Review Article - (2023) Volume 14, Issue 10
Abstract
The use of alternative microbiological methods has been encouraged by the United States Pharmacopeia (USP) guidelines, since they offer several advantages over the reference methods in terms of specificity, sensibility, accuracy, precision, and adaptability to technology. Generally, these alternative microbiological methods are automated systems that are less labor-intensive, allowing online measurement registration, real-time microbial monitoring, ensuring data integrity, traceability of the whole analysis and producing automated quality reports with all the parameters required in the pharmaceutical industry, thus reducing routine manual data transcription, which risks user mistakes. Nonetheless, in order to implement these innovative alternative methods within the pharmaceutical sector, there is a need to successfully perform the validation of these methods. The main purpose of this review is to show that an alternative method’s performance on the whole is not inferior to that of the conventional plate-count method for the detection and quantification of yeasts and molds. Therefore, following USP chapters 1223 and 1225, a quantitative validation was carried out in order to highlight essential quantitative validation parameters, such as equivalence of results, operating range, linearity, precision, accuracy, robustness, ruggedness, specificity, limit of detection, and limit of quantification. Thus the alternative microbiological method was found to meet all the validation criteria needed to be considered to be a viable method for yeasts’ and molds’ quantification in a tested antacid oral suspension.
Keywords
Alternative Microbiological Methods (AMM), Plate-Count Method (PCM), Rapid Microbiological Methods (RMM), Soleris Technology (ST)
Introduction
The implementation of innovative Alternative Microbiological Methods (AMMs) and Rapid Microbiological Methods (RMM) has been growing over the last decade, since they can offer benefits in execution, monitoring, and automation while improving accuracy, specificity, sensitivity, and precision (Johnson PJ, 2023; Miller MJ, 2012; Denoya CD, et al. , 2010; Prada-Ramírez HA, et al. , 2023). Because these alternative methods are usually automated systems, they enable a more rapid and efficient response in case of adverse microbiological results (Johnson PJ, 2023; Miller MJ, 2012; Denoya CD, et al. , 2010; Prada-Ramírez HA, et al. , 2023). Furthermore, implementation of innovative technologies significantly reduces the microbiological process time, leading to more rapid release of the pharmaceutical products into the market, allowing a significant reduction of company warehousing costs as well as an improvement in efficiency in inventory control (Prada-Ramírez HA, et al. , 2023). Additionally, these methods exhibit high performance and the ability to analyze a large number of simultaneous samples, with automated results, allowing real-time analysis and the possibility of early detection of contamination (Prada-Ramírez HA, et al. , 2023). However, their use in the pharmaceutical industry has tended to be delayed, because these new technologies used to be expensive and time consuming, these issues being the primary obstacles for their adoption (PradaRamírez HA, et al. , 2023). Moreover, pharmaceutical regulators used to be overly cautious in endorsing these alternative methods as an integral part of routine product release (Prada-Ramírez HA, et al. , 2023). Therefore, there is a need to perform a robust validation of an alternative microbiological method for the detection and quantification of yeasts and molds using a pharmaceutical and personal care matrix in which essential validation parameters are tested, such as equivalence of results, linearity, operative range, precision, accuracy, robustness, ruggedness, specificity, limit of detection,
and limit of quantification (Prada-Ramírez HA, et al. , 2023; USP, 2020; USP, 2023; PDA, 2013).
Literature Review
It is widely known that the drug product matrix is an important factor for validation results because it has an impact on the kinetics of recovery (Prada-Ramírez HA, et al. , 2023). These specific product impacts have led to differences in the correlation curve models between the tested product types. So before performing the validation of AMM, first of all the suitability of the method should be proven. This means that the preservative of any antimicrobial substance needs to be neutralized in order to undertake an optimal microorganism recovery. In the present review, a widely-used pharmaceutical product and several personal care products have been used to show the performance of the AMM in quantifying yeasts and molds (Prada-Ramírez HA, et al. , 2023). The validation method described in this review is used to reduce the microbiological process time for yeast and mold counts from 5-7 days, as the Plate-Count Method (PCM) which usually takes 72 hours using the AMM (Prada-Ramírez HA, et al. , 2023). So the microbiological assessment for the pharmaceutical products is intended to be done in 3 days for the total aerobic microbial count and analysis of pathogens such as E. coli (using the conventional method), and three days for the total yeast and mold count (using the Soleris ® system).
Briefly, the Soleris ® Technology (ST) used as an AMM is an automated growth system based on the microbial metabolism as the yeasts and molds grow and spread into the Soleris ® DYM-109C vial and produce Carbon Dioxide (CO2), which diffuses from the growth medium through a gas-permeable layer into the indicator portion of the Soleris ® vial (Prada-Ramírez HA, et al. , 2023; Pereault M, et al. , 2014; Foti D, et al. , 2012; McCormick PJ, et al. , 2013; Alles S, et al. , 2015; Montei C, et al. , 2014; Miller MJ, et al. , 2010; Mozola M, et al. , 2013; Alles S, et al. , 2009). Because of the presence of antibiotics such as chloramphenicol, the Soleris vial exclusively allows the growth of yeasts and molds, preventing the proliferation of bacteria. It is important to mention that only gasses can enter into the reading zone; microorganisms, the medium, and particulates are blocked. Dissolved CO2 leads to the formation of carbonic acid, reducing the pH and resulting in a change in color of the chemical indicator (thymolphthalein) over time (Pereault M, et al. , 2014; Foti D, et al. , 2012; McCormick PJ, et al. , 2013; Alles S, et al. , 2015; Montei C, et al. , 2014; Miller MJ, et al. , 2010; Mozola M, et al. , 2013; Alles S, et al. , 2009). This colorimetric change is detected and recorded by the equipment’s software and corresponds to the Detection Time (DT), indicative of a positive test result (Prada-Ramírez HA, et al. , 2023; Pereault M, et al. , 2014; Foti D, et al. , 2012; McCormick PJ, et al. , 2013; Alles S, et al. , 2015; Montei C, et al. , 2014; Miller MJ, et al. , 2010; Mozola M, et al. , 2013; Alles S, et al. , 2009).
As demonstrated by Prada-Ramírez HA, et al. , 2023 this automated growth-based system could be an alternative quantitative method for the detection of yeasts and molds through the construction of calibration curves that allow the establishment of numerically equivalent results between enumeration data from the standard reference method and the alternative method. Correlation curves (log CFU vs. DT) should fulfill USP chapter-1223 demands, such as the determination coefficient (R2 ≥ 0.9025) and correlation coefficients (CC ≥ 0.95) (USP, 2020; USP, 2023). DT data from Soleris ® equipment software for the automatic routine assessment of pharmaceutical articles for yeasts and molds through a direct comparison with the correlation curve, which ought to be specific for each product, will allow converting Detection Time (DT) into its CFU enumeration equivalent at a 95% confidence level (Prada-Ramírez HA, et al. , 2023). Therefore, the AMM will have the same regulatory implications as the traditional method for microbiological specifications, because all the Soleris ® results will be represented in CFUs.
The main purpose of this study is to summarize several research studies that have proven that the AMM’s entire performance is not inferior to the conventional PCM. Therefore, this innovative technology could replace the reference standard method, leading to a reduction in process time while maintaining good laboratory practices.
So taking into account that PCM and AMM yield quantifiable values of different kinds (CFU vs. DT), an equivalence of results must be performed through the construction of correlation curves. Therefore, essential validation criteria such as linearity, operative range, equivalence of results, accuracy, Limit of Detection (LOD) and Limit of Quantification (LOQ), precision, ruggedness, and specificity were established in accordance with the United States Pharmacopeia guideline (USP, 2020; USP, 2023).
Validation of the alternative microbiological method
Suitability of the method: In order to guarantee a successful microbiological recovery from different pharmaceutical products, the suitability of the method should be shown (Prada-Ramírez HA, et al. , 2023). Thus suppression of antimicrobial activity needs to be done for all the pharmaceutical and cosmetic articles tested (Figure 1). Generally, polysorbate 80 can be used as a neutralizer agent that chemically deactivates the preservative included in the formulation. Once the suitability of the method has been proven, it is possible to go on to the quantitative validation process (PradaRamírez HA, et al. , 2023).
Discussion
Linearity, operative range, and equivalence of results
As is outlined in the USP guidelines, it is usually observed that the PCM and the AMM yield quantifiable values of different magnitudes (CFUs vs. DT); therefore, an equivalence of results must be carried out through the construction of correlation curves (Prada-Ramírez HA, et al. , 2023; USP, 2020; USP, 2023; Limberg BJ, et al. , 2016). In order to establish a quantitative equivalence of results, correlation curves for yeasts and molds were derived by plotting DT values with their respective equivalents on log CFU (Figure 1). Linear regression analysis yielded the relationship between DTs and log CFU values (USP, 2020; USP, 2023; Limberg BJ, et al. , 2016). The linearity observed in the regression line must be consistent with USP requirements (R2 ≥ 0.9025) (Table 1). Taking into account that the data modeled in the linear regression has a Poisson distribution, an χ² goodness-of-fit was performed (Prada-Ramírez HA, et al. , 2023). Thus the χ² goodness-of-fit test demonstrated a statistical association between the microbial concentration and Colony-Forming Units (CFUs) and the DT values for all the PCP tested (p ≤ 0.05) (Table 1) (Prada-Ramírez HA, et al. , 2023). Quantification ranges were established from the correlation curves for each pharmaceutical product tested (Figure 1).
According to chapters-1223 and 1225, the ability of the alternative method to produce signals that depend on the microbial threshold is a key parameter for successfully achieving the AMM’s validation (USP, 2020; USP, 2023; Limberg BJ, et al. , 2016). As has previously been shown by Prada-Ramírez HA, et al. , 2023, in several validation procedures performed on pharmaceutical and personal care products, DT data from the Soleris ® equipment’s software for microbiological assessment will automatically allow, through a direct comparison with the calibration curve, translating Detection Time (DT) into its CFU enumeration equivalence (Prada-Ramírez HA, et al. , 2023). In this way, the alternative method has the same regulatory implications as the traditional method for microbiological specifications, because all the Soleris ® results will be represented in CFUs (Prada-Ramírez HA, et al. , 2023).
Similarly, according to the Personal Care Product Council (PCPC) and the Scientific Committee on Consumer Safety (SCCS), there is a need to develop an innovative alternative method that exhibits the same performance as that observed for the standard method, in order to safely release the product more quickly into the market (Prada-Ramírez HA, et al. , 2023). For instance, the PCPC and SCCS have established that cosmetic products should be classified into two large groups (category 1 and category 2). Products classified as category 1, which includes all cosmetics applied around the eyes, those of mucosal contact, and products specifically intended for infants, must have a total viable aerobic microorganism count <102 CFU/ml or g. Category 2 includes the remaining cosmetic products, which must have a total viable count of aerobic microorganisms <10 3 CFU/mL or g (Prada-Ramírez HA, et al. , 2023). So the enforcement of an innovative method that allows precise quantification of colony-forming units, according to the category, will have a huge impact on the cosmetic industry (Prada-Ramírez HA, et al. , 2023).
| Pharmaceutical and cosmetic products | R2 | CC | χ2 (p ≤ 0.05) | LDD (UFC) | Upper range (UFC) | % Recovery | Goodness of fit test (p ≥ 0.05) | Fisher test | Standard deviation | Coefficient of variation AMM | ANOVA (p ≥ 0.05) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antacid oral suspension Ab | 0.93 | 0.95 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 117 | p ≥ 0.05 | p ≥ 0.05 | 4 | 13.1 | p ≥ 0.05 |
| Antacid oral suspension Ca | 0.92 | 0.96 | p ≤ 0.05 | 1 | 1.9 × 10 3 | 106 | p ≥ 0.05 | p ≥ 0.05 | 4 | 17.5 | p ≥ 0.05 |
| Sunscreen Zahara ® | 0.91 | 0.96 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.3 | 3.9 | p ≥ 0.05 |
| Mouthwash zero alcohol ® | 0.94 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.8 | 5.4 | p ≥ 0.05 |
| Dermoprotective cream ® | 0.95 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 117 | p ≥ 0.05 | p ≥ 0.05 | 1.6 | 5.4 | p ≥ 0.05 |
| Intimate female bathroom ® | 0.95 | 0.95 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 106 | p ≥ 0.05 | p ≥ 0.05 | 2.9 | 9.5 | p ≥ 0.05 |
| Rollon deodorant T24 for men ® | 0.97 | 0.97 | p ≤ 0.05 | 1 | 1.8 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 0.6 | 2 | p ≥ 0.05 |
| Colonia lotion Tersura ® | 0.95 | 0.98 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.5 | 5.4 | p ≥ 0.05 |
| Honey shampoo Pequitas ® | 0.97 | 0.98 | p ≤ 0.05 | 1 | 1.9 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1 | 3.5 | p ≥ 0.05 |
| Chamomile shampoo Pequitas ® | 0.96 | 0.98 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 2.8 | 9 | p ≥ 0.05 |
| Sunscreen Dermavive ® | 0.96 | 0.98 | p ≤ 0.05 | 1 | 1.9 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.1 | 3.9 | p ≥ 0.05 |
| Toothpaste prodent ® | 0.94 | 0.97 | p ≤ 0.05 | 1 | 1.4 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.3 | 4.7 | p ≥ 0.05 |
| Moisturizing gel Zahara ® | 0.97 | 0.99 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 115 | p ≥ 0.05 | p ≥ 0.05 | 0.7 | 2.2 | p ≥ 0.05 |
| Sabila cream fascination ® | 0.93 | 0.97 | p ≤ 0.05 | 1 | 1.9 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.1 | 3.3 | p ≥ 0.05 |
| Sunscreen Tacoa ® | 0.98 | 0.99 | p ≤ 0.05 | 1 | 1.9 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 2.2 | 7 | p ≥ 0.05 |
| Extra-moisturizing cream ® | 0.91 | 0.95 | p ≤ 0.05 | 1 | 1.5 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.1 | 3.8 | p ≥ 0.05 |
| Mouthwash original ® | 0.97 | 0.98 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.8 | 5.9 | p ≥ 0.05 |
| Rinse Pequitas ® | 0.93 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.1 | 3.6 | p ≥ 0.05 |
| Insect repellent Repeblanc ® | 0.97 | 0.98 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 2.6 | 8.8 | p ≥ 0.05 |
| Mouthwash cool mint ® | 0.92 | 0.95 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 99 | p ≥ 0.05 | p ≥ 0.05 | 0.9 | 3.2 | p ≥ 0.05 |
| Mouthwash calculus control ® | 0.95 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 0.4 | 1.9 | p ≥ 0.05 |
| Moisturizing cream Crelim ® | 0.93 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 0.9 | 3.5 | p ≥ 0.05 |
| Intimate female bathroom ® | 0.94 | 0.99 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.2 | 4.6 | p ≥ 0.05 |
| Talcum powder T4 ® | 0.99 | 0.99 | p ≤ 0.05 | 1 | 1.8 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.2 | 3.9 | p ≥ 0.05 |
| Tersura oil ® | 0.94 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 0.8 | 3.5 | p ≥ 0.05 |
| OVY shampoo ® | 0.98 | 0.99 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 2.5 | 7.7 | p ≥ 0.05 |
| Talcum powder Pequitas ® | 0.92 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.9 | 5.8 | p ≥ 0.05 |
| Soothing gel Oxitrex ® | 0.96 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 0.3 | 1.3 | p ≥ 0.05 |
| Rollon deodorant T24 ® | 0.95 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 2.7 | 9.7 | p ≥ 0.05 |
| Ultrapure tersure shampoo ® | 0.91 | 0.95 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 93 | p ≥ 0.05 | p ≥ 0.05 | 0.4 | 1.3 | p ≥ 0.05 |
| Talcum for women ® | 0.94 | 0.96 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 7.3 | 14.7 | p ≥ 0.05 |
| Talcum for men ® | 0.95 | 0.97 | p ≤ 0.05 | 1 | 2.0 × 10 3 | 100 | p ≥ 0.05 | p ≥ 0.05 | 1.4 | 6.8 | p ≥ 0.05 |
Table 1: Summarized quantitative validation parameters for AMM (Prada-Ramírez HA, et al., 2023)
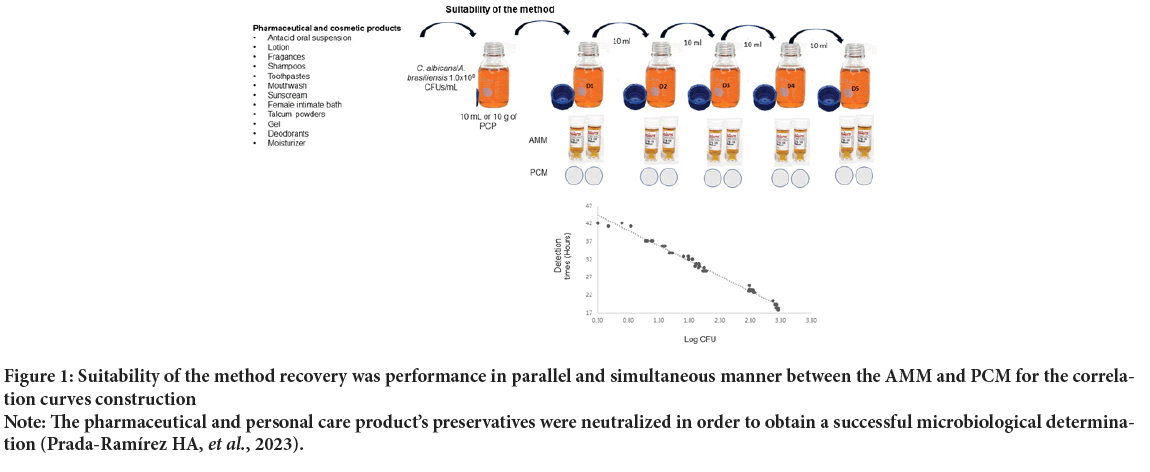
Figure 1: Suitability of the method recovery was performance in parallel and simultaneous manner between the AMM and PCM for the correlation curves construction
Note: The pharmaceutical and personal care product’s preservatives were neutralized in order to obtain a successful microbiological determination (Prada-Ramírez HA, et al., 2023).
Accuracy
As was previously reported by Prada-Ramírez et al. 2023, the accuracy was measured through the percent of recovery (USP, 2020; USP, 2023; Limberg BJ, et al. , 2016). Thus using the equation derived from linear regression, detection time (DT) values were used to determine log CFU (Limberg BJ, et al. , 2016). The mean accuracy log CFU derived from the alternative method DT was ≥ 70% of the parallel plate count. At the same time, the Pearson goodness-of-fit test was chosen to assess the proximity of the results obtained by the AMM and those observed for PCM for each microorganism (Table 1). In this way, the automated system was able to predict CFUs from DT values using a 95% confidence interval (p ≥ 0.05) (PradaRamírez HA, et al. , 2023). Supporting these results, yeast and mold correlation curves each showed a coefficient of correlation (CC>0.95), fulfilling USP requirements (CC>0.95). So a high correlation coefficient is a reliable indication of the accuracy that the quantifiable data acquired via the PCM (in CFUs) and can be calibrated to DT units generated by the alternative method (PDA, 2013). The percent of recovery of the AMM was more than 70% using different pharmaceutical and cosmetic products, showing the reliability and robustness of the method.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
As outlined in the International Council of Harmonization (ICH) Q2A validation of analytical procedures, the LOD and LOQ were calculated using the standard deviation of data obtained for the fewest number of recoverable microorganisms (<10 CFUs) and the slope of the corresponding standard curve(s) (Limberg BJ, et al. , 2016). As has been shown in several research studies using pharmaceutical products and personal care products, the LOQ for the AMM is <10 CFUs. The LOD for the AMM was 1 CFU/sample (Table 1). According to USP chapter-1111 requirements’ acceptance criteria for the microbiological quality of nonsterile dosage for all aqueous preparations for oral use, there should be an absence of Esche- richia coli (1 g or 1 mL) and a total count for yeasts and molds of less than 20 CFUs in order to fall under the microbiological specification before the product’s release for sale (USP, 2016; USP, 2016). In this way, the AMM was shown to have the ability to detect 1 CFU during the assay time (72 hours), thus ensuring the microbiological assessment quality for yeast and mold analysis as specified by the USP.
Intermediate precision robustness and ruggedness
In order to proceed with the AMM validation, the intermediate precision was estimated. As is outlined in the USP guidelines, the precision of the alternative quantitative microbiological method is the degree of agreement among individual test outcomes when the experimental design is applied repeatedly to several samples across the range of the test (USP, 2020; USP, 2023). The repeatability and the intermediate precision were estimated independently for yeasts and molds. Ruggedness could be interpreted as a type of intra-laboratory precision involving the effect of different lots and operators on the test result variability, as well as repeatability. In order to observe the effect of these operational variables on the average DT for each bioburden level, a multifactorial Analysis of Variance was performed (ANOVA) (Prada-Ramírez HA, et al. , 2023).
It can be seen that for all the pharmaceutical and cosmetic products tested, different lots and operators do not have a significant effect on the mean of the DTs (ANOVA, p>0.05) (Table 1) (Prada-Ramírez HA, et al. , 2023). So the AMM has shown its ability to be unaffected by the effect of different variables such as different lots and users (ANOVA, p>0.05) (Table 1). Similarly, the values from the AMM have been shown to exhibit a high degree of concordance at the microbial threshold that recovered 10-100 CFU (SD<5) (Table 1). It may be useful to note that the correlation curves constructed for yeasts and molds during the validation process included all these variations for routine assessment of yeasts and molds in pharmaceutical and cosmetic products (χ² square p ≤ 0.05) (Table 1). The robustness parameter was assessed by the manufacturer. According to the Neogen supplier’s information, the detection of yeasts and molds for the Soleris ® equipment has demonstrated its capacity to remain unaffected by small but deliberate variations in the method’s parameters, such as temperature variation (28°C +/-0.5°C), the algorithm detection parameter (10 optical units +/-2 optical units), and different Soleris ® equipment.
Specificity
As has been shown in previous research, all the pathogenic bacteria tested (E. coli, S. thyphimurium, S, aureus, P. aeruginosa, and B. subtilis ) were unable to grow in the Soleris ® DYM 109C vials, whereas C. albicans and A. brasiliensis were able to grow as expected (Table 2). The DYM Soleris ® vials inhibited bacterial growth such as E. coli, P. aeruginosa, Bacillus cereus, and Staphylococcus aureus because of the presence of chloramphenicol and oxytetracyclin, thus ensuring that the growth observed inside the vials corresponded exclusively to yeast and mold growth. These results show the high specificity of the alternative method to exclusively detect the growth of yeasts and molds.
| Microorganisms | Soleris ® vial DT/ND | Plate count (CFU) |
|---|---|---|
| Pseudomonas aeruginosa | ND | 0 |
| Escherichia coli | ND | 0 |
| Staphylococcus aureus | ND | 0 |
| Salmonella typhimurium | ND | 0 |
| Bacillus subtilis | ND | 0 |
| Candida albicans | 30.4 | 89 |
| Aspergillus brasiliensis | 26.1 | 96 |
Note: ND: Not Detected within 72 hours
Table 2: Results for specificity testing for the Soleris ® yeasts and molds method (Prada-Ramírez HA, et al., 2023)
Conclusion
As has been shown in various research studies, the construction of correlation curves enables carrying out a quantitative equivalence of results between quantifiable values from the PCM and AMM, which allows demonstrating that the Soleris ® automated growth system can be used as a replacement for the standard reference method for the quantification of yeasts and molds in finished products. This method reduced the assessment time from 5-7 days, as is usual for the reference method, to a maximum of 72 hours or less, because the limits of detection (1 CFU) for both microorganisms could be reached in maximum incubation times of three days for microbiological assessment of yeasts and molds. These results show the good performance of the AMM in detecting the lowest microbiological contamination, ensuring an accurate microbiological assessment of finished pharmaceutical products. Moreover, the LOD and LOQ were not statistically different between the alternative and the standard method (Fisher’s test p>0.05), showing that the AMM’s performance is not inferior to that of the PCM. All these results show that AMMs are an important tool for more quickly manufacturing pharmaceutical products. The evidence demonstrates that this alternative automated method yields accurate quantitative results equivalent to those of the PCM (R2>0.9025, CC>0.95, and % recovery >70%). The AMM shows a high repeatability and reproducibility in all the pharmaceutical and personal care products tested. Indeed, its ability to remain unaffected by different operational variables such as different lots and users is evidence of its reliability and stability. At the same time, the method has good specificity in detecting target organisms such as yeasts and molds and excluding non-target bacteria such E. coli, S. aureus, and P. aeruginosa .
The enforcement of such alternative innovative methodologies results in a reduction of company warehousing costs, improved efficiency in inventory control, and the ability to respond more quickly to adverse microbiological results. Likewise, this proposed alternative method is potentially more sensitive and specific than PCM for assessing yeasts and molds in several pharmaceutical and cosmetics products.
Acknowledgment
The authors thank Catver SAS and Neogen Corporation, Mexico for their technical support and helpful advice for the validation testing.
Funding
The study was funded by Laboratorios Coaspharma SAS.
References
- Johnson PJ. Overview of alternative rapid microbiological technologies. CRC Press. 2003: 35-47.
- Miller MJ. Case study of a new growth-based Rapid Microbiological Method (RMM) that detects the presence of specific organisms and provides an estimation of viable cell count. Am Pharm Rev. 2012; 15(2): 18-25.
- Denoya CD, Saghee MR, Sandle T, Tidswell EC. Alternative microbiological methods and new pharmaceutical microbiology curriculum. 2010: 491-518.
- Prada-Ramírez HA, BeltrÁn-Osuna ÁA, Celeita S, Fonseca JC. Performance equivalence and validation of a rapid microbiological method for detection and quantification of yeast and mold in an antacid oral suspension. PDA J Pharm Sci Technol. 2023; 77(4): 268-280.
[Crossref] [Google Scholar] [Pubmed]
- Prada-Ramírez HA, Celeita S, Fonseca JC. Validation of a rapid microbiological method for the detection and quantification of the Burkholderia cepacia complex in an antacid oral suspension. J AOAC Int. 2023; 106(5): 1288-1294.
[Crossref] [Google Scholar] [Pubmed]
- Prada-Ramírez HA, Celeita S, Fonseca JC. Efficacy comparison of an automated growth-based system and plate-count method on the detection of yeasts and molds in personal care products. J AOAC Int. 2023: qsad075.
[Crossref] [Google Scholar] [Pubmed]
- Validation of alternative microbiological methods (Chapter-1223). United States Pharmacopeia (USP). 2020.
- Validation of compendial methods (Chapter-1225). United States Pharmacopeia (USP). 2023.
- Technical report No. 33: Evaluation, validation and implementation of alternative and rapid microbial methods. Parental Drug Association (PDA). 2013.
- Pereault M, Alles S, Caballero O, Sarver R, McDougal S, Mozola M, et al. Validation of the soleris® direct yeast and mold method for semiquantitative determination of yeast and mold in a variety of foods. J AOAC Int. 2014; 97(4): 1084-1091.
[Crossref] [Google Scholar] [Pubmed]
- Foti D, Romano L, Alles S, Mozola MA. Validation of the soleris® E. coli method for detection and semi-quantitative determination of Escherichia coli in foods. J AOAC Int. 2012; 95(3): 786-794.
[Crossref] [Google Scholar] [Pubmed]
- McCormick PJ, Schoene MJ, Dehmler MA, Kaiser JJ, Norton SE, Pizon A, et al. Evaluation of a rapid microbiological method with a mixed culture biofilm model. PDA J Pharm Sci Technol. 2013; 67(5): 512-532.
[Crossref] [Google Scholar] [Pubmed]
- Alles S, McDougal S, Caballero O, Mozola M, Rice J. Validation of a minor modification to the soleris® direct yeast and mold vial and selective supplement. J AOAC Int. 2015; 98(5): 1286-1289.
[Crossref] [Google Scholar] [Pubmed]
- Montei C, McDougal S, Mozola M, Rice J. Semiquantitative determination of mesophilic, aerobic microorganisms in cocoa products using the soleris® NF-TVC method. J AOAC Int. 2014; 97(1): 155-158.
[Crossref] [Google Scholar] [Pubmed]
- Miller MJ. The implementation of rapid microbiological methods. Eur Pharm Rev 2010; 15(6): 27-31.
- Mozola M, Gray LR, Feldpausch J, Alles S, McDougal S, Montei C, et al. Validation of the Soleris® NF-TVC method for determination of total viable count in a variety of foods. J AOAC Int. 2013; 96(2): 399-403.
[Crossref] [Google Scholar] [Pubmed]
- Alles S, Shrestha N, Ellsworth A, Rider A, Foti D, Knickerbocker J, et al. Validation of the soleris® yeast and mold test for semiquantitative determination of yeast and mold in selected foods. J AOAC Int. 2009; 92(5): 1396-1415.
[Crossref] [Google Scholar] [Pubmed]
- Limberg BJ, Johnstone K, Filloon T, Catrenich C. Performance equivalence and validation of the soleris automated system for quantitative microbial content testing using pure suspension cultures. J AOAC Int. 2016; 99(5): 1331-1337.
[Crossref] [Google Scholar] [Pubmed]
- Microbiological examination of nonsterile products: Acceptance criteria for pharmaceutical preparation and substances for pharmaceutical use (Chapter-1111). United States Pharmacopeia (USP). 2016.
- Microbiological examination of nonsterile products: Microbial enumeration tests. United States Pharmacopeia (USP). 2016.
- Validation of microbial recovery from pharmacopeial articles (Chapter-1227). United States Pharmacopeia (USP). 2023.
Author Info
Harold Alexis Prada-Ramirez*Citation: Prada-Ramírez HA: Review on Enforcement of Alternative Microbiological Methods in the Pharmaceutical Industry
Received: 11-Sep-2023 Accepted: 25-Sep-2023 Published: 05-Oct-2023, DOI: 10.31858/0975-8453.14.10.616-621
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






