the Minister in charge of Health based on their competence and qualifications in areas related to the agency's functions (article 9 of Decree 1999/190).
The Scientific Council of the Agency differs from the board of directors. It is composed of representatives of the National Council for Health Ethics, the National Council for Medical Ethics, pharmaceutical workers, pharmacy organizations, patient associations, scientific and pharmaceutical associations, and university professors. It also contains five experts other than members of specialized committees appointed by the Minister for Health based on their competence and qualifications in areas relevant to the agency's functions (article 21 of Decree 1999/190).
It is necessary to note that the IAEA (International Atomic Energy Agency) Scientific Council is an advisory body, providing views and recommendations on matters relating to the agency's activity. It can make suggestions on strategies for developing the pharmaceutical sector, and can propose measures that would encourage production in pharmaceuticals and medical supplies. It might also express its opinion on all matters relating to the scientific and pharmaceutical fields relevant to the activity of the agency including draft legislative and regulatory texts governing pharmaceuticals and medical supplies (article 20 of Decree No).
It must be made clear that the director of the agency is appointed by presidential decree on the proposal of the Minister in Charge of Health. Assisted in his duties by a secretary-general and sub-directors (article 16 of Decree 19/190), the director is responsible for implementing the decisions of the board of directors and the views of the Scientific Board of the Agency.
It is necessary to emphasize that the National Pharmaceutical Agency has exclusive competence in the area of registration, medicines, and pharmaceuticals. The agency is responsible for the registration, validation, and control of pharmaceuticals and medical supplies and participates in the implementation of the National Policy on Pharmaceuticals and Medical Supplies Used in Human Medicine.
It is also responsible for the control of pharmaceuticals and medical supplies and is responsible for causing or requesting the competent authorities to take the necessary measures aimed at maintaining public health in the event of a pharmaceutical substance or medical requirement that constitutes or may pose a threat to human health (Article 5 of Decree No). To achieve its objectives, the agency may express an opinion on provisional licenses for the use of unregistered medicines. It also contributes to the definition of good practice rules for the manufacture, storage, distribution, and disbursement of pharmaceuticals.
The agency's field inspection and inspection functions are carried out by inspectors including, in particular, the monitoring of the application of rules of pharmaceutical good practice and standards of medical supplies following the legislation and regulations in force. It also conducts scientific assessments of the benefits, risks, and therapeutic value of pharmaceuticals and medical supplies, as well as their economic aspects. It must be noted that its tasks include:
• Contributing to the preparation and updating of pharmaceutical codes and medical supplies.
• Contribution to the preparation of the list of pharmaceuticals and essential medical supplies.
• Contribution to the preparation of the National Pharmaceutical Registry and the Pharmaceutical Constitution.
• Issuance of the drug price certificates upon registration as soon as it is determined by the Joint Sectoral Commission on Medicines.
• Licensing the promotion and publicity of registered pharmaceuticals for health professionals.
• Giving opinions on the standards, rules of good practice, procedures, and methods applicable to medical studies in relation to pharmaceuticals and medical supplies.
• Undertaking any study, research, or formative or information activities in the fields of its competence, and to contribute to the promotion of scientific research in the field of pharmaceuticals and medical supplies and the establishment of databases relating thereto.
• Participation in the preparation of the list of medicines compensable by social security bodies.
Role of the Ministry of Pharmaceutical Industry in maintaining public health
The most recent Presidential Decree No. 20-163 of 23 June 2020 appointing members of the Government as the Ministry of the Pharmaceutical Industry, under the auspices of the Ministry of Health, has now been abandoned, but the scientific approach requires that its functions be recognized.
Regarding the above-mentioned decree, it enjoyed extensive powers that overlapped with the functions of the National Agency for the Pharmaceutical Industry, such as:
• Preparation, development, monitoring, and monitoring of pharmaceutical industry policy.
• Developing and proposing pharmaceutical strategies aimed at promoting national production, implementing such strategies, and ensuring their follow-up.
• Prepare and propose policies for the promotion and development of investment in the pharmaceutical industry.
• Prepare and propose policies for the conduct of state contributions to the public sector of the pharmaceutical industry and ensure their implementation.
• Develop and propose measures and actions aimed at the abundance, quality, and availability of pharmaceuticals and medical supplies.
• Encourage the completion of investment projects in the pharmaceutical industry and ensure their facilities, in particular alternative productive investment.
• Regulation of the framework for exploration and promotion of strategic and technological vigilance in the pharmaceutical industry.
• Proposal and adoption of any measures aimed at ensuring the control of pharmaceutical activities, in particular in the area of the registration and certification of pharmaceuticals and medical supplies.
• Proposal and adoption of any measures aimed at controlling the activities of pharmaceutical enterprises in the fields of production, import, export, exploitation, and distribution.
• Accreditation of pharmaceutical enterprises in the production, import, export, exploitation, and distribution of pharmaceuticals and medical supplies, as well as medical promotion companies and service providers.
In the area of industrial policy, and in the promotion of national production and investment, it prepared the industrial policy of the pharmaceutical branch and assessed its impact, proposing necessary adjustments, in liaison with the parties concerned. It also promoted and harmonized the productive capacities of pharmaceutical enterprises to produce pharmaceuticals and medical supplies following specific objectives and national priorities. In addition, every measure was taken to achieve the objectives set by the pharmaceutical industry policy and to follow up the implementation of its development programs. It also encouraged the development of production inputs to create and promote the industrial fabric of users necessary for the integration of the pharmaceutical industry.
It identified the mechanisms necessary to promote innovation and technological development in the pharmaceutical industry. All actions aimed at developing training and rehabilitation capacities in the professions of the sector could be proposed and implemented in liaison with the parties concerned. All measures for investment promotion could be proposed, and whatever contributed to improving the environment for the pharmaceutical industry.
It also ensured the regulation of investment projects towards the production of high-value-added essential pharmaceuticals. It facilitated the establishment of industrial pharmaceutical enterprises and the promotion of contracts and partnerships between the public sector and the national and foreign private sectors in the pharmaceutical industry, in particular through the development, monitoring, and evaluation of a partnership program for industrial public enterprises (article 3 of Decree 2000/271).
It should be noted that the Ministry has powers in the area of the supply of pharmaceuticals and medical supplies. The Ministry prepares the registration and certification policy and ensures its development and implementation, particularly in its targeting of high-value-added items from national production. The existing legislation and regulations on the quality, effectiveness, and security of pharmaceuticals and medical supplies are respected. In addition, all measures are taken to ensure the availability of pharmaceuticals and medical supplies, particularly in the area of market control.
Temporary licenses for the use of unregistered medicines are also received in accordance with the legislation and regulation in force. Such legislation ensures that import programs for pharmaceuticals and medical supplies are carried out in integration with national production. Any measure aimed at controlling the national production of pharmaceuticals and medical supplies is proposed through the national territory (Article 4 of Decree 2000/271).
It is necessary to point out that the Ministry assigned to the pharmaceutical industry plays an important role in the area of promotion of studies, research, and development under article 8 of Decree No. 20/271, by promoting research and development within producing pharmaceutical enterprises and by proposing all incentive measures for Research and Development activity in the pharmaceutical industry. It also promotes innovation in the pharmaceutical industry and ensures the promotion and development of clinic studies and the delivery of licenses.
The Ministry also has several powers in the area of strategic vigilance, in accordance with article 7 of Decree No. 20/271, which ensures that it follows the development and direction of the national, regional, and international pharmaceutical markets and takes all measures to ensure their balance. It ensures that all technological security devices are developed in the field of pharmaceutical industry activities.
It also works on the establishment of a data bank and the preparation of periodic and circumstantial reports on the evaluation of the pharmaceutical industry. It promotes any measure that facilitates and enables clients to access new technologies in the pharmaceutical industry. The list of pharmaceuticals and essential medical supplies, as well as the National Pharmaceutical Registry, the Pharmaceutical Constitution, and the National Codes of Pharmaceuticals and Medical Supplies, are also prepared and updated.
National Health Security Agency
Following the emergence of the Corona pandemic, Algeria established the National Agency for Health Security by Presidential Decree No. 20/158 of 13 June 2020. This agency serves as an advisory body for strategic vigilance in the service of Algeria's public authority. It is both a private and a public institution with legal personality and financial independence.
It is an institution for monitoring, consultation, and oversight of the National Health Security Strategy and for ensuring its implementation. The agency's primary task is to prepare and implement the national health security strategy.
The agency is responsible for coordinating national programs for the prevention and control of threats and risks of health crises. The agency is the scientific adviser to the public authorities in the area of health security and the reform of the national public health system.
It is important to note that the agency has advisory, scientific guidance, and strategic vigilance bodies composed of scientific personalities, experts, and specialists recognized as competent in their fields.
The role of the private sector
In Algeria, the pharmaceutical industry is not only confined to public institutions and agencies but to private institutions as well. Within this framework, the Algerian legislator, in article No.184 of the law No.08-13 relating to the protection and promotion of health, has exclusively designated the pharmaceutical public institutions and the accredited private ones as producers, importers, and exporters of the pharmaceutical products.
In that context, the creation of a pharmaceutical company to produce medicines requires prior approval for completion and another approval for opening. These two approvals are exclusively delivered by the minister in charge of the pharmaceutical industry as stipulated in article No.17 of the executive decree No.21-82 relating to the pharmaceutical institutions and their accreditation requirements.
Accordingly, many private actors like (Biopharm, Hydrapharm-Beker, Inpha-Médis-Biocare, LDM (Laboratoires de Diagnostic Maghrébins), Pharmalliance, etc) play a significant role in the production, import, export, distribution of medicines in Algeria. However, the contribution of these private players remains insufficient compared to the needs of the local market and the facilities provided by the public authorities in terms of legislation and real estate.
Carrying out their work, these private companies may sign agreements with foreign laboratories to create a common project in Algeria. For example, the private pharmaceutical company Biopharm signed an agreement worth 15 million USD with the Indian group Cipla in February 2015 to market and distribute medicines for respiratory diseases. Biopharm also signed in the same year another agreement with the Anglo-Swedish AstraZeneca laboratory of over 50 million USD for the construction of a pharmaceutical manufacturing plant in Algeria (Algerian economic review Investment opportunities, U.N.I.D.O (United Nations Industrial Development Organization), Humilis, Tokyo November 2015. P.36).
It is noted that the Algerian authorities in the last years are working to reduce the bill of the medicinal imports through encouraging private investments and pushing efforts towards the generic products. The aim of this is to reduce the dependency on foreign companies and to minimize the penetration of the Algerian pharmaceutical market by the world pharmaceutical companies.
Conclusion
The research produced the following findings:
• Algeria has established an integrated system for framing the pharmaceutical industry at the national level. Algeria reserves its right to intervene in pharmaceutical economic activity through national industrial institutions. At the same time, Algeria allows private Algerian or foreign companies to become active in the pharmaceutical industry.
• Algeria has adopted institutions with considerable powers to frame the pharmaceutical industry to maintain public health, the aim of which is to protect society.
• Under its legislative policy in the pharmaceutical industry, Algeria has struck a balance between the need to protect health on the one hand and the protection of wealth on the other.
• Algeria still has several stages to invest in all legislative and institutional capacities in the pharmaceutical industry.
• Algeria still has several stages to invest in all legislative and institutional capacities in the pharmaceutical industry.
• Algeria responded positively to the situation to deal with the Corona pandemic and established the National Health Security Agency. It became the State Adviser for Strategic Vigilance for Future Medical Emergencies.
Recommendations
In order to strengthen the role of the private pharmaceutical companies, it is highly recommended that:
• Public authorities should provide continuous information about the support and facilities provided to pharmaceutical investors.
• Public and private partnerships should be given priority within the pharmaceutical industry of the state.
• Startup pharmaceutical companies should be encouraged and accompanied by the public authorities.
• Pharmaceutical incubators should also be given priority in order to contribute effectively to the development of pharmaceutical research.
Acknowledgement
All authors of this Article would like to thank the Governance and Policy Design Research Lab (GPDRL) of Prince Sultan University (PSU) for financial and academic support to conduct this research and publish it in Sustainability Journal.
References
- Winslow CE. The untilled fields of public health. Science. 1920; 51(1306): 23-33.
[CrossRef] [Google Scholar] [Pubmed]
- Maynard A, Bloor K. Regulation of the pharmaceutical industry: Promoting health or protecting wealth?. J R Soc Med. 2015; 108(6): 220-222.
[CrossRef] [Google Scholar] [Pubmed]
- Abraham J. The pharmaceutical industry as a political player. Lancet. 2002; 360(9344): 1498-1502.
[CrossRef] [Google Scholar] [Pubmed]
- Dukes MG. Accountability of the pharmaceutical industry. Lancet. 2002; 360(9346): 1682-1684.
[CrossRef] [Google Scholar] [Pubmed]
- Henry D, Lexchin J. The pharmaceutical industry as a medicines provider. Lancet. 2002; 360(9345): 1590-1595.
[CrossRef] [Google Scholar] [Pubmed]
- Nomani MZ, Alhalboosi AK, Rauf M. Legal and intellectual property dimension of health & access to medicines in India. Indian J Forensic Med Toxicol. 2020; 14(1).
- Lohman D, Schleifer R, Amon JJ. Access to pain treatment as a human right. BMC Med. 2010; 8(1): 1-9.
[CrossRef] [Google Scholar] [Pubmed]
- Ayati N, Saiyarsarai P, Nikfar S. Short and long term impacts of COVID-19 on the pharmaceutical sector. DARU J Pharm Sci. 2020; 28(2): 799-805.
[CrossRef] [Google Scholar] [Pubmed]
- Boukhari M. Performance and restructuring: The saïdal case. The Cread. 2012; 101.
- Laouisset DE. Algeria import substitution policy: The case of the pharmaceutical industry. The Cread. 2021; 37(1): 5-39.
- Lall S, Bibile S. The political economy of controlling transnationals: The pharmaceutical industry in Sri Lanka (1972-1976). World Dev. 1977; 5(8): 677-697.
[CrossRef] [Google Scholar] [Pubmed]
- Mooney KG. Challenges faced by the pharmaceutical industry: Training graduates for employment in pharmaceutical R and D. Eur J Pharm Sci. 2001; 12(4): 353-359.
[CrossRef] [Google Scholar] [Pubmed]
- Kirim AS. Reconsidering patents and economic development: A case study of the Turkish pharmaceutical industry. World Dev. 1985; 13(2): 219-236.
- Barshikar R. COVID 19-Impact and new normal for pharmaceutical industry (Part-I). J Generic Med. 2020; 16(3): 112-119.
- Benabderazek K. The role of industrial policies in the development of pharmaceutical industry in Algeria. Journal of Economic and Financial Research. 2018; 468(6080): 1-21.
[CrossRef]
Author Info
Tareck Alsamara1, Ghazi Farouk2* and Abbaci Adel22Department of Law, Annaba Badji Mokhtar University, Annaba, Algeria
Citation: Alsamara T: Public Health and the Legal Regulation of the Pharmaceutical Industry in Algeria
Received: 07-Feb-2022 Accepted: 21-Feb-2022 Published: 28-Feb-2022, DOI: 10.31858/0975-8453.13.2.74-78
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3