Review Article - (2022) Volume 13, Issue 1
Abstract
SEDDS (Self Emulsifying Drug Delivery System) have been broadly classified based on the basis of droplet size obtained after dispersion. If the droplet size of dispersion is in range of 100-250 nm then the SEDDS are termed as SMEDDS (Self Micro Emulsifying Drug Delivery System) while those having droplet size below 100 nm are called SNEDDS (Self Nano Emulsifying Drug Delivery System). Selection of lipid, surfactant and co-surfactant is done based on solubility of drug in lipid, surfactant and co-solvent. A ternary phase diagram of the three components is plotted by keeping oil, surfactant and co-surfactant on three different axis. Based on the clarity of dispersion, emulsification time and droplet size, the entire plot is divided into four regions namely phase separation, SEDDS, SMEDDS and SNEDDS. Self-emulsification is a phenomenon that occurs spontaneously during the formation of SEDDS. It occurs when entropy change that favours dispersion is greater than energy required to increase the surface area of emulsion. Physical adsorption of L-SEDDS (Liquid- Self Emulsifying Drug Delivery System) on the solid carriers is one of the simplest technique of solidification. In this process, L-SEDDS are added on solid carrier and mixed either via physical blending with hand or motor pestle on lab scale or via use of blenders.
Keywords
Self Emulsifying Drug Delivery System (SEDDS), Non-dispersing, Hydrophilic lipophilic balance, Self-emulsification, Self-Nano Emulsifying Drug Delivery System (SNEDDS)
Introduction
SNEDDS have been broadly classified based on the basis of droplet size obtained after dispersion. If the droplet size of dispersion is in range of 100-250 nm then the SEDDS are termed as SMEDDS (Self Micro Emulsifying Drug Delivery System) while those having droplet size below 100 nm are called SNEDDS (Singh B, et al., 2009; Tarate B, et al., 2014). However, it is important to note that there is no consensus on the droplet size of SMEDDS and SNEDDS and they may vary according to literature (Garg V, et al., 2016; Singh B, et al., 2009; Tarate B, et al., 2014).
Literature Review
Based on the type of constituents, lipid formulations are categorized in four different types as described below:
Type I
These are basically non-dispersing systems. They consist of oils like triglycerides or mixed glycerides and form coarse dispersion on dilution. As they are non-dispersing themselves, their absorption takes place through digestion via gastric enzymes. These are suitable for molecules exhibiting higher solubility in oils so that their required dose can be incorporated in the optimum quantity of oil (Tarate B, et al., 2014).
Type II
These are composed of single or mixed triglycerides along with lipophilic surfactants having HLB (hydrophilic lipophilic balance) values less than 12 (Pouton CW, 2000). Self-emulsification usually occurs when surfactant concentration is between 20%-60%. On dispersion, large interfacial areas are generated which lead to optimum partitioning of the bio-molecules between both the phases (Hauss DJ, et al., 1998; Porter CJ, et al., 2008; Pouton CW and Porter CJ, 2008).
Type III
These consist of oil and hydrophilic surfactants with HLB values more than 12 (Sapra K, et al., 2012). These form SNEDDS (Singh B, et al., 2009). Cosolvents may also be added to improve the formulation characteristics. Commonly used co-solvents include ethanol, Propylene Glycol (PG) and Polyethylene Glycols (PEG) etc. Type III emulsions may further be classified as type III A and type III B. Type III A consists of more amount of lipids (40%-80%) while type III B contains less amount of lipids (upto 20%) and more surfactants. Type IIIB formulations are capable of achieving greater dispersion rates as compared to Type IIIA although the risk of drug precipitation on dispersion of the formulation is higher due to their lower lipid content (Garg V, et al., 2016; Singh B, et al., 2009).
Type IV
These are emulsions without oils. They constitute water soluble and insoluble surfactants along with co-solvents. The resultant droplet size of dispersion is generally less than 50 nm. These are generally opalescent or transparent in nature (Garg V, et al., 2016; Singh B, et al., 2009).
Composition of Snedds
Selection of lipid, surfactant and co-surfactant is done based on solubility of drug in lipid, surfactant and co-solvent (Kohli K, et al., 2010). A ternary phase diagram of the three components is plotted by keeping oil, surfactant and co-surfactant on three different axis. Based on the clarity of dispersion, emulsification time and droplet size, the entire plot is divided into four regions namely phase separation, SEDDS, SMEDDS and SNEDDS. A ratio should be selected from a particular relevant region of this plot to achieve the desired type of formulation (Singh B, et al., 2009). For selection of oil, surfactants and co-surfactants, solubility and affinity of drug in each of these components, their compatibility with each other at concentration in which they are selected are the important parameters to be evaluated. However, the most important parameter is the self-emulsification ability of the resultant formulation (Garg V, et al., 2016; Rahman MA, et al., 2013).
Mechanism of Formation of Snedds
Self-emulsification is a phenomenon that occurs spontaneously during the formation of SEDDS. It occurs when entropy change that favours dispersion is greater than energy required to increase the surface area of emulsion (Kohli K, et al., 2010; Singh B, et al., 2009). Free energy of an emulsion is considered as a direct function of the energy required to create a new surface between any two immiscible phases. The two immiscible phases of an emulsion exhibit a tendency to separate so as to reduce interfacial area to minimum and thus, to minimize free energy of system. These systems are stabilized by use of emulsifying agents that reduce the interfacial tension (Parmar N, et al., 2011; Singh B, et al., 2009).Thus, for SEDDS, such kind of emulsifiers and co-solvents need to be selected that will be able to reduce the interfacial tension. This, in turn, will lower the free energy required by SEDDS so that when they come in contact with aqueous medium in GIT, the self-emulsification process sets (Garg V, et al., 2016; Kohli K, et al., 2010; Singh B, et al., 2009).
Categorization of Snedds
L-SEDDS (Liquid-Self Emulsifying Drug Delivery System)
These are self-emulsifying isotropic mixtures of oil, surfactant, and co-surfactant in liquid state. These offer the advantages of enhanced solubility of drugs and their increased lymphatic absorption. However, due to their liquid state, they are difficult to be dispensed as dosage form. To make the dosage form more convenient, they need to be incorporated into soft gelatin capsules. This, in turn, adds to the cost of formulation (Garg V, et al., 2016; Singh B, et al., 2009).
Super saturable SEDDS
The concentration of surfactants in the SEDDS formulation is usually in the range of 20-60%. From safety point of view, use of such high concentration of surfactant becomes a concern for the formulator, as their higher concentration is known to lead to some adverse effects (Garg V, et al., 2016). To overcome this problem, the concept of super saturable SEDDS was created. In these, the concentration of surfactants is reduced by the inclusion of water soluble Polymeric Precipitation Inhibitor (PPI). These formulations maintain a supersaturable metastable state in vivo by reducing precipitation of drug using PPI. Hydroxypropyl Methylcellulose (HPMC) of different grades of viscosity have been widely reported to prevent crystallization as PPI in supersaturable SEDDS (Garg V, et al., 2016; Gao P and Morozowich W, 2006; Gao P, et al., 2003; Raghavan SL, et al., 2000).
S-SEDDS (Solid-Self Emulsifying Drug Delivery System)
Self-emulsifying drug delivery systems were initially developed in liquid form. However these L-SEDDS faced the difficulty of stability, formation of unit dosage form, high production costs, low stability and portability, low drug loading and few choices of dosage forms. Irreversible drugs/excipients precipitation may also be problematic. S-SEDDS come as a superior alternative to the L-SEDDS. S-SEDDS along with advantages of L-SEDDS provide better stability, ease of handling, ease of conventional dosage forms like tablets and capsules (Mohsin K, et al., 2012).
Techniques Used for Solidification of Snedds
Physical adsorption
Physical adsorption of L-SEDDS on the solid carriers is one of the simplest technique of solidification. In this process, L-SEDDS are added on solid carrier and mixed either via physical blending with hand or motor pestle on lab scale or via use of blenders. The loading factor is calculated as the amount of solid carrier required for adsorption of L-SEDDS so that homogenous powder is obtained. After this, weighed amount of both L-SEDDS and carrier are mixed together until a homogenous solid powder is formed via adsorption of L-SEDDS over solid carriers. This powder should be passed through sieves to break any lumps, if present. The resultant powder can be directly filled into capsules or can be compressed into tablets via addition of some other excipients used for tableting (Zidan AS, et al., 2015). Several carriers like silicon dioxide, syloid have the capacity to absorb large amount of L SEDDS (Tarate B, et al., 2014).
Hydrophilic/hydrophobic nature of carrier on which L-SEDDS have to be adsorbed affect the properties of drug e.g. L-SNEDDS of ezetimibe were prepared with Capryol 90, Lauroglycol FCC, ethyl laurate, Cremophor EL and Transcutol® P and adsorbed on hydrophobic colloidal silicon dioxide to form Self Nano Emulsifying Granules (SNEG). X-Ray Diffraction (XRD) indicates that drug is in its amorphous form, but when the same SNEDDS were loaded on magnesium sterate a eutectic mixture is resulted (Dixit RP and Nagarsenker MS, 2008).
Melt granulation
Melt granulation is a method in which S-SEDDS are prepared in a single step. In this method, there is no need to prepare L-SEDDS and then adsorb on the solid carrier. In this method, oil e.g. goat fat, or surfactant which are solid at room temperature are used. All the mixture of oil and surfactant is taken in the desired quantity and heated above the melting point. In this melted mixture drug is added and mixed to form homogenous mixture (Attama AA and Mpamaugo VE, 2006).
Pour moulding method
Self-emulsifying suppositories and tablets can be prepared via pour moulding method. In this method, oil and surfactant are taken and heated together until they homogenize completly. Drug is added into this homogenous mixture and stir thoroughly. This mixture is now poured into moulds and allowed to settle at a temperature of 4°C. The tablets or suppositories with self-emulsifying ability are taken out from mould and stored at cool place (Attama AA, et al., 2003).
Spray congealing
Self-emulsifying microparticles can be produced by spray congealing technology. Fluidized bed equipment is utilized for this purpose. It uses two fluid atomisers with a wide orifice opening i.e. pneumatic nozzle. External mixing of fluid and air or gas occurs outside nozzle orifice, thus atomisation can be varied by changing the air pressure without affecting the liquid flow rate to enable the spraying of high concentration or viscous products. The temperature of feed tank containing molten fluid should be kept higher than melting temperature. Congealing chamber should be cooled using refrigerator system for solidification of droplets.
Spray drying
Spray drying is one of the commonly used technique for the formation of S-SEDDS. Spray dryer consists of following components viz. feed delivery system, atomizer, heated air supply, drying chamber, solid-gas separator, and product collection system. In this technique, drug, L-SEDDS and carrier are dissolved or suspended in a solvent to form a homogenous system. This solution is now atomized to produce liquid droplets with the help of spray nozzle in spray dryer. The atomizer, the temperature, the most suitable air flow pattern and the drying chamber design are important variables affecting product characteristics (Alinaghi A, et al., 2015; Czajkowska-Kośnik A, et al., 2015; Tarate B, et al., 2014).
Extrusion-spheronization
S-SEDDS can also be formulated in the form of pellets via extrusion-spheronization. This process includes wet granulation of L-SEDDS with solid excipients, followed by extrusion of wet mass, spheronization of extrudates, drying of the spheroids, sizing, and optionally coating of the pellets. The bottom plate is grooved to provide the equipment-particle interactions for rounding the cylindrical pellets (Abdalla A, et al., 2008; Tarate B, et al., 2014).
Lyophilization
Lyophilization can also be used for formulating S-SEDDS. In this process, water is evaporated directly via sublimation. It includes several steps i.e. freezing, primary drying, and secondary drying. In this process both carrier and L-SEDDS are dissolved in a common solvent followed by freezing and sublimation process. This method gives a solid product (Tarate B, et al., 2014). Jain AK, et al. 2014 prepared S-SNEDDS using lyophilization technique. SNEDDS were diluted in minimum quantity of deionized water and thoroughly mixed with Aerosil® 200.
Self-emulsifying solid dispersion
Self-emulsifying solid dispersions can also be prepared by melting method. In this method, drug, surfactant and fatty acids are homogenously mixed and slightly heated to get a melted mixture. This melted mixture is then added to a suitable adsorbent like Aerosil® 200 and kept at cool temperature. Solid mass obtained is crushed and passed through sieve of suitable size to obtain fine powder (Tran TH, et al., 2014).
Positively charged SEDDS
Most of the absorptive cells present in the human body carry a negative charge. Due to this reason, positively charged SEDDS have been reported to show better bioavailability as compared to conventional SEDDS. Oppositely charged SEDDS have more time to interact with gastric mucosa via increased adhesion.
Drug transport mechanism of SEDDS
SEDDS offer bioavailability of water insoluble drugs even through oral administration. Once they reach the GIT, they undergo three processes; i.e. digestion, absorption, and circulation. During digestion, SEDDS form a coarse emulsion, which undergoes enzymatic hydrolysis at oil water interphase and thereby gets ready for absorption phase. After formation of mixed micelles, due to interaction of fatty acids with bile, digestion process stops. The next phase of drug absorption then starts. These colloids are taken up by passive diffusion or active transport through enterocyte membrane (Stremmel Q, 1988) (Figure 1).
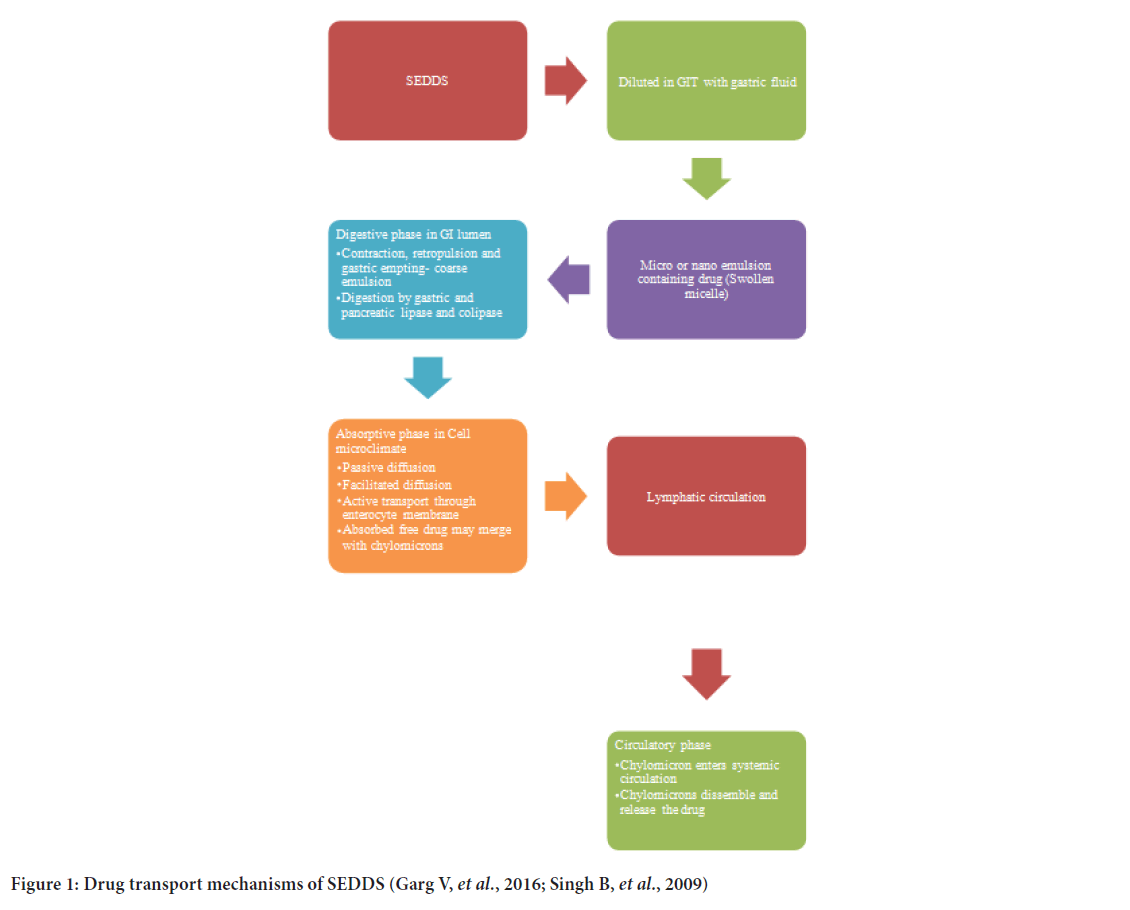
Figure 1:Drug transport mechanisms of SEDDS (Garg V, et al.,2016; Singh B, et al., 2009)
Characterization of Snedds
Dispersibility test
It is conducted to check the phase separation and clarity. In dispersibility test USP dissolution apparatus II is used. SEDDS (1 mL) is added to 500 mL of water at 37 ± 0.5°C at 50 rpm. Emulsion formed is genarally examined visually and graded accordingly i.e. Emulsion, micro emulsion, nano-emulsion or no emulsion (Singh B, et al., 2009).
Droplet size, morphology and zeta potential
Droplet size has an important role in stability of an emulsion. They not only affect the bioavailability but also drug loading. Droplet size also helps in categorisation of emulsions like SEDDS, SMEDDS and SNEDDS. Charge plays an important role in stability of emulsion. In conventional SEDDS usually negative charge is present, however depending upon the requirements desirable charge can be added by the selection of proper emulsifier (Garg V, et al., 2016; Kohli K, et al., 2010; Muller BW and Muller RH, 1984; Singh B, et al., 2009).
Transmission Electron Microscopy (TEM)
Electron microscopy studies like Scanning Electron Microscopy (SEM), TEM and cryo-TEM are used to generate information on sample topography, composition, morphology, shape, texture, size etc. One drop of diluted samples was deposited on a film coated copper grid and then stained with one drop of 2% aqueous solution of Phosphotungstic Acid (PTA), allowing to dry before observation under the electron microscope (Garg V, et al., 2017; Setthacheewakul S, et al., 2010).
Turbidity measurements
It helps in determining whether equilibrium has reached in dispersion. It helps in calculation of emulsification time. Turbidity meters like Hach turbidity meters are popularly used to measure turbidity. These meters can also be connected to dissolution apparatus and can measure turbidity at frequent intervals to know the clarity of micro or nano-emulsion with respect to time (Garg V, et al., 2016; Kohli K, et al., 2010; Singh B, et al., 2009).
Stability study
Phase separation and clarity study: Physical and chemical stability of emulsions containing drug should be studied via phase separation and clarity study. Emulsions are centrifuged for specific time and store at different temperature (5°C, 15°C, 25°C and 37°C). Normally emulsions should be kept at 15°C or higher. Usually 5°C emulsions show turbidity and phase separation (Kohli K, et al., 2010; Singh B, et al., 2009; Verwey EJ, 1947).
Thermodynamic stability studies: Thermodynamic stability can be studied by keeping the samples a number of times between 4°C and 45°C and then centrifuging them for 30 min at 3500 rpm followed by freez thaw cycles between -2°C and +25°C in triplicate. All formulations should be kept at each temperature for not less than 48 h (Singh B, et al., 2009).
Robustness to dilution: Dilution robustness is an important stability parameter for SEDDS. SEDDS should form micro or nano emulsion with any dissolution media simulating GIT at any volume. There should be no change in the emulsion even after dilution. It should neither show phase separation nor drug precipitation even after 12 hr (Garg V, et al., 2016; Singh B, et al., 2009).
Applications of SNEDDS
The technique of formation of SEDDS to achieve the optimum delivery has been widely reported. A number of bio-actives and phyto constituents have been prepared in the form of various types of SEDDS using variable combinations of the available excipients to achieve their optimum delivery (Garg V, et al., 2016).
Certain protein compounds have been formulated as SNEDDS for achieving the oral delivery of these molecules by providing protection against gastric enzymes (Rao SV and Shao J, 2008). The peptide fluorescent labeled beta-lactamase, was studied for its formulation which was subsequently tested for its in vitro transport and in vivo oral absorption (Rao SV, et al., 2008; Rao SV and Shao J, 2008).
A single dose of self-emulsifying formulation of vitamin E resulted in quicker and higher absorption as compared to the conventional formulation available as soft gelatin capsules. This was attributed to finer dispersion size and the resultant larger surface area in self-emulsifying systems (Julianto T, et al., 2000).
Taha E, et al. 2007 carried out the bioavailability assessment of L- and S-SNEDDS of vitamin A and compared it to that of the conventional oil filled capsules of vitamin A. An optimized oily formulation containing a mixture of vitamin A, soybean oil (16.17 mg), Cremophor EL (43.62 mg) and Capmul MCM C8 (42.53 mg) were used in liquid form. S-SNEDDS were prepared using Avicel PH105 as absorbent, 4% talc powder as lubricant and compressing the SNEDDS in the form of tablets.
Ginkgo biloba Extract (GBE) was formulated in the form of SNEDDS prepared from Tween 80, Cremophor EL 35, 1,2-propanediol and ethyl oleate. Dissolution rate of SNEDDS was found to be significantly higher in this formulation as compared to conventional tablets. Relative bioavailability of SNEDDS for bilabolide and ginkgolide A and B was reported to be 162.1,154.6, and 155.8% respectively as compared to the reference tablets in dogs (Tang B, et al., 2008).
CRM, a naturally active constituent has been used as anti-tumor, anti-inflammatory, anti-viral, anti-oxident and anti-HIV, with promising clinical application. In a latest study, a combination of four naturally occurring enzyme inhibitors (piperine, quercetin, tangeretin and silibinin) was co-formulated with CRM to prepare its SMEDDS (Grill AE, et al., 2014). Both ex vivo and in vivo studies indicated the superiority of the co-delivery of enzyme inhibitors with CRM in the form of self-emulsifying delivery system (Garg V, et al., 2016).
Vinpocetine, the water insoluble active alkaloids of Vinca were formulated into SMEDDS to increase its bioavailability (Cui J, et al., 2009). The formulation prepared using ethyl oleate, Solutol HS and Transcutol® P was found to increase the bioavailability of the active constituent by approximately 1.7 folds. Another SMEDDS formulation prepared using Labrafac, oleic acid, Cremophor EL, Transcutol® P, and gum acacia was compared to a solid dispersion of the drug for its dissolution as well as pharmacokinetic parameters (Chen Y, et al., 2009). The SMEDDS formulation was reported to be superior to the solid dispersion formulation in terms of its solubility, dissolution, permeability, absorption and oral bioavailability (Garg V, et al., 2016).
An alkaloidal drug obtained from Sophora roots, matrine has been reported to exhibit anti-tumor activities in a variety of cancer models (Liu Y, et al., 2014). However, the use of drug is limited by its poor bioavailability. To enhance its oral bioavailability, a two way approach of preparing its phospholipid complexes and then formulating these complexes as SNEDDS was adopted (Ruan J, et al., 2010). A substantial increase in the bioavailability of the drug was achieved in rat model with the SNEDDS formulation, prepared by using Lauroglycol FCC, Cremophor EL and Transcutol® HP. The use of this technique was also made in preparing SNEDDS of morin wherein the phospholipid complexes of the drug were prepared (Chavan RB, et al., 2015).
Conclusion
Drug discovery programs provided many new chemical species that are poorly water-soluble. The use of lipid-based formulations in general and SNEDDSs in particular shows great potential in enhancing aqueous solubility, stability, oral absorption and in minimizing inter/intra-patient dose variability. SNEDDSs improve the absorption of drugs by several pathways, including increasing membrane fluidity, bypassing the first-pass effect, and inhibition of P-gp efflux. Following this process, micelles along with other colloidal structures made of phospholipids, bile salt, and triglycerides are formed, which increase the transport of the drug through the intestinal barrier. The submicron size of the system with enhanced surface activity allows more robust drug transport through the GI boundary layer, ultimately resulting in better drug absorption and a rapid onset of action.
Previously, SNEDDSs formulations were used to overcome issues related to low aqueous solubility and oral bio-availability drugs. However, the scope of SNEDDSs is far beyond the solubility and dissolution issues. Presently, they have evolved into mucus-permeating, supersaturated, solid and targeted SNEDDSs to tackle issues related to classical SNEDDSs and to make new changes for several applications.
References
- Singh B, Bandopadhyay S, Kapil R, Singh R, Katare OP. Self-Emulsifying Drug Delivery Systems (SEDDS): Formulation development, characterization, and applications. Crit Rev Ther Drug Carrier Syst. 2009; 26(5).
[CrossRef] [Google Scholar] [Pubmed]
- Tarate B, Chavan R, K Bansalk A. Oral solid self-emulsifying formulations: A patent review. Recent Patents on Drug Deliv Formul. 2014; 8(2): 126-143.
[CrossRef] [Google Scholar] [Pubmed]
- Garg V, Gupta R, Kapoor B, Singh SK, Gulati M. Application of Self-Emulsifying Drug Delivery Systems for effective delivery of nutraceuticals. Emulsions. 2016; 479-518.
- Pouton CW. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘Self-Micro Emulsifying’ Drug Delivery Systems. Eur J Pharm Sci. 2000; 11: 93-98.
[CrossRef] [Google Scholar] [Pubmed]
- Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, et al. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998; 87(2): 164-169.
[CrossRef] [Google Scholar] [Pubmed]
- Porter CJ, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Del Rev. 2008; 60(6): 673-691.
[CrossRef] [Google Scholar] [Pubmed]
- Pouton CW, Porter CJ. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv Drug Del Rev. 2008; 60(6): 625-637.
[CrossRef] [Google Scholar] [Pubmed]
- Sapra K, Sapra A, Singh SK, Kakkar S. Self Emulsifying Drug Delivery System: A tool in solubility enhancement of poorly soluble drugs. Indo Global J Pharm Sci. 2012; 2(3): 313-332.
- Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-Emulsifying Drug Delivery Systems: An approach to enhance oral bioavailability. Drug Discov Today. 2010; 15(21-22): 958-965.
[CrossRef] [Google Scholar] [Pubmed]
- Rahman MA, Hussain A, Hussain MS, Mirza MA, Iqbal Z. Role of excipients in successful development of Self-Emulsifying/Micro Emulsifying Drug Delivery System (SEDDS/SMEDDS). Drug Dev Ind Pharm. 2013; 39(1): 1-9.
[CrossRef] [Google Scholar] [Pubmed]
- Parmar N, Singla N, Amin S, Kohli K. Study of co-surfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) Self Nano Emulsifying Drug Delivery System. Colloids Surf B: Biointerfaces. 2011; 86(2): 327-338.
[CrossRef] [Google Scholar] [Pubmed]
- Gao P, Morozowich W. Development of supersaturatable Self-Emulsifying Drug Delivery System formulations for improving the oral absorption of poorly soluble drugs. Exp Opin Drug Del. 2006; 3(1): 97-110.
[CrossRef] [Google Scholar] [Pubmed]
- Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W, et al. Development of a supersaturable SEDDS (S‐SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003; 92(12): 2386-2398.
[CrossRef] [Google Scholar] [Pubmed]
- Raghavan SL, Trividic A, Davis AF, Hadgraft J. Effect of cellulose polymers on supersaturation and in vitro membrane transport of hydrocortisone acetate. Int J Pharm. 2000; 193(2): 231-237.
[CrossRef] [Google Scholar] [Pubmed]
- Mohsin K, Shahba AA, Alanazi FK. Lipid based self-emulsifying formulations for poorly water soluble drugs-an excellent opportunity. Ind J Pharm Edu Res. 2012; 46(2): 88-96.
- Zidan AS, Aljaeid BM, Mokhtar M, Shehata TM. Taste-masked orodispersible tablets of cyclosporine self-nanoemulsion lyophilized with dry silica. Pharm Dev Technol. 2015; 20(6): 652-661.
[CrossRef] [Google Scholar] [Pubmed]
- Dixit RP, Nagarsenker MS. Self-nano emulsifying granules of ezetimibe: Design, optimization and evaluation. Eur J Pharm Sci. 2008; 35(3): 183-192.
[CrossRef] [Google Scholar] [Pubmed]
- Attama AA, Mpamaugo VE. Pharmacodynamics of piroxicam from self-emulsifying lipospheres formulated with homolipids extracted from Capra hircus. Drug Deliv. 2006; 13(2): 133-137.
[CrossRef] [Google Scholar] [Pubmed]
- Attama AA, Nzekwe IT, Nnamani PO, Adikwu MU, Onugu CO. The use of solid self-emulsifying systems in the delivery of diclofenac. Int J Pharm. 2003; 262(1-2): 23-28.
[CrossRef] [Google Scholar] [Pubmed]
- Alinaghi A, Tan A, Rao S, A Prestidge C. Impact of solidification on the performance of lipid-based colloidal carriers: Oil-based versus self-emulsifying systems. Curr Drug Deliv. 2015; 12(1): 16-25.
[CrossRef] [Google Scholar] [Pubmed]
- Czajkowska-Kośnik A, Szekalska M, Amelian A, Szymańska E, Winnicka K. Development and evaluation of liquid and solid Self-Emulsifying Drug Delivery Systems for atorvastatin. Molecules. 2015; 20(12): 21010-21022.
[CrossRef] [Google Scholar] [Pubmed]
- Abdalla A, Klein S, Mäder K. A new Self-Emulsifying Drug Delivery System (SEDDS) for poorly soluble drugs: Characterization, dissolution, in vitro digestion and incorporation into solid pellets. Eur J Pharm Sci. 2008; 35(5): 457-464.
[CrossRef] [Google Scholar] [Pubmed]
- Jain AK, Thanki K, Jain S. Solidified self-nano emulsifying formulation for oral delivery of combinatorial therapeutic regimen: Part I, formulation development, statistical optimization, and in vitro characterization. Pharm Res. 2014; 31(4): 923-945.
[CrossRef] [Google Scholar] [Pubmed]
- Tran TH, Guo Y, Song D, Bruno RS, Lu X. Quercetin-containing Self-Nano Emulsifying Drug Delivery System for improving oral bioavailability. J Pharm Sci. 2014; 103(3): 840-852.
[CrossRef] [Google Scholar] [Pubmed]
- Stremmel W. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J Clin Invest. 1988; 82(6): 2001-2010.
[CrossRef] [Google Scholar] [Pubmed]
- Muller BW, Muller RH. Particle size analysis of latex suspensions and microemulsions by photon correlation spectroscopy. J Pharm Sci. 1984; 73(7): 915-918.
[CrossRef] [Google Scholar] [Pubmed]
- Garg V, Kaur P, Singh SK, Kumar B, Bawa P, Gulati M, et al. Solid Self-Nano Emulsifying Drug Delivery Systems for oral delivery of polypeptide-k: Formulation, optimization, in-vitro and in-vivo antidiabetic evaluation. Eur J Pharm Sci. 2017; 109: 297-315.
[CrossRef] [Google Scholar] [Pubmed]
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-micro emulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm. 2010; 76(3): 475-485.
[CrossRef] [Google Scholar] [Pubmed]
- Verwey EJ. Theory of the stability of lyophobic colloids. J Phys Coll Chem. 1947; 51(3): 631-636.
[CrossRef] [Google Scholar] [Pubmed]
- Rao SV, Shao J. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for oral delivery of protein drugs: I, formulation development. Int J Pharm. 2008; 362(1-2): 2-9.
[CrossRef] [Google Scholar] [Pubmed]
- Rao SV, Agarwal P, Shao J. Self-Nano Emulsifying Drug Delivery Systems (SNEDDS) for oral delivery of protein drugs: II, In vitro transport study. Int J Pharm. 2008; 362(1-2): 10-15.
[CrossRef] [Google Scholar] [Pubmed]
- Julianto T, Yuen KH, Noor AM. Improved bioavailability of vitamin E with a self emulsifying formulation. Int J Pharm. 2000; 200(1): 53-57.
[CrossRef] [Google Scholar] [Pubmed]
- Taha E, Ghorab D, Zaghloul AA. Bioavailability assessment of vitamin A Self-Nano Emulsified Drug Delivery Systems in rats: A comparative study. Med Princ Pract. 2007; 16(5): 355-359.
[CrossRef] [Google Scholar] [Pubmed]
- Tang B, Cheng G, Gu JC, Xu CH. Development of solid Self-Emulsifying Drug Delivery Systems: Preparation techniques and dosage forms. Drug Discov Today. 2008; 13(13-14): 606-612.
[CrossRef] [Google Scholar] [Pubmed]
- Grill AE, Koniar B, Panyam J. Co-delivery of natural metabolic inhibitors in a Self-Micro Emulsifying Drug Delivery System for improved oral bioavailability of curcumin. Drug Deliv Transl Res. 2014; 4(4): 344-352.
[CrossRef] [Google Scholar] [Pubmed]
- Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, et al. Enhancement of oral absorption of curcumin by Self-Micro Emulsifying Drug Delivery Systems. Int J Pharm. 2009; 371(1-2): 148-155.
[CrossRef] [Google Scholar] [Pubmed]
- Chen Y, Li G, Huang JG, Wang RH, Liu H, Tang R. Comparison of Self-Micro Emulsifying Drug Delivery System (SMEDDS) versus solid dispersion technology used in the improvement of dissolution rate and bioavailability of vinpocetine. Acta Pharm Sin. 2009; 44(6): 658-666.
[Pubmed]
- Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q, et al. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor Biol. 2014; 35(6): 5111-5119.
[CrossRef] [Google Scholar] [Pubmed]
- Ruan J, Liu J, Zhu D, Gong T, Yang F, Hao X, et al. Preparation and evaluation of Self-Nano Emulsified Drug Delivery Systems (SNEDDSs) of matrine based on drug-phospholipid complex technique. Int J Pharm. 2010; 386(1-2): 282-290.
[CrossRef] [Google Scholar] [Pubmed]
- Chavan RB, Modi SR, Bansal AK. Role of solid carriers in pharmaceutical performance of solid supersaturable SEDDS of celecoxib. Int J Pharm. 2015; 495(1): 374-384.
[CrossRef] [Google Scholar] [Pubmed]
Author Info
Sandip Badadhe G* and Nilam DalaviCitation: Badadhe SG: Review on Self Nano Emulsifying Drug Delivery System
Received: 15-Dec-2021 Accepted: 29-Dec-2021 Published: 05-Jan-0022, DOI: 10.31858/0975-8453.13.1.63-68
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






