Review Article - (2022) Volume 13, Issue 8
Abstract
A pilot plant is the pre-commercial production system which includes new production technology and produces small volumes of new technology based products. Scale up is the process of increasing the batch size or a procedure for applying the same process to different output volumes. Process scale up for a technology is one the most common reasons to build a pilot plant. It is a connection between Research and Development (R and D) Industry. It is a Hybrid development facility and Manufacturing unit. Even though commercialization is the primary motivation for pilot plant projects, there can be multiple, complex objectives that will vary by project organization and goals. It is a place where the 5M’S like Money, Material, Man, Method and Machine are brought together for the manufacturing of the products. In pilot plant before investing a large sum of money on a production unit it is a small preliminary lab scale formula to be carried out on a model of proposed plant. Pilot plant scale up gives information about examination of formula can be done, review of range of relevant processing equipment, the specification of the raw materials can be understood, we can know the production rate, the physical space needed can be checked. It can store appropriate records and reports for analysis to support GMP process. Pilot plant scale up considerations for solids, liquids, semisolids is discussed here. The main objective of pilot plant is “Find mistakes on a small scale and make profit on large scale”.
Keywords
Pilot plant, Solid, Liquid, Semisolid, Equipment
Introduction
Pilot plant and scale-up techniques are both integral and critical to drug discovery and development process for new medicinal products. The speed of drug discovery and development has been accelerating at an exponential rate. From the past two decades, particular have witnessed amazing inventions in pharmaceutical research, resulting in the ability to produce new drugs faster than even before. The New Drug Applications (NDAs) and Abbreviated New Drug Applications (ANDA) are all time high. Also different types of laboratories have been motivated to adopt new processes and technologies in an effort to stay at the forefront scientific innovation (Lachman L, et al., 1976).
A pilot plant allows investigation of a product and process on an intermediate scale before largely are committed to full scale production. Pilot plant scale up techniques consist manufacture of experimental formulation on high speed production instrumentation, with an efficient manner. It is a requirement for equipment analysis and establishes documented evidence with high degree of assurance that produce a product which meets predetermined specifications and quality attributes. Pilot plant is a pharmaceutical plant, which is used to obtain experimental data on a new process, to produce a new dosage form. The rapid development of modern economy and technology the energy crisis, air pollution from the combustion of fossil fuels, volcanoes and wildfires (Jiaqiang E, et al., 2018). The solid and liquid particles suspended in air (aerosols) and water pollution from waste water because of Marine Dumping, Oil leaks and Spills and Global Warming have become increasingly serious. So the current study of pilot plant was designed to obtain the pharmaceutical using of water efficiency using water conservation strategy and at source effluent treatment for reusable in manufacturing unit and save the maximum water, because water is the main source of pilot plant.
Pilot plant scale up consideration of solid dosage form refers essentially to pharmaceutical drug product in the form of tablets, capsules, powders, granules, lozenges and suppositories containing an active drug component and excipients. It includes Material handling, dry blending, granulation, drying, and reduction of particle size, blending, granulation handling and feed system, compression for Tablets. The Solid dosage form of Capsule includes Mixing of Ingredient, granulation and lubrication, making of capsules, filling of capsule, uniformity testing and packaging and labeling (Ramasubramaniyan P, et al., 2014).
Pilot plant scale up for liquid dosage forms consists of a mixture of active drug components and non-drug component (Excipients) in the form in which it is marketed for use. Liquid state forms are meant for internal, parenteral or external use. They are available in mono phasic liquid dosage form. The liquid which consists of 2 phases are biphasic liquid dosage form (Jain NK and Sharma SN, 2016).
Pilot plant scale up for semisolid dosage forms are complex formulation. They are compose of two phases (Oil and Water), one of which is external phase and other is internal phase. The active ingredient dissolves in one phase, although occasionally the drug is not fully soluble in the system and is dispersed in one or both phases, thus creating a three phase system (Figure 1) (Shaikh R, et al., 2018).
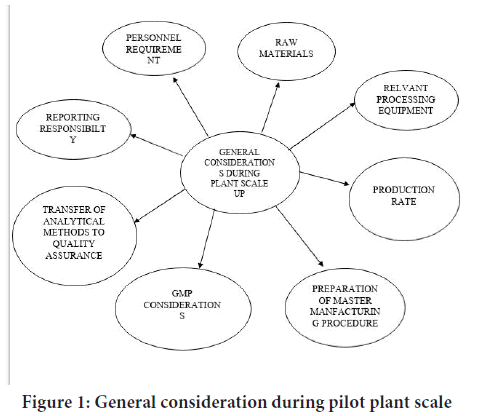
Figure 1: General consideration during pilot plant scale
Literature Review
Solid dosage form
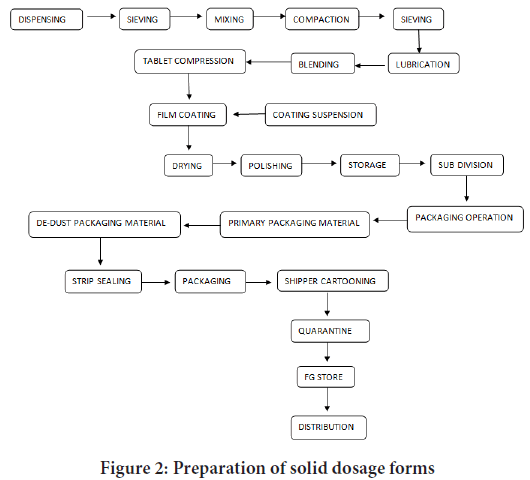
Pilot plant scale up techniques for solid dosage form involve in the process for large scale manufacturing (Figure 2). It play vital role in large scale manufacturing. In past two decades pilot plants have particularly witnessed amazing inventions and innovations in pharmaceutical research, resulting in the ability to produce new drug faster than before. The scale up of solid dosage form would be better appreciated if one understands the pilot plant scale up techniques used in pharmaceutical manufacturing. It is considered to be intermediate form of batch size in large scale production; various types of size and equipment are used. Solid dosage form refers essentially to pharmaceutical drug products in the form of tablets, capsules, powders, granules containing active drug component or a mixture of active drug component (Active pharmaceutical ingredient) and non-drug component (excipients) (Allen L and Ansel HC, 2013). Tablets are composed of solid unit dosage form containing medicament. Which are usually circular, may be flat or biconvex in shape. It contains medicament with or without excipients. They are prepared by compressing drugs or mixture of drugs with or without diluents. Types of tablets are compressed tablets, sugar coated tablets, and film coated tablets, effervescent tablet, enteric coated tablets, and chewable tablets, buccal and sub-lingual tablets (Ennis BJ and Litster JD, 1997). Capsules are solid dosage form in which the drug substance is present either in hard or soft soluble container or shell of suitable form of model like gelatin. The drug which is in powder or any other forms encloses gelatin or other polymers. Hard capsules are generally used for powder or solid fills. Soft capsules are generally used for semisolid or liquid fills. Powders are intimate mixture of drug that may be intended for internal or external use. The flow properties of powder are depends upon the particle size, nature of particle, shape and moisture content. It can be determined by angle of repose, bulk or tapped density. Granules are small powder particles are gathered together to form agglomerates. It is necessary to include adhesive substances to achieve cohesion between the powders. It increases the flow properties of powders. During tabulating it prevents segregation of powder components. During manufacture process it reduces cross contamination and hazard associated with generation of toxic dust. All the formulation ingredients should be uniformly distributed in the granules (Boylan JC and Swarbrick J, 2001).
Figure 2: Preparation of solid dosage forms
Equipments of solid dosage form (Tablets) are material handling, it is mechanical equipment used for movement, storage, protection, of material and products throughout the process of manufacturing, distribution, consumption. System proper handling of material is necessary for large scale production ingredients should be delivered to the destination in accurate quantity. The characteristic of the materials depends on the selection of the type of system. It has four different types they are transport equipment, positioning equipment, unit load formation equipment and storage equipment (Faure A, et al., 2001).
Conveyor:
Belt conveyor: It is a simple device that is very useful here a motor is used to turn the pulleys, thus moving the belt.
Chain conveyor: It consists of moving chain to carry products instead of having rollers or a belt it is used to carry large items.
Blending: It is the action of mixing or combining things together (Schwartzbach H, 2010).
Dry blending: It is the process to produce a well-mixed dry product by in-corporating dry ingredients. If desired it is also possible to add controlled amount of liquids for some blenders. It also have temperature control can be used to heat the bed of powder furthermore temperature controls which can heat the liquid stream that is added to the bed. To ensure proper distribution of drug granulated powder are well blended. Due to insufficient blending of powders could result in uneven portion of the batch which result in either high or low in potency. All ingredients should be free of lumps and agglomerates prior to blending. The proper steps should be taken care or else flow problems can occur. Before blending, screening of the ingredients should be done (Gohel MC, et al., 2007).
Ribbon blender: The machine consists of U-shaped horizontal trough. It rotates up to approximately 300 feet/min. the liquid ingredient can be added through a charge port on the cover but for critical application. It is also used in preparation of flow able slurries or pastes such as food extrusion operation.
Paddle blender: It is U-shaped trough. It provides low shear and less heat development.
Tumble blender: It is a double cone or V-shaped. It is designed with asymmetric vessels to reduce blend time and improve uniformity. It operates a speed of 5 to 25 rpm (Sitompul JP, et al., 2013).
Granulation: It is a unit operation where powder particles are combined together to form granules.
• Dry granulation is the process of granulating without the use of liquid. It is slugging and roll compaction method.
• Wet granulation method is the process of granulating with use of liquid. It also involves additional steps of wet massing, wet screening and drying (Meyer T, 2003).
Drying: It is the process which removes the presence of solvents in the formulation with presence of heat. The final product of drying is a dry solid mass or powders. This process impact on the quality attributes of the API. It will not have impact on the drug safety and efficiency, thus providing high quality final product. Drying of granulation takes place by heating either by steam or electricity in a hot air oven. For each product drying times as specified temperature and air flow rates. Drying of wet soils is done by fluidized bed dryer to obtain good contact between the warm drying air and wet particles. It improves the efficiency of heat transfer and vapor removal, as compared with the older static tray dryer. It also allows the efficient transfer of the latent heat of evaporation from the air and into the drying solid. For solutions and suspensions spray drying is most useful method. As it disperse the liquid to a spray of small droplets. In spray dryer it atomizes the liquid into small droplets thus creating a large surface area for heat and mass transfer. It is most beneficial for thermo liable materials. Spray drying is capable of producing spherical particles in the respirable range of 1-7 micrometer that are necessary for the delivery of drugs from dry powder inhalers (Benedek I, 1998).
Reduction of particle size: During hand screening with a small scale mill ing equipment is used to obtain the desired particle size distributions prior to compression or encapsulation.
Hammer mill: the material is impacted by the hammer bars and is there by shredded and expelled through screens in the drum of a selected size (Marshall K, 1986).
Compression: It is the process of granulation can be compressed on high speed tablet press and produce tablets with the help of compressing machine. Granules are compressed and produce tablets. By combined pressing extinction of two punches and die tablet formation takes place. The principle behind tablet compression is hydraulic pressure. It is divided into 4 distinct stages filling, metering, compression and ejection.
Coating: To a solid dosage form a thin polymer-based coat is applied. The thickness of a coating is typically between 20-100 micrometers.
Standard coating pan: It contains a metal pan which is circular in shape rotated on its horizontal axis by a motor. Heated air is directed into the pan and on to the tablet bed surface and is exhausted by means of ducts through the front of the pan. It is improved by pellegrini pan, the immersion sword, and immersion tube system (Hoag SW, 2017).
Capsules
Two types of gelatins are hard gelatin and soft gelatin. Hard gelatin capsules are made up of two separate parts called body and cap where soft gelatin capsules are hermetically sealed one piece capsule which cannot be separated. The manufacturing of hard gelatin capsule are produced in two steps where shell is manufactured by one type of machine and then filling is done by another machine but soft are manufactured in one step.
The manufacturing process of hard gelatin capsule is shell composition-gelatin which is used for shell composition it is produced from the collagen by hydrolysis or by extraction process. Generally they are two types of gelatin, which are differentiated by isoelectric point and by nature of viscosity and film forming capacity. To produce optimize shell the combination of pork skin and bone gelatin is often used. Here the coloring agent is used for color the drug, opaquing agent such as titanium dioxide is preferred for protection against light. Preservatives such as paraben are often used.
Shell manufacture:
Dipping: The pairs of stainless pins are dipped in solution to form caps and bodies. The pins will be at ambient temperature; where solution temperature is about 50°C in a heater, jacketed dipping pan.
Rotation: After dipping the pins are elevated and rotated continuously for 2-12 times until they are faced upward.
Trimming: The stripped portion are delivered and collected which are firmly held. Then it will rotate, and then knives are brought against shell to cut them for equal length.
Joining: The two portions are aligned concentrically in channels and then pushed together slowly.
Sorting: The moisture content of capsule as from the machine they will be in the range of 15%-18% w/w then they are passed through light moving conveyor and examined for defects.
Printing: They are printed just before filling which are done by offset rotator presses which have a high capability of printing. Size and shapes they are different range of capacities.
Sealing: They are sealed and reshaped by a seal process. This is a thermal welding process where cap overlap the body.
Storage: They are normally containing moisture content 13%-16%, relative humidity 40%-60%.
Soft gelatin capsules-plasticizer: It is made up of soft shell which is elastic and pliable. Commonly used plasticizer is made up of glycol, sorbital, and propylene glycol-400. It is should be minimum interaction between liquid fill material and soft gel shell. The presence of water is 30%-40% of the wet formulation and it is presence is importance during gel preparation (Augsburger LL, 1990). The colorants which may be natural and synthetic for desire shell color. An opacifier is also added for preparation of opaque shell commonly used opacifier is titanium dioxide. The preservative which is used to prevent growth of microbes is of concentration range of 0.2% commonly used preservative is methyl paraben and propyl paraben. The following agent which is used to mask bitter taste commonly used flavoring are ethyl vanillin, essential oil and sucrose is used.
Manufacturing plate process: Here the warmed sheet of gelatin sheet is placed over die plates which have moulds then sheet is drawn into the moulds by vacuum. A measured liquid medicament is pour over. Then another plate of mould is placed and then by applying pressure the plate is combined. Then it will shape, filled, sealed and cut into individual unit. Here it has moisture content of 20%-40%.
Rotary die process: Here the production of gel mass by dissolving gelatin in water at 80°C under vacuum, followed by adding excipients like opacifier, flavor, color, preservative are added. Here the 57°C-60°C is maintained in melting tank. Then hot gel transfer into encapsulate machine by heated transfer pipes by casting method. This forms two separate gelatin ribbons. Then production of gelatin ribbons by metering device. Then checked correct thickness where two ribbons are then carried by roller and rotator die encapsulation (Augsburger LL, 1990). The liquid fill matrix is prepared separately. The drug which is a non-liquid vehicle filled using a conventional mixer homogenizer. The two ribbons of gelatin are fed between rollers and it sealed after pass through roller with pressure forms capsule encapsulation. Liquid gelatin which is passing overhead tank is passed through continuous ribbon by rotating drum and then it brought together by twin rotating dies. The injection of liquid between the ribbons, thereby gel expands and into the packets of die, which is used in maintaining the size and shape of soft gel.
Reciprocatingdieprocess:This is similar to rotator process but different encapsulating process.
Accogel: It is another rotator process which consists of measuring roll, die roll and sealing roll. Here the measuring roll rotates directly over the die roll and the pockets in two rolls are aligned. With each other powder fill material in pockets by applying vacuum. Then plasticized sheet is placed over as measured roll and die roll rotate which is transfer to gelatin lined pockets of die roll.
Seamless gelatin capsules: The apparatus consist of two concentric tubes, where inner tube is filled with medicament and through surrounding outer tube, the gelatin solution. The formed capsules are dropped into liquid paraffin where gelatin becomes insoluble. The capsules are subsequently degreased and dried. Generally soft gelatin capsules are used as ophthalmic soft gelatin capsule/ ophthalmic ointments, chewable soft gelatin capsule, enteric coated soft gelatin (Augsburger LL, 1990).
The solid packaging area as per schedule ‘M’ for acillinary area square meters 60, 30, 20, 10 area requirement, environment requirement, temperature requirement 25°C-5°C humidity, 55%-10% RH pressure in ware house area, 10 Pascal pressure in weighing area, 20 Pascal pressure in tabulating area, 15 Pascal pressure in central corridor. The advantages of solid dosage form are they have strong onset of action, formulation is simpler than liquid and semisolid dosage forms, it has high precision, lowest variability and accurate dosing, doses available according to patients requirements, easy for packaging, transport and does not require special conditions for storage, it does not harm the GIT due to its easy and rapid digestion, unpleasant taste and odor can be marked by using capsules and sugar coating for tablets, they are stable in chemical, physical and microbiological properties. The disadvantages of solid dosage form are it is not easy to swallow, it cannot be given to the unconscious patient, encapsulation of tablets, complex process of capsules may increase production cost and hygroscopic drugs are not suitable for these types of dosage forms.
Liquid dosage form
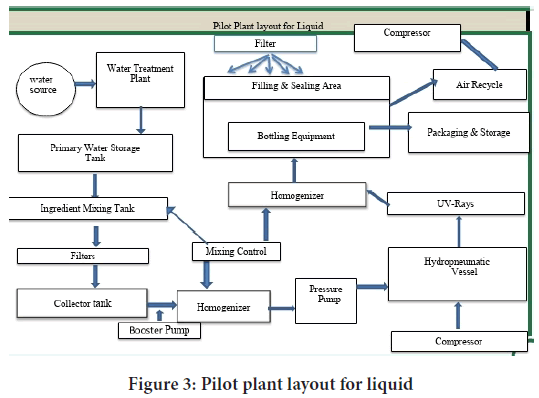
The Physical form of a drug product that is pourable displays Newtonian or Pseudo plastic flow behavior and conforms to its container at room temperature. Liquid dosage forms may be dispersed systems or solutions. In dispersed systems there is two or more phase; where one phase is distributed in another (World Health Organization, 2007) (Figure 3).
Figure 3: Pilot plant layout for liquid
The oral liquid dosage forms classify as Monophasic and Biphasic. The Monophasic is of simple solutions. The Biphasic is suspension and emulsion. Liquid preparation for oral use is usually Solutions, Emulsions or Suspensions containing one or more drug forms in suitable vehicle. The preparation for oral use are either supplied in finished form or with excipients or it may also prepare just before use by dissolving powder in vehicle stated on label.
The liquid preparation for oral use consists of antimicrobial preservative, antioxidants, dispensing agent, suspending agent, thickening agent, emulsifying agent, buffering wetting, solubilizing, stabilizing, flavoring and sweetening with suitable coloring agent. They may be single dosage or multiple dosage preparation. The devices used are spoon or cup, oral syringe, dropper, etc. The liquid of should be uniformity of mass, uniformity of mass of doses delivered by measuring devices, container with proper labeling.
The Oral suspension consists of active ingredient suspended in suitable liquid or vehicle. It should be readily dispersed when shaking and stable to give or enable correct dose to be delivered. Oral emulsion is active ingredients which are stabilized in oil in water dispersion or water in oil dispersion. It also readily disperses while shaking. Oral drops which are used in small volumes with the aid of suitable measuring device. The drops should be free from precipitate. The instability can be seen through flocculants, sediments, change in color, etc. Powders for oral solutions consists one or more dose preparation consist of solid loose, dry particle with high degree of fitness. It should be readily disperse in solution and should not form cake and the instability can be check through texture. Examples are clumping. Presentation for granules for oral solutions are intended to be issued to patient as granules to be swallowed, as such to be chewed or taken through water. They are dry aggregate of powder particles sufficiently resistant to withstand handling. It has noticeable changes in physical appearance when there is disability in the drug (Remington JP, 2006).
Formulation aspects of liquid orals are suspensions, emulsions, solutions. They are facilitating the connection between API and Vehicle. Steps involved in manufacturing process include planning of material requirements, liquid preparation, filling and packing, quality assurance (World Health Organization, 2007).
The Equipment’s used are mixers or mixing tank, homogenizer, filtration assembly, bottling assembly. The mixing tank and storage tank for liquid oral contains a bottom out let with electric or steam heating also available. Storage tank is manufactured from stainless steel 304/stainless steel 316 and is argon welded. They are made up with suitable thickness with smooth finish. It has a valve at bottom and lid at top. It has capacity of 50-10000 liters. The tank will be mounted on 4 legs with castors for movement. It is used or proper mixing of drug with excipients. Stainless steel tank with stirrer is with stainless steel steam jacketed and insulating with stainless cladding. They are different types of stirrer such as paddle/anchor/propeller. The electric heating of liquid is possible for small scale. It has capacity of 100-10000 litres. Colloidal mill is use for superfine grinding and simultaneous emulsifying, dispersing and homogenizing within one process. The homogenizer spring crude mixture inlet outlet homogenized liquid valve cover, valve seating. It is used for making suspension and emulsion. It consists of head of spring arrangement made out of stainless steel ultrasonifier inlet orifice vibrating blade outlet. The filter press is where the liquid to be filtered is pumped to tank where it enters into individual plates then passes through filter media like paper and crystal clear filtrate comes out through a central channel formed by interlocking cup then the pure form of liquid is obtained. Bottle washing machine consists of four inner and one outer wash. The first wash with water, second wash with detergent then wash with demineralized water and dry in inverted position or dry under hot air oven.
The liquid packaging area as per schedule ‘M’ include bottle washing area, filling and capping area, bottle labeling and box filling area. Bottle washing area here the tank should be filled with fresh demineralized water and then place in bottle into aluminum tray and then for washing area. The bottles should be inspected and rejected. Then the correct size should be chosen and fix on stainless steel platform and washing process is semiautonomous platform and washing process is semi-automatic. The washed bottles should be inverted on empty nozzles and then unloading of washed bottle in aluminum clean perforated tray in inverted position. So the water should be drained completely then dried at oven for 120°C for 1 hour. The filling and capping area contains the bulk containers which are used for filling. Only washed bottles and cleaned caps are used. The filled volumes record should be recorded and maintained as prescribed. The box filling area consists of the sealed and filled bottles are passed through convey belt and filled boxes consists labeling.
The layout of pilot plant of liquids consists of the equipment such as tanker, mixer, homogenizer, filtration assembly.
Tanker: It should be according to batch size preparation of the drug. It should not produce any additive to the product. It is made up of stainless steel of different grades and lined with Teflon and glass if high viscosity liquid then high electrical stirrers are used.
Mixer: Here simple mixing is done to increase mixing of liquid. There should be proper adequate clean up procedure. At high viscosity air entrapment occur it can be minimized by reduce agitator speed by caring out mixing process in closed tank under vacuum homogenizer. There should be a variety of equipment should be used for better results. Filtration and Clarification should require careful evaluation to exhibit high purity of drug as their laboratory counterparts. It should be checked periodically to know the purity of substance.
The advantages of liquid dosage form are immediate available for absorption in the body, easy route of administration, they can be taken easily and can be add color, flavor, sweetener as per required, they are used to change the dose daily easily, they can be given for children and old people, better for patient who have trouble swallowing, expiration, than other, more flexibility in achieving the proper dosage of medication. The disadvantages of liquid dosage form are they are bulky form than capsules or tablets, so they are difficult to carry, they are less stable and some are cannot store in room temperature, they are incompatibility than solid dosage form, there will be accident breakage of container, they have shorter half-life, they are harder to measure accurate dose, they are easily affected by microbes. “The visual inspection of solution is should be clear and free from any precipitation. A change in physical or chemical form of drug such as cloudiness of solutions may indicate chemical degradation or microbial contamination. It should be avoided for safety of the patient.”
Semi-solid dosage forms
Semi solid dosage forms are the topical dosage forms that are intended for the therapeutic, protective or cosmetic functions. Few examples are Ointments, paste, creams, plasters, suppositories, gels and rigid foams. Semisolids are the complex formulations which are having complex structural elements. They are of two phases-oil in water one is a continuous phase also known as external phase and the other is a dispersed phase also known as internal phase. Semi solid dosage form products are mostly administered topically or by the insertion method into an orifice of the body. During mixing process an active ingredient of a semi solid product attains uniformity. The consistency and viscosity of these products, once the active pharmaceutical ingredient is distributed in the manufacturing batch, the API is less prone to segregation than solid dosage form. The physical properties of the semi-solid dosage form depends upon numerous factors such as interfacial tension between the phases, size of the dispersed particles, partition coefficient of the active ingredient between the phases and also the product rheology. These factors merge to determine the release characteristics of the drug, as well as other characteristics.
The semi-solid dosage forms are classified as Suppositories. Suppositories are introduced into orifices of human body, which are of various weights and shapes these are the solid drug delivery systems. When suppositories are introduced into orifice, the external membrane will typically melt or dissolve at the body temperature that allows the active ingredient absorbed by the surrounding tissue. Suppositories bases that are usually employed are hydrogenated vegetable oils, mixtures of polyethylene glycols, glycerinated gelatin, fatty acid esters of polyethylene glycol. The suppository base has a marked impact on the release of active ingredient which is incor porated in it. Ointments are semi solid preparations. Ointments are homogenous, viscous, translucent semi solid preparations that are intended for external applications to the skin or mucus membranes. Ointments may be medicated or non-medicated. The bases of ointments are used as vehicle for transfer of drug into skin. Depending on the carrier of the drug or base used for its formulation, ointments can be classified as follows Hydrocarbon or oleaginous bases, absorbent or anhydrous base, emulsion or water miscible base and water soluble base. Creams are viscous semi solid emulsion with an opaque appearance. Creams are emulsions of water and oil classified as oil in water (o/w) or water in oil (w/o) emulsions. Oil in Water creams spread easily and do not leave the skin greasy and sticky, whereas Water in Oil creams is greasy and more emollient. Medical topical cream formulations also contain the suitable excipients such as emulsifiers and preservatives. Pastes generally contain a larger portion of solid material (such as 25%) than ointments and therefore they are stiffer. Pastes are prepared by incorporating the solids directly into a congealed system by levigation with a portion of base to form the paste like mass. They have good adhesion on skin and are less greasy. Gels are typically transparent or translucent, water-based semisolid dosage forms. They exhibit good spreading properties. Many gel products are turbid (Sud S and Kamath A, 2013).
The Equipment’s used in semi-solid dosage form are:
Agitator mixer: This agitator is a machine used in a tank for mixing numerous process media together. It works through mechanical mean by rotating an impeller to impart energy to the media which interact and mix the ingredients. An agitator consists of shaft, impellers, motor and gear box.
Roller mill: It is a form of compression mill which use single, double or triple cylindrical wheels arranged horizontally. It is rotated through there long axis in opposite pairs or against flat plates which is used to crush or grind several materials. One roller is run by motor and other by friction. The stress and attrition are employed in the procedure of milling which are rotate at distinct speeds.
Ribbon agitator: The mechanism involved is shear that is transfer by moving blades in a fixed shell. The functions are paste mixers, vacuum dryer, granulators. The mixing is completed within 15 mins or less. It consists of U-shaped shell containing a double helical ribbon agitator.
Colloidal mill: It is used to reduce the particle size of solid forms of the pharmaceutical ingredients which are present in different liquid or solid forms. It works on the principal of rotor-stator. Generally it is used in production of sterile products.
Sigma mixer: It works on the principle of shearing and tearing.
The plant layout requirements for semi solid dosage form as per schedule ‘M’ include external preparation, Quantitative layout, and Specific requirement for the manufacturing process. External preparation here it is recommended to have a minimum of 30 square meters area for basic installation of 10 square meters for ancillary area. It is necessary to provide a separate area for the formulation aimed for external and internal use, so that it can be avoidance of mix-up for suppositories, there should be a minimum area of 20 square meters for the basic installation. Quantitative layout for cream it has to be 18.5 square meters, 25 Lts to 10000 Lts for raw material storaging area; 40.8 square meters for the manufacturing area; tube and machine filling area should be 27 square meters; 46.8 square meters for packaging and labeling area and for the final product storage area should be 14 square meters. Specific requirements for the manufacturing of topical preparation here the manufacturing area temperature should not exceed 30°C. The area should be under suitable air lock. Insectocutors shall be installed outside the air lock. The air is filtered through 20 micrometer air filters and air conditioning at the manufacturing area. A suitable capacity of an exhaust system is used. There should be no usage of rags and dusters in the cleaning and drying process. Water used in compounding should be purified and the powders are sieved suitably before use. The heating of vehicles and bases should be done in separate mixing area. The advantages of semi-solid dosage form are it can be applied directly on to the affected area, it can be administered easily at any conditions, the active pharmaceutical ingredient present in the dosage can be directly deliver to target system through skin or other physical membrane, it reduce risk of unwanted side effects, the first pass effect is avoided, it is stable than liquid dosage form, it is convenient for patients who have difficulty in oral administration, it is suitable dosage form for bitter drugs. The disadvantages of semi-solid dosage form are it cause irritation or allergy to some patients, the accuracy of dosage form cannot be measured, it is bulky to handle, the physiochemical properties are less stable than solid dosage form, it is easily contaminates when applied with fingers.
Discussion and Conclusion
Pilot plant scale up considerations for solids, liquids, semisolids is discussed here. The main objective of pilot plant is “Find mistakes on a small scale and make profit on large scale”. The significance of pilot plant scale up studies gives review of range of relevant processing equipment, Optimization and control of production rate, Information on infrastructure of equipment’s. This also gives detail information about dosage form such as solids, liquids and semisolid dosages their types, equipment’s used in their preparations, infrastructure of pilot plant layout.
References
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Philadelphia: Lea and Febiger. 1976: 681-710.
- Jiaqiang E, Zhang Z, Chen J, Pham M, Zhao X, Peng Q, et al. Performance and emission evaluation of a marine diesel engine fueled by water biodiesel-diesel emulsion blends with a fuel additive of a cerium oxide nanoparticle. Energy Conv Manag. 2018; 169: 194-205.
- Ramasubramaniyan P, Shibinraj C, Nagarajan P, Sherly D, Subramanian L, Solairaj P. Pilot scale-up techniques for solid dosage form: An overview for tablets. World J Pharm Res. 2014; 3(8): 925-931.
- Jain NK, Sharma SN. A text book of professional pharmacy. Vallabh Prakashan. 2016; 215-218.
- Shaikh R, O’Brien DP, Croker DM, Walker GM. The development of a pharmaceutical oral solid dosage forms. Computer Aided Chemical Engineering. 2018: 41; 27-65.
- Allen L, Ansel HC. Ansel's pharmaceutical dosage forms and drug delivery systems. Lippincott Williams and Wilkins. 2013.
- Ennis BJ, Litster JD. Particle size enlargement. Perry's Chemical Engineers' Handbook. 1997: 20-56.
- Boylan JC, Swarbrick J. Encyclopedia of pharmaceutical technology. Marcel Dekker. 2001; 12: 171-186.
- Faure A, York P, Rowe RC. Process control and scale-up of pharmaceutical wet granulation processes: A review. Eur J Pharm Biopharm. 2001; 52(3): 269-277.
[Crossref] [Google Scholar] [Pubmed]
- Schwartzbach H. The possibilities and challenges of spray drying. Pharm Technol Eur. 2010; 22: 5-8.
- Gohel MC, Parikh RK, Padshala MN, Sarvaiya KG, Jena DG. Formulation and optimization of directly compressible isoniazid modified release matrix tablet. Indian J Pharm Sci. 2007; 69(5): 640.
- Sitompul JP, Lee HW, Kim YC, Chang MW. A scaling-up synthesis from laboratory scale to pilot scale and to near commercial scale for paste-glue production. J Eng Tech Sci. 2013; 45(1).
- Meyer T. Scale-up of polymerization process: A practical example. Org Process Res Dev. 2003; 7(3): 297-302.
- Benedek I. Development and manufacture of pressure-sensitive products. CRC Press. 1998.
- Marshall K. Compression and consolidation of powdered solids. The theory and practice of industrial pharmacy. 1986; 3: 66-99.
- Hoag SW. Capsule’s dosage form: Formulation and manufacturing considerations. Developing solid oral dosage forms. 2017; 723-747.
- Augsburger LL. Hard and soft gelatin capsules. Drugs and the pharmaceutical sciences. 1990; 40: 441-490.
- World Health Organization. Forty-second report of the WHO Expert Committee on specifications for pharmaceutical preparations. World Health Organization. 2007.
- Remington JP. Remington: The science and practice of pharmacy. Lippincott Williams and Wilkins. 2006.
- Sud S, Kamath A. Methods of size reduction and factors affecting size reduction in pharmaceutics. Int Res J Pharm. 2013; 4(8): 57-64.
Author Info
R Shireesh Kiran*, Mohammed Ahmer Ali, K Geetha, G S Sharma and T Rama RaoCitation: Kiran RS: Review on Pilot Plant Scale up Techniques Used in Solid, Liquid and Semisolids
Received: 01-Jul-2022 Accepted: 25-Jul-2022 Published: 01-Aug-2022, DOI: 10.31858/0975-8453.13.8.557-562
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3