Research Article - (2022) Volume 13, Issue 5
Abstract
Ulcerative Colitis (UC) is a common condition resulting in inflammation of the colon. Current treatments for this condition result in side effects in a significant proportion of patients and consequently alternative treatments are being sought. Probiotics are live microorganisms which have been used to treat other inflammatory conditions such as gastroenteritis and pouchitis.
In this study, the evidence for the use of probiotics for the treatment of ulcerative colitis has been investigated. The current research suggests that conventional treatment combined with probiotic therapy does not provide any additional benefit over conventional treatment alone in patients with mild to moderate ulcerative colitis. There is limited evidence that probiotics may reduce disease activity. However there is not enough evidence to recommend the use of probiotics for the treatment of ulcerative colitis. Larger, well designed randomized controlled trials are needed to determine whether probiotics are of benefit for the treatment of ulcerative colitis.
Keywords
Ulcerative colitis, Probiotics, Gastroenteritis, Pouchitis, Inflammatory condition
Introduction
Ulcerative colitis
Inflammatory Bowel Disease (IBD) is a chronic recurrent disease, which mainly consists of ulcerative colitis and Crohn’s disease (Katz J, 2006; Singh S, et al., 2015). One of the most common forms of IBD is Ulcerative Colitis (UC), a chronic condition that is characterized by mucosal inflammation and ulcers on the inner lining of the human colon and rectum (Waal MB, et al., 2018). It is estimated that worldwide 150 out of every 100,000 people suffer from ulcerative colitis (Sang LX, et al., 2010; Cui HH, et al., 2004). Ulcerative colitis is a common condition resulting in inflammation of the colon (Mallon P, et al., 2007). Ulcerative colitis is a chronic, immune-mediated disease of the intestinal tract of which etiology and pathogenesis have not been definitively elaborated (Palumbo VD, et al., 2016). Intestinal microflora has long been implicated as an important initiating factor in the pathogenesis of Inflammatory Bowel Disease (IBD) (Huynh HQ, et al., 2009). Ulcerative colitis most often begins gradually and can become worse over time. Symptoms can be mild to severe (Chibbar R and Dieleman LA, 2015). Most people have periods of remission-times when symptoms disappear-that can last for weeks or years (NIH, 2020). Ulcerative colitis can occur in people of any age. However, it is more likely to develop in people who are between the ages of 15 and 30, older than 60 and those who have a family member with inflammatory bowel disease (Shanahan F, 2005; Mohanta S, et al., 2019).
Materials and Methods
Study design
Subjects were seen at baseline, weeks 2, 4, and 8 and were asked to record in a diary their daily symptoms, adverse events, and medications taken. Subjects were asked to record stool frequency, urgency of defecation, signs of blood in stool, extra-colonic manifestations, and general well-being. At baseline and final (week 8) visit colonoscopy/sigmoidoscopy and histologic assessment of disease activity were determined .Blood, urine, and stool were collected pre-and post-treatment for hematology (a complete blood count with differential and Erythrocyte Sedimentation Rate (ESR); biochemistry (electrolytes, creatinine, alkaline phosphates, albumin, and C-Reactive Protein (CRP)), and urinalysis (pH, protein, glucose, ketone, blood, and microscopic sediment examination). A stool sample was obtained to exclude infection (including Clostridium difficile toxin assay, bacterial pathogen culture, and parasite examination). Blood serum was collected pre and post-treatment for the cytokine profile (Tumor Necrosis Factor-alpha (TNF-ἀ), BD Biosciences, San Jose, CA); Interferon-gamma (IFN-γ) (R and D Systems, Minneapolis, MN) using Enzyme-Linked Immunosorbent Assay (ELISA) according to the manufacturer’s instructions. Pre-and post-rectal biopsies were also collected for microbial profiling based on bacterial signatures encoded in 16S ribosomal RNA.
Procedure
Randomised Controlled Trials (RCTs) is to identify comparative studies of probiotics in ulcerative colitis.
Selection of patients
Twenty patients (12 male and 8 female, mean age 40 years; range 30 ± 65), intolerant or allergic to oral 5-Aminosalicyclic Acid (5-ASA), with ulcerative colitis in remission, have been studied. Patients are eligible for the treatment for mild to moderate ulcerative colitis as indicated in Table 1 which ranged from a minimum of 3 to a maximum of 11 and who had duration of exacerbated symptoms lasting less than 4 weeks (Cui HH, et al., 2004; Huynh HQ, et al., 2009). Patients infected with enteric pathogen, diagnosed with CD or pouchitis, were excluded. Patients who received a high dose of oral prednisone (>10 mg/day) within the last 4 weeks or antibiotics within the last 2 weeks prior to entry were excluded (Zigra PI, et al., 2007). Patients who had a change in dose of oral 5-Aminosalicyclicacid (5-ASA) containing products within the last 4 weeks or a change in dose of rectal 5-ASA or steroids with in 7 days prior to study entry were also excluded (Venturi A, et al., 1999; Matthes H, et al., 2010; Jia K, et al., 2018). Patients with known hepatic, renal, endocrine, respiratory, neurological, or cardiovascular diseases or patients who required imminent surgery or those who had a severe disease as defined by an SCCAI of 12 or greater were excluded. In addition, patients who were pregnant or lactating were also excluded (Shen J, et al., 2014; Asto E, et al., 2019; Kianifar H, et al., 2014).
| Simple clinical colitis activity index | |
|---|---|
| Symptoms | Score |
| Bowel frequency (day) | |
| 1-3 | 0 |
| 4-6 | 1 |
| 7-9 | 2 |
| >9 | 3 |
| Bowel frequency (night) | |
| 1-3 | 1 |
| 4-6 | 2 |
| Urgency of defecation | |
| Hurry | 1 |
| Immediately | 2 |
| Incontinence | 3 |
| Blood in stool | |
| Trace | 1 |
| Occasionally frank | 2 |
| Usually frank | 3 |
| General well being | |
| Very well | 0 |
| Slightly below par | 1 |
| Poor | 2 |
| Very poor | 3 |
| Terrible | 4 |
| Extracolonic features | |
| Uveitis, Pyoderma, Gangrenosum erythema nodosum, Arthropathy | 1 per manifestation |
Table 1: Ulcerative colitis activity index (Huynh HQ, et al., 2009; Sood A, et al., 2009)
Concomitant therapy
Concurrent medications were recorded and patients were allowed to continue to maintain a stable dose of oral 5-Aminosalicyclicacid (5-ASA) and a low dose of corticosteroids, provided dosage was stable for at least 4 weeks prior to study entry. Patients receiving stable doses of immunosuppressive medications such as azathioprine or 6-mercaptopurine for at least 3 months prior to entry were eligible. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and antidiarrheal agents (loperamide, diphenoxylate, and opiates) were not permitted throughout the 8-week trial (Peng L, et al., 2019).
Study medication
Patients were treated twice daily for 8 weeks with a dose of probiotic based on their age. The dose of probiotics for age is determined by scaling doses shown to be efficacious and safe for the treatment of ulcerative colitis in the adult trial as recommended by the manufacturer. The concentration of bacteria used in this trial exceeded previous pediatric trials by 10-20 billion per dose (Tursi A, et al., 2010).
Outcome measures
The colonoscopy/sigmoidoscopy assessment was based on the Mayo endoscopic scoring system for ulcerative colitis ranging from mild, moderate, to severe (1-3, respectively).
Results and Discussion
Types of ulcerative colitis
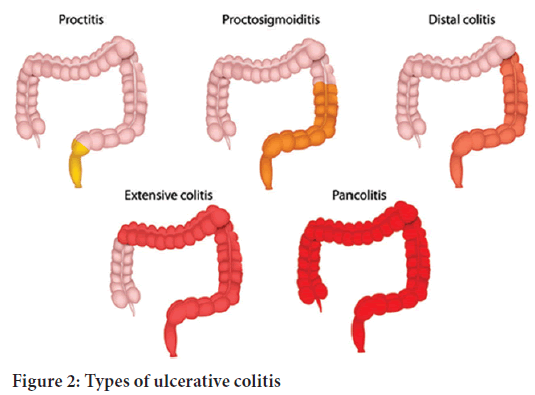
In ulcerative colitis, the inflammation extends from the rectum in a circumferential manner, typically affecting both sides of the colon in an uninterrupted pattern (Figure 1). Inflammation affects the rectum in over 95% of the cases. Depending on the extent of the inflammation, ulcerative colitis can be classified as indicated below and as shown in Figure 2(Health engine, 2003; IBD relief, 2022).
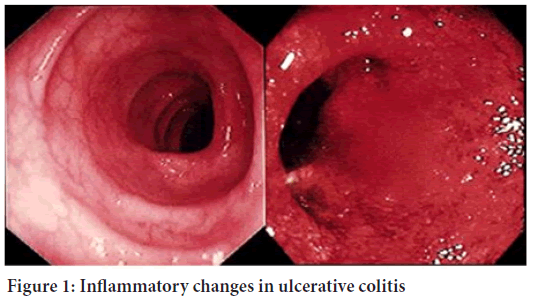
Figure 1: Inflammatory changes in ulcerative colitis
Figure 2: Types of ulcerative colitis
• Proctitis-the least severe form of the disease, proctitis is characterized by inflammation of the rectal mucosa
• Left-sided colitis-characterized by limited inflammation of the colon
• Extensive colitis-characterized by extensive inflammation of the colon
• Pancolitis-characterized by inflammation involving the entire colon
UC may also be classified according to symptoms, as either:
• Mild-When the person passes <4 stools daily (with or without blood) and no evidence of systemic toxicity.
• Moderate-When the person passes >4 stools daily with minimal systemic toxicity.
• Severe-When the person passes >6 bloody stools per day with signs of systemic toxicity, which may include fever, tachycardia (rapid heartbeat), and anaemia.
• Fulminant-When the person passes >10 stools per day, continuous rectal bleeding needing blood transfusion, abdominal tenderness and distension, and systemic toxicity.
Signs and symptoms
The most common signs and symptoms of ulcerative colitis are diarrhea with blood or pus and abdominal discomfort, rectal bleeding, loss of appetite, fever, weight loss and fatigue. Other signs and symptoms include an urgent need to have a bowel movement, feeling tired, anaemia-a condition in which the body has fewer red blood cells than normal (Shanahan F, 2005). Furthermore, UC may manifest itself outside of the gastrointestinal tract, for instance in the form of episcleritis, uveitis, arthropathy or sclerosing cholangitis (NIH, 2020).
Risk factors
There are various risk factors associated with ulcerative colitis such as family history, abnormal immune response, age, personality type, diet, urban living etc.
Diagnosis of ulcerative colitis
The different tests through which ulcerative colitis can be diagnosed are blood test, urine test, liver function test, gut test, stool sample, colonoscopy and biopsy.
Treatment for ulcerative colitis
Primary therapy for ulcerative colitis is usually a combination of sulfasalazine and glucocorticoids. Sulfasalazine can be given alone or in combination with other drugs. However, a large number of patients are resistant or intolerant to sulfasalazine (Palumbo VD, et al., 2016; Yoshimatsu Y, et al., 2015; Gionchetti P, et al., 2002). Sulfasalazine, mesalazine, and immune-modulators promote remission maintenance, but are not adequately effective. Moreover, an appreciable number of patients cannot tolerate these drugs, and immune-modulators can cause serious adverse events. Recently, probiotic therapy has been acknowledged to be potentially effective and safe in patients with ulcerative colitis. The use of probiotics has been proposed for providing benefits to human health for a long time but in recent years there has been increased interest for their use in ulcerative colitis due to the microbiome role in ulcerative colitis pathogenesis. Probiotics are being ingested by patients with ulcerative colitis sometimes through the advice of the physician but mostly self-prescribed as a form of alternative medicine. The reasons for their usage seem to be mostly related to severity of disease, side effects of treatments and health beliefs. Recent reports suggest that patient’s sufferings from ulcerative colitis are found to be experimenting probiotics about 50% more as compared to earlier (Mack DR, 2011; Meier J and Sturm A, 2011).
Probiotics
Probiotics are defined as a live microbial feed nutritional supplement that beneficially affects the host by improving the balance of the intestinal flora. Studies of animal models of colitis have suggested that the intestinal flora has an important role in the pathogenesis of colitis (Sheil B, et al., 2007; Bernstein CN, 2014; Zigra PI, et al., 2007; Guo Q, et al., 2019; Floch MH, et al., 2011). Probiotics are live microorganisms which have been used to treat other inflammatory conditions such as gastroenteritis, ulcerative colitis and pouchitis. Probiotics are viable, non-pathogenic microorganisms that exert health benefits beyond basic nutrition by improving microbial balance. Probiotics have been shown to be safe, as the enterococcal strains used are normal inhabitants of the gastrointestinal tract (Huynh HQ, et al., 2009; Indrio F, et al., 2014; Kruis W, et al., 2004; Sullivan A, 2002).
Products sold as probiotics include foods (such as yogurt), dietary supplements, and products that aren't used orally, such as skin creams. There are large numbers of microorganisms live on and in our bodies (Kruis W, et al., 2004). Many of the microorganisms in probiotic products are the same as or similar to microorganisms that naturally live in our bodies (Elaheh V, et al., 2013). Probiotics have also been investigated in relation to atopic eczema and complications of liver cirrhosis (Derikx LP, et al., 2016). Although there is some clinical evidence for the role of probiotics in lowering cholesterol, the results are conflicting (NIH, 2019; Ong TG, et al., 2019; Rojas MA, et al., 2012).
The history of probiotics
The concept behind probiotics was introduced in the early 20th century, when Nobel laureate Elie Metchnikoff, known as the “father of probiotics,” proposed that consuming beneficial microorganisms could improve people’s health. Researchers continued to investigate this idea, and the term “probiotics”-meaning “for life” eventually came into use (NIH, 2019; WHO, 2021). The term probiotic is a relatively new word meaning “for life” and it is currently used to name bacteria associated with beneficial effects for humans and animals. The original observation of the positive role played by some selected bacteria is attributed to Eli Metchnikoff, the Russian born Nobel Prize recipient working at the Pasteur Institute at the beginning of the last century, who suggested that “The dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful microbes”. At this time Henry Tissier, a French paediatrician, observed that children with diarrhoea had in their stools a low number of bacteria characterized by a peculiar, Y shaped morphology. These “bifid” bacteria were, on the contrary, abundant in healthy children. He suggested that these bacteria could be administered to patients with diarrhoea to help restore a healthy gut flora.
The concept behind probiotics was introduced in the early 20th century, when Nobel laureate Elie Metchnikoff, known as the “father of probiotics,” proposed that consuming beneficial microorganisms could improve people’s health. Researchers continued to investigate this idea, and the term “probiotics”-meaning “for life” eventually came into use (NIH, 2019; WHO, 2021). The term probiotic is a relatively new word meaning “for life” and it is currently used to name bacteria associated with beneficial effects for humans and animals. The original observation of the positive role played by some selected bacteria is attributed to Eli Metchnikoff, the Russian born Nobel Prize recipient working at the Pasteur Institute at the beginning of the last century, who suggested that “The dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful microbes”. At this time Henry Tissier, a French paediatrician, observed that children with diarrhoea had in their stools a low number of bacteria characterized by a peculiar, Y shaped morphology. These “bifid” bacteria were, on the contrary, abundant in healthy children. He suggested that these bacteria could be administered to patients with diarrhoea to help restore a healthy gut flora.
Mechanisms of action of probiotics
Prebiotics affect intestinal bacteria by increasing the numbers of beneficial anaerobic bacteria and decreasing the population of potentially pathogenic microorganisms. Probiotics affect the intestinal ecosystem by stimulating mucosal immune mechanisms and by stimulating nonimmune mechanisms through antagonism and competition with potential pathogens (Guarner F, et al., 2011; Dunne C, et al., 2004). These phenomena are thought to mediate most beneficial effects, including reduction of the incidence and severity of diarrhoea, which is one of the most widely, recognized uses for probiotics (Shen ZH, et al., 2018). Probiotics reduce the risk of colon cancer in animal models, probably due to their role in suppressing the activity of certain bacterial enzymes that may increase the levels of procarcinogens, but this has not been proven in humans (O’Hara AM and Shanahan F, 2007).
Selection of probiotic strains for human use
Probiotics must be able to exert their benefits on the host through growth and/or activity in the human body. However, it is the specificity of the action, not the source of the microorganism that is important. Indeed, it is very difficult to confirm the source of a microorganism. Infants are born with none of these bacteria in the intestine, and the origin of the intestinal microflora has not been fully elucidated. It is the ability to remain viable at the target site and to be effective that should be verified for each potentially probiotic strain. There is a need for refinement of in vitro tests to predict the ability of probiotics to function in humans. The currently available tests are not adequate to predict the functionality of probiotic microorganisms in the intestine (WHO, 2021).
Probiotic strains
The various probiotic strains are of Lactobacillus species, Bifidobacteriumspecies and few others are also considered as the probiotic strains, the list of which is mentioned in Table 2(Mahajan B and Singh V, 2014).
| Probiotics strains | Species names |
|---|---|
| Lactobacillus species | L. acidophilus |
| L. plantarum | |
| L. casei subspecies rhamnosus | |
| L. brevis | |
| L. delbreuckii subspecies bulgaricus | |
| Bifidobacterium species | B. adolescentis |
| B. bifidum | |
| B. longum | |
| B. infantis | |
| B. breve | |
| Others | Streptococcus salivarius ssp. thermophilus |
| Lactococcuslactis ssp. lactis | |
| Escherichia coli | |
| Lactococcuslactis s ssp. cremoris | |
| Enterococcus faecium | |
| Leuconostoc mesenteroides ssp. dextranicum | |
| Propionibacterium freudenreichii | |
| Pediococcus acidilactici | |
| Saccharomyces boulardii |
Table 2: List of various probiotic strains
Characteristics of probiotics
• Able to survive the passage through the digestive system
• Able to attach the intestinal epithelia and colonize
• Able to maintain good viability
• Able to utilize the nutrients and substrates in a normal diet
•Non-pathogenic and non-toxic
• It should be safe to the host
• It should have anti-carcinogenic activity
• It should stimulate the immune system of the body
• It should have the ability to colonize the gastrointestinal tract (Prantera C and Scribano ML, 2009).
Clinical applications
Colon cancer: The SYNCAN study tested the effect of oligo-fructose plus two probiotic strains in patients at risk of developing colonic cancer. The results of the study suggest that a symbiotic preparation can decrease the expression of biomarkers for colorectal cancer (WHO, 2001).
Diarrhoea: It has been confirmed that different probiotic strains, including L.reuteri,L.rhamnosus,L.casei,and Saccharomycescerevisiae(boulardii) are useful in reducing the severity and duration of acute infectious diarrhoea in children. The oral administration of probiotics shortens the duration of acute diarrheal illness in children by approximately 1 day.
Several meta-analyses of controlled clinical trials have been published that show consistent results in systematic reviews, suggesting that probiotics are safe and effective. The evidence from studies on viral gastroenteritis is more convincing than the evidence on bacterial or parasitic infections. Mechanisms of action are strain-specific: There is evidence for efficacy of some strains of lactobacilli (e.g., Lactobacillus casei and Lactobacillus reuteri) and for Saccharomyces boulardii. The timing of administration is also of importance (Floch MH, 2003).
Inflammatory bowel disease:
• Ulcerative colitis-The probiotic E. coli Nissle strain may be equivalent to mesalazine in maintaining remission of ulcerative colitis. The probiotic mixture VSL#3 has shown efficacy to induce and maintain remission in children and adults with mild-to-moderate ulcerative colitis.
• Crohn’s disease-Studies of probiotics in Crohn’s disease have been disappointing, and the Cochrane systematic review concluded that there is no evidence to suggest that probiotics are beneficial for maintenance of remission in Crohn’s disease.
• Pouchitis-There is good evidence for the usefulness of probiotics in preventing an initial attack of pouchitis (VSL#3), and in preventing further relapse of pouchitis after the induction of remission with antibiotics. Probiotics can be recommended to patients with pouchitis of mild activity, or as maintenance therapy for those in remission.
Eradication of Helicobacter pylori: Several Lactobacilli and Bifidobac- terial strains as well as Bacillus clausii, appear to reduce the side effects of antibiotic therapies and improve patient compliance. Several strains were effective in decreasing side effects, but did not have effects on the eradication rate. A recent meta-analysis of 14 randomized trials suggests that supplementation of anti-H. pylori antibiotic regimens with certain probiotics may also be effective in increasing eradication rates and may be considered helpful for patients with eradication failure. There is currently insufficient evidence to support the concept that a probiotic alone, without concomitant antibiotic therapy, would be effective. In summary, there is literature suggesting that certain probiotics may be helpful as adjuvant therapy with antibiotics in the eradication of H. pylori infection.
Cirrhosis: Prebiotics such as lactulose are commonly used for the prevention and treatment of this complication of cirrhosis. Minimal hepatic encephalopathy was reversed in 50% of patients treated with a symbiotic preparation (four probiotic strains and four fermentable fibres, including inulin and resistant starch) for 30 days.
Allergy: The strongest evidence is for the prevention of atopic dermatitis when certain probiotics are administered to pregnant mothers and newborns up to 6 months of age. However, a recent clinical trial did not confirm these results. With regard to the treatment of allergic disease, a few well-designed studies have provided evidence that specific probiotic strains can be effective in the treatment of a subset of patients with atopic eczema. Little is known about the efficacy of probiotics in preventing food allergy.
Irritable Bowel Syndrome (IBS): Several studies have demonstrated significant therapeutic gains with probiotics in comparison with placebo. A reduction in abdominal bloating and flatulence as a result of probiotic treatments is a consistent finding in published studies; some strains may ameliorate pain and provide global relief (B. infantis) in addition. Lactobacillus reuteri may improve colicky symptoms within one week of treatment, as shown in a recent trial with 90 breastfed babies with infantile colic. In summary, there is literature suggesting that certain probiotics may alleviate symptoms in persons with functional abdominal pain.
Prevention of systemic infections: There is insufficient evidence to support the use of probiotics and synbiotics in critically ill adult patients in intensive-care units.
Cardiovascular disease: The use of probiotics/prebiotics for preventative medicine and decreasing risk of cardiovascular disease is still unproven.
Lactose malabsorption: Streptococcusthermophilusand Lactobacillusdel-brueckii(subsp. Bulgaricus) improve lactose digestion and reduce symptoms related to lactose intolerance. This was confirmed in a number of controlled studies with individuals consuming yogurt with live cultures.
Advantages of probiotics
• Produce lactic acid-lowers the pH of intestines and inhibiting bacterial villains such as Clostridium, Salmonella, E.coli, etc.
• Lead to improved appetite and/or growth performance.
• Decreases the production of a variety of toxic or carcinogenic metabolites.
• Aid absorption of minerals, especially calcium, due to increased intestinal acidity.
• Production of beta-D-galactosidase enzymes that break down lactose.
• Produce a wide range of antimicrobial substances.
• Produce vitamins (especially vitamin B and vitamin K).
• Act as barriers to prevent harmful bacteria from colonizing the intestines.
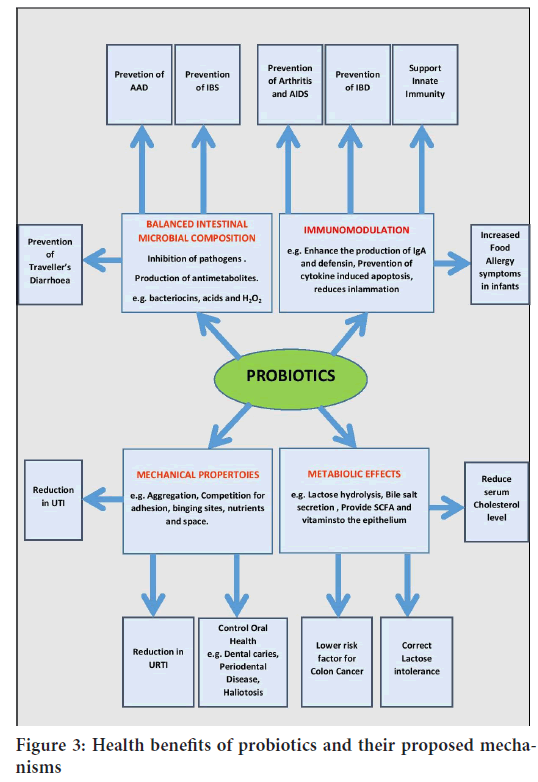
The health benefits of probiotics and their mechanisms are as indicated in Figure 3.
Figure 3: Health benefits of probiotics and their proposed mechanisms
Disadvantages of probiotics
• There is still no consensus on the most effective dose of a probiotics.
• Probiotic products manufactured by established pharmaceutical companies typically have the stated concentration and bacterial or fungal strain listed on the label.
Preparation of probiotics
Probiotic preparations consists of 4 strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus), 3 strains of Bifidobacteria (B. longim, B. breve and B. infantis), and 1 strain of Streptococcus salivarius subsp. thermophilus. Few of the marketed preparations of probiotics are as mentioned in Table 3(Huynh HQ, et al., 2009).
| Company | Probiotic strain | Marketed product |
|---|---|---|
| BioGaia | L. reuteri | Drops |
| BioGaia | L. reuteri | Chewable tablets |
| Nestle | B. lactis | Probiotic |
| Lichu Drug House | B. adolescentis | - |
| Shanghai Sinyi Drug Pte. Ltd. | B. longum, B. adolescentis and Enterococcus faecium | - |
| Mongolian Shuanchi Drug Co. Ltd. | B. longum and L. delbreuckii subspecies bulgaricus | - |
| Nestle | B. lactis | Baby Cereals |
| Bio-LiFE | L. acidophilus and B.lactis | A.B.Pre and Pro |
| Vitis Pharma | L. rhamnosus | Dicoflor 30 |
| OptiBac | L. acidophilus, B. infantis and B. bifidum | Probiotics |
| EvoraKidsTM | B. longum, B. bifidum and B. infantis | Biomilk with probiotics (Babio) |
| MXI Corp. TM | L. helveticus and B. longum | XoBioticTM Squares |
| Chocolate Crisp® | L. acidophilus, L. casei and B. lactis | Chocolate probiotic bars |
| Bunker hill cheese company | L. acidophilus, L. casei, B. lactis | Cheese |
Table 3: Marketed products
Probiotics as maintenance therapy in ulcerative colitis
Only a few probiotic products either combined as blends or administered as single strain monotherapy have been studied in ulcerative colitis maintenance trials with 3 of the single probiotic trials utilizing E. coli strain (Mack DR, 2011). With a background of up to 70% relapse rate over a 1-year period for those with ulcerative colitis not taking any form of maintenance therapy, many of the trials have been for one year and studied remission rates in comparison with 5-aminosalicylate products. One of these 12-month probiotic versus 5-aminosalicylate trials was initiated with active ulcerative colitis patients and followed those achieving remission for a 12-month period. In this study the relapse rates were high in both the group maintained with E. coli and those maintained on 1.5 g of daily mesalazine (67% and 72%, respectively). The other 12-month trials were initiated in participants with quiescent disease. In these studies, maintenance of remission rates varied between 45% and 75% and studies in those receiving 5-aminosalicylates as a control group had a similar maintenance of remission rate as the probiotic intervention group. Interestingly in the trial comparing monotherapy L. rhamnosus strain, monotherapy mesalamine (2.4 g per day) and combination probiotic and mesalamine, no synergistic benefit was derived from combination therapy but all three groups had equivalent rates for maintenance of remission. The studies comparing probiotic with 5-aminosalicylates have used different total daily amounts (1.5-2.4 g per day). Nevertheless, currently there is not currently clinical evidence of a direct dose-dependent maintenance benefit above 1.6 g daily dosing of 5-aminosalicylate.
Efficacy and safety of probiotics
In spite of inherent difficulties establishing good measures of probiotic efficacy, studies on lactose intolerance, diarrhoea and colon cancer show that a daily dose of about 1-10 billion bacteria is needed for any measurable effect (Romach AE, et al., 2008). Unfortunately, the concentration of probiotics in food products varies tremendously and there are currently no national standards of identity for levels of bacteria required in yogurt or other fermented products. Concentration of bacteria contained in food products is generally left up to the manufacturer, who may or may not accept recommendations from industry groups (Hoolihan KL, 2001). The “Live active culture” seal established by the National Yogurt Association requires 108 viable lactic acid bacteria per gram at the time of manufacture for refrigerated yogurt and 107 per gram for frozen yogurts. However, these counts may not accurately indicate probiotic content as they do not differentiate probiotic bacteria from starter culture bacteria such as S. thermopliilus. Culture manufacturers recommend including approximately 106 probiotic bacteria per gram for yogurt and acidophilus milk at the end of shelf-life (Doron S and Snydman DR, 2015). As research in this field progresses and consumers demand tighter regulation, requirements for probiotic concentration and viable counts at the time of consumption will undoubtedly become more standardized for these products (Rijkers GT, et al., 2011).
Due to their long history of use in food fermentation, the FDA has designated many probiotics to be Generally Recognized As Safe (GRAS). Even for those without GRAS status, the industry has used probiotic bacteria in food fermentations with the assumption that their history of use implies their safety. The occasional reports of bacteremias and endocarditis associated with Lactobacillus have generally been in severely immunocompromised individuals. Epidemiological data on the safety of dairy products and a thorough review of the safety data on probiotics suggests no evidence of probiotics being involved with human infections. However, there always remains the possibility that probiotic consumption can cause infection and that individuals will respond in different ways to a specific probiotic strain. The food industry will need to carefully assess the safety and efficacy of all new species and strains of probiotics before incorporating them into food products. As a practitioner it may be prudent to advise clients to incorporate well-known species into their diet gradually, building up to the recommended daily levels needed over a period of two to three weeks, to minimize any potential deleterious effects.
From a scientific perspective, the suitable description of a probiotic product as reflected on the label should include (Guarner F, et al., 2011).
• Genus and species identification, with nomenclature consistent with current scientifically recognized names
• Strain designation
• Viable count of each strain at the end of shelf-life
• Recommended storage conditions
• Safety under the conditions of recommended use
• Recommended dose, which should be based on induction of the claimed physiological effect
• An accurate description of the physiological effect, as far as is allowable by law
• Contact information for post-market surveillance
Drug interactions
Since probiotics contain live microorganisms, concurrent administration of antibiotics could kill a large number of the organisms, reducing the efficacy of the Lactobacillus and Bifidobacterium species (Williams NT, 2010). Patients should be instructed to separate administration of antibiotics from these bacteria-derived probiotics by at least two hours. Similarly, S. boulardii might interact with antifungals, reducing the efficacy of this probiotic. Probiotics should also be used cautiously in patients taking immunosuppressants, such as cyclosporine, tacrolimus, azathioprine, and chemotherapeutic agents, since probiotics could cause an infection or pathogenic colonization in immunocompromised patients.
Future implications of probiotics
In spite of the problems with dosage and viability of probiotic strains, lack of industry standardization and potential safety issues, there is obviously considerable potential for the benefits of probiotics over a wide range of clinical conditions (Hoolihan KL, 2001; Playne MJ, 2005). On-going basic research will continue to identify and characterize existing strains of probiotics, identify strain-specific outcomes, determine optimal doses needed for certain results and assess their stability through processing and digestion (Wolvers D, et al., 2010; Vanderhoof JA, 2001). Gene technology will certainly play a role in developing new strains, with gene sequencing allowing for an increased understanding of mechanisms and functionality of probiotics. In addition to such basic research, industry-centered research will focus on prolonging the shelf-life and likelihood of survival through the intestinal tract, optimizing adhesion capacity and developing proper production, handling and packaging procedures to ensure that the desired benefits are delivered to the consumer.
Conclusion
Ulcerative Colitis (UC) is a chronic disease and easy to relapse, its etiology and pathogenesis have not been definitively elaborated. The pathogenesis of UC is closely related to intestinal microbiota, although the exact bacteria that contribute to UC have not been determined. The use of probiotics in UC is currently being investigated. Probiotics may help normalize the imbalance of intestinal microbiota, improve the micro ecological environment, enhance intestinal mucosal barrier function, and reduce gastrointestinal infections. Use of probiotics in UC is provocative and suggests potential for benefit in selected patients but concerns remain about the evidences of treatment from trials.
References
- Katz J. The role of probiotics in IBD. Gastroenterol Hepatol. 2006; 2(1): 16-18.
[Google scholar] [PubMed]
- Singh S, Am S, Sd H, Wj S, Ds P. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis (Review) summary of findings for the main comparison. 2015; 11:10-13.
- Waal MB, Flach, J, Pamela D, Vaart IB, Claassen E, Burgwal HM, et al. Probiotics for improving quality of life in ulcerative colitis: Exploring the patient perspective. Biochem Pharmacol. 2018;
- Sang LX, Chang B, Zhang WL, Wu XM, Li XH, Jiang M. Remission induction and maintenance effect of probiotics on ulcerative colitis: A meta-analysis. World J Gastroenterol. 2010; 16(15): 1908-1915.
[Crossref] [Google scholar] [PubMed]
- Cui HH, Chen CL, Wang J de, Yang YJ, Cun Y, Wu JB, et al. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004; 10 (10): 1521-1525.
[Crossref] [Google scholar] [PubMed]
- Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007; 4: CD005573.
[Crossref] [Google scholar] [PubMed]
- Palumbo VD, Romeo M, Gammazza AM, Carini F, Damiani P, Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed Pap. 2016; 160(3): 372-377.
[Crossref] [Google scholar] [PubMed]
- Huynh HQ, de Bruyn J, Guari L, Diaz H, Li M, Girgis S, et al. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: A pilot study. Inflamm Bowel Dis. 2009; 15(5): 760-768.
[Crossref] [Google scholar] [PubMed]
- Chibbar R, Dieleman LA. Probiotics in the management of ulcerative colitis. J Clin Gastroentero. 2015; 49(Suppl 1): S50-S55.
[Crossref] [Google scholar] [PubMed]
- Ulcerative colitis. NIH (NIDDK). 2020.
- Shanahan F. Physiological basis for novel drug therapies used to treat the inflammatory bowel diseases. I pathophysiological basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005; 288(3): 417-421.
[Crossref] [Google scholar] [PubMed]
- Mohanta S, Singh SK, Kumar B, Gulati M, Kumar R, Yadav AK, et al. Efficacy of co-administration of modified apple polysaccharide and probiotics in guar gum-eudragit S100 based mesalamine mini tablets: A novel approach in treating ulcerative colitis. Int J Biol Macromol. 2019; 126: 427-435.
[Crossref] [Google scholar] [PubMed]
- Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009; 7(11): 1202-1209e1.
[Crossref] [Google scholar] [PubMed]
- Zigra PI, Maipa VE, Alamanos YP. Probiotics and remission of ulcerative colitis: A systematic review. Neth J Med. 2007; 65(11): 411-418.
[Crossref] [Google scholar] [PubMed]
- Venturi A, Gionchetti P, Rizzello F, Johansson, Zucconi E, Brigidi P, et al. Impact on the composition of the faecal flora by a new probiotic preparation: Preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999; 13(8): 1103-1108.
[Crossref] [Google scholar] [PubMed]
- Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli nissle 1917 (ecn). BMC Complement Altern Med. 2010; 10.
[Crossref] [Google scholar] [PubMed]
- Jia K, Tong X, Wang R, Song X. The clinical effects of probiotics for inflammatory bowel disease: A meta-analysis. Medicine (Baltimore). 2018; 97(51): e13792.
[Crossref] [Google scholar] [PubMed]
- Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014; 20(1): 21-35.
[Crossref] [Google scholar] [PubMed]
- Asto E, Mendez I, Audivert S, Codina FA, Espadaler J. The efficacy of probiotics, prebiotic inulin-type fructans, and synbiotics in human ulcerative colitis: A systematic review and meta-analysis. Nutrients. 2019; 11(2): 293.
[Crossref] [Google scholar] [PubMed]
- Kianifar H, Ahanchian H, Grover Z, Jafari S, Noorbakhsh Z, Khakshour A, et al. Synbiotic in the management of infantile colic: A randomised controlled trial. J Paediatr Child Health. 2014; 50(10): 801-805.
[Crossref] [Google scholar] [PubMed]
- Peng L, Zhong Y, Wang A, Jiang Z. Probiotics combined with aminosalicylic acid affiliates remission of ulcerative colitis: A meta-analysis of randomized controlled trial. Biosci Rep. 2019; 39(1): BSR20180943.
[Crossref] [Google scholar] [PubMed]
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti G M, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010; 105(10): 2218-2227.
[Crossref] [Google scholar] [PubMed]
- Ulcerative colitis. Health engine. 2003.
- Types of ulcerative colitis. IBD relief. 2022.
- Probiotics: What you need to know. NIH, 2019.
- Yoshimatsu Y, Yamada A, Furukawa R, Sono K, Osamura A, Nakamura K, et al. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J Gastroenterol. 2015: 21(19): 5985-5994.
[Crossref] [Google scholar] [PubMed]
- Gionchetti P, Amadini C, Rizzello F, Venturi A, Campieri M. Review article: Treatment of mild to moderate ulcerative colitis and pouchitis. Aliment Pharmacol Ther Suppl. 2002; 16(4): 13-19.
[Crossref] [Google scholar] [PubMed]
- Mack DR. Probiotics in inflammatory bowel diseases and associated conditions. Nutrients. 2011; 3(2): 245-264.
[Crossref] [Google scholar] [PubMed]
- Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol. 2011; 17(27): 3204-3212.
[Crossref] [Google scholar] [PubMed]
- Sheil B, Shanahan F, Mahony LO. Probiotic effects on inflammatory bowel disease. J Nutr. 2007; 137(3): 819S-824S.
[Crossref] [Google scholar] [PubMed]
- Bernstein CN. Antibiotics probiotics and prebiotics in IBD. Nestle Nutr Inst Workshop Ser. 2014; 79: 83-100.
[Crossref] [Google scholar] [PubMed]
- Guo Q, Goldenberg JZ, Humphrey C, Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019; (4).
[Crossref] [Google scholar] [PubMed]
- Floch MH, Walker WA, Madsen K, Sanders ME, Macfarlane GT, Flint HJ, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011; 45 SUPPL 3:168-171.
[Crossref] [Google scholar] [PubMed]
- Indrio F, Mauro DA, Riezzo G, Civardi E, Intini C, Corvaglia L, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation a randomized clinical trial. JAMA Pediatr. 2014; 168(3): 228-233.
[Crossref] [Google scholar] [PubMed]
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli nissle 1917 is as effective as with standard mesalazine. Gut. 2004; 53(11): 1617-1623.
[Crossref] [Google scholar] [PubMed]
- Sullivan A. Probiotics in human infections. J Antimicrob Chemother. 2002; 50(5): 625-627.
[Crossref] [Google scholar] [PubMed]
- Elaheh V, Brian MA, Joyce WL, Alexander ZD. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J Clin Gastroenterol. 2013; 47(5): 437-439.
[Crossref] [Google scholar] [PubMed]
- Derikx LP, Dieleman LA, Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol. 2016; 30(1): 55-71.
[Crossref] [Google scholar] [PubMed]
- Ong TG, Gordon M, Banks SSC, Thomas MR, Akobeng AK. Probiotics to prevent infantile colic. Cochrane Database Syst Rev. 2019; 13(3): CD012473.
[Crossref] [Google scholar] [PubMed]
- Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon M A, Bastidas JA, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics. 2012; 130(5).
[Crossref] [Google scholar] [PubMed]
- Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. WHO. 2001.
- Guarner F, Khan A, Garisch J. Probiotics and prebiotics. World gastroenterology organisation global guidelines. 2011.
[Crossref] [Google scholar] [PubMed]
- Dunne C, Kelly P, Soden D, Bennett M, Kiely B, Collins J K, et al. Mechanisms of adherence of a probiotic lactobacillus strain during and after in vivo assessment in ulcerative colitis patients. Microb Ecol Health Dis. 2004; 16(2-3): 96-104.
- Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018; 24(1): 5-14.
[Crossref] [Google scholar] [PubMed]
- O’Hara AM, Shanahan F. Mechanisms of action of probiotics in intestinal diseases. Sci World J. 2007; 7: 31-46.
[Crossref] [Google scholar] [PubMed]
- Mahajan B, Singh V. Recent trends in probiotics and health management: A review. Int J Pharm Sci. 2014; 5(5): 1643-1652.
- Prantera C, Scribano ML. Antibiotics and probiotics in inflammatory bowel disease: Why when and how. Curr Opin Gastroenterol. 2009; 25(4): 329-333.
[Crossref] [Google scholar] [PubMed]
- Floch MH. Probiotics irritable bowel syndrome, and inflammatory bowel disease. Curr Treat Options Gastroenterol. 2003; 6(4): 283-288.
[Crossref] [Google scholar] [PubMed]
- Romach AE, Uni Z, Reifen R. Therapeutic potential of two probiotics in inflammatory bowel disease as observed in the trinitrobenzene sulfonic acid model of colitis. Dis Colon Rectum. 2008; 51(12): 1828-1836.
[Crossref] [Google scholar] [PubMed]
- Hoolihan KL. Prophylactic and therapeutic uses of probiotics: A review. J Am Diet Assoc. 2001; 101(2): 229-241.
[Crossref] [Google scholar] [PubMed]
- Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015; 60 (Suppl 2): S129-S134.
[Crossref] [Google scholar] [PubMed]
- Rijkers GT, de vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: Bridging science and marketing. Br J Nutr 2011; 106(9): 1291-1296.
[Crossref] [Google scholar] [PubMed]
- Williams NT. Probiotics. Am J Heal Pharm. 2010; 67(6): 449-458.
[Crossref] [Google scholar] [PubMed]
- Playne MJ. Probiotics marketed as pharmaceutical products in China. Curr Pharm. Des. 2005.
- Wolvers D, Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, et al. Guidance for substantiating the evidence for beneficial effects of probiotics: Current status and recommendations for future research. J Nutr. 2010; 671-676.
[Crossref] [Google scholar] [PubMed]
- Vanderhoof JA. Probiotics: Future directions. Am J Clin Nutr. 2001; 73(6): 1152-1155.
[Crossref] [Google scholar] [PubMed]
Author Info
Himali J Prajapati, Manish P Patel*, Mansi N Athalye and Praful D BharadiaCitation: Prajapati HJ: Role of Probiotics in Ulcerative Colitis
Received: 01-Apr-2022 Accepted: 29-Apr-2022 Published: 06-May-2022, DOI: 10.31858/0975-8453.13.5.334-341
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3