Research Article - (2022) Volume 13, Issue 10
Abstract
Objective: Salivation is a double-edged sword. It is desired for its protective function to the oral structures but undesirable due to the need for isolation during dental procedures. Atropine sulphate drops are known to decrease salivary secretion due to their muscarinic antagonistic activity. The present study was undertaken to quantitatively assess decrease in salivary secretion in children post administration of atropine sulphate drops sublingually, during routine dental procedures.
Methods: A total of sixty children between the ages of 7-17 years were divided into two groups. Case group was given atropine sulphate drops sublingually in a dose dependent manner and control group received distilled water in a similarly calculated dose. The stimulated and unstimulated salivary flow rate was quantitatively assessed at three-time intervals (0 min, 60 min and 90 mins) along with the heart rate and blood pressures.
Results: It was found that the unstimulated salivary flow rates in case group dropped by 80.3% and stimulated flow rates reduced by 79.4% in 90 minutes. The study also showed that time of onset of action was within 60 minutes and effect lasted beyond 90 minutes.
Conclusion: The present study translated into proving that the dose administered was adequate to elicit the antisialogogue effect via chosen route in children and the duration of effect was adequate for routine dental procedures.
Clinical relevance: The duration of effect of atropine drops, the route of administration, and reversibility of effect in children can be interpreted as adopting the said drug as an adjunct to conventional saliva control measures.
Trial registration: The present study bearing CTRI number-CTRI/2020/05/025447 was registered with the Clinical Trial Registry-India under ICMR-National Institute of Medical Statistics on 29/05/2020 prospectively.
Keywords
Isolation, Atropine sulphate, Salivary flow rate, Sublingual drops
Introduction
In dental practice saliva, although a natural protective barrier in the oral environment, poses a huge deterrent at the altar of most of the dental procedures which demand complete isolation. Paediatric dentistry is no exception where children often because of limited maturity to follow instructions, make it difficult for us as clinicians to ensure a dry and clean area of operation. The newer methods of both direct and indirect isolation lead to additional discomfort and anxiety in the children leading to an already fearful patient to become uncooperative. With the recent advent of multiple diseases citing saliva and oral secretions as the portal of transmission, including the recent pandemic of COVID-19, it becomes even more important to decrease the amount of saliva we encounter whilst performing routine dental procedures.
Atropine is a white crystalline alkaloid extracted from roots and leaves of dianqiecao (Belladonnae Herba) and mantuoluo (Mandala). It is a blocker of typical M-choline receptor. As per the current understanding, stimulation of muscarinic subtype 3 receptors (M3) is responsible for salivation (Sapkos SW, 1984). It is primarily mediated by parasympathetic innervation of the salivary glands. Acetylcholine is the active neurotransmitter, binding at muscarinic receptors at the salivary glands. Thus, cholinergic muscarinic receptor antagonists such as atropine are well established to treat glandular hypersecretion (Joseph MC and Vale RJ, 1960; Mushin WW, et al., 1953).
Sublingual delivery of atropine sulphate has numerous advantages namely ease of delivery and ready absorption by the mucosal membrane. It does not require any special skill for use is inexpensive and is reversible in nature (Mushin WW, et al., 1953). Also, in the absence of invasive techniques like syringes or additional armamentarium, it is acceptable by children of all ages and intellectual range.
The published body of work on the use of sublingual atropine drops as an antisialogogue in children and its subsequent application in dentistry is a little researched territory.
• The primary aim of this study was to evaluate the reduction in salivary secretion post administration of atropine sulphate drops locally on the muscarinic receptors on the salivary glands.
• Secondary aim was to estimate the effective dosage and duration of efficacy of the said medication in intraoral practice.
• Additionally, the present study endeavored to determine if the use of atropine drops intraorally, is a viable adjunct to the established dental isolation and salivary control methods.
Materials and Methods
The present study was undertaken as a prospective, parallel group, randomized placebo-controlled, open label trial conducted as a single centre study. For the present trial, the recruitment span and duration of the trial was 30/05/2020 to 17/08/2020. No changes were made in the methods after trial commencement. The present study only enrolled healthy human volunteers who satisfied the following-
Inclusion criteria
• Children between ages of 7-17 years
• Children belonging to group 1-2 (Healthy or minor disease) according to the ASA (American society of Anaesthesiologists) physical status classification system.
• Children with normal salivary flow above baseline
Exclusion criteria
• Children with oral mucosal lesions
• Children undergoing or history of chemotherapy or radiotherapy.
• Children on medications due to underlying systemic conditions.
• Known cases of congenital or cardiac conditions.
• Children with known allergy to atropine
According to power analysis, the sample size was calculated to be 30 for each group i.e., cases and controls at confidence level of 95%. A total of 60 patients satisfying the inclusion criteria were enrolled into the study. Thereafter, computer randomizations were used to allocate the participants into two groups indiscriminately. The primary researcher enrolled the patient and generated the random allocation sequence, and the secondary researcher assigned the participants to interventions. The intervention and recording of data were done by primary researcher. The participants were blinded regarding the kind of intervention they received so as to rule out bias. The intent-to treat principle was included in the written consent forms and explained to the parents and caregivers before starting the trial.
• Before the start of the study a complete demographic, dental and medical history was recorded of the patient.
• At commencement, patient’s heart rate was recorded using a portable heart rate monitor (uniformly the left index finger was used at random).
• Blood pressure was checked and noted using digital blood pressure monitor to establish baseline (left arm was cuffed, with a paediatric sized cuff, commercially available).
Atropine sulphate 1% w/v drops (available commercially-Jawa Pharmaceuticals (India) Pvt. Ltd) as per the pre-set dosage of 0.01 mg/kg of body weight was given to the case group whereas, placebo group received drops of distilled water in equal proportions sublingually. The patients were asked to open their mouths and touch the tip of the tongue to the palate and then the drops were placed sublingually using a Pasteur’s pipette. The patient was asked to not swallow for 1 minute and collect saliva. After one minute patient was allowed to swallow.
Collection of unstimulated whole saliva
Patient was asked to swallow once and void the mouth of collected saliva. Then requested to lean their head forward with mouth positioned over the neck of calibrated test tube passively drooling all the pooled saliva in the test tube for a period of one minute. This level was marked and noted. For the purpose of the present study, unstimulated flow rate of 0.3 ml/min was taken as the baseline for normal function.
Collection of stimulated whole saliva
Patient was given an unflavoured paraffin wax block to chew till soft (for a period of 30 seconds) after which patient was asked to spit the entire saliva collected in their mouth into the tube without swallowing. This level was marked and recorded. For this study, the Stimulated flow rate accepted was a minimum of 0.2 ml/min.
This procedure of collecting stimulated and unstimulated saliva was repeated in the same order at three intervals-0 minutes, 60 minutes and 90 minutes. In the end a total of six samples were collected per patient. The recording of salivary flow at 3 intervals was to quantitatively assess the variation in salivary flow over the 90 minutes-this was in accordance with the primary aim of the study.
Following this, the drug or placebo was administered to the participants. To maintain participant blind, both drug and placebo was dispensed in identical clear unmarked test tubes. The intervention was then made in front of patient using Pasteur’s pipette to ensure exact dosing. After the administration of the drops, patients were taken up for the required dental procedure as planned. Salivary flow rate was checked once more after 60 and 90 minutes. The heart rate and blood pressure were also checked and noted at this time.
At the end of the study, recorded results were tabulated and statistically analyzed using the SPSS-21 software. The intergroup analysis was done using student t-test and intragroup comparisons done with one-way ANOVA testing.
Results
The present study included a total of 60 children, with 30 boys and 30 girls across both groups who all received the elective dental treatment they had reported to the dental clinic for, irrespective of the study group they were assigned.
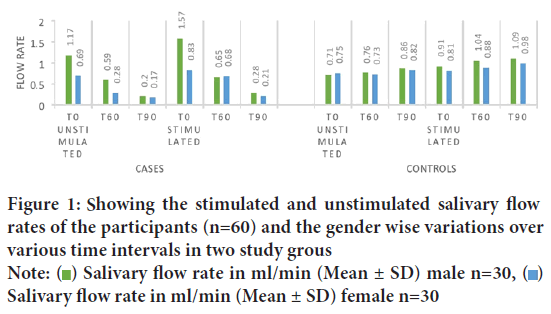
The stimulated and unstimulated salivary flow rates of the participants and the disparity gender wise over various time intervals is charted in the Figure 1.
Figure 1: Showing the stimulated and unstimulated salivary flow rates of the participants (n=60) and the gender wise variations over
various time intervals in two study groups
Note:  Salivary flow rate in ml/min (Mean ± SD) male n=30,
Salivary flow rate in ml/min (Mean ± SD) male n=30,  Salivary flow rate in ml/min (Mean ± SD) female n=30
Salivary flow rate in ml/min (Mean ± SD) female n=30
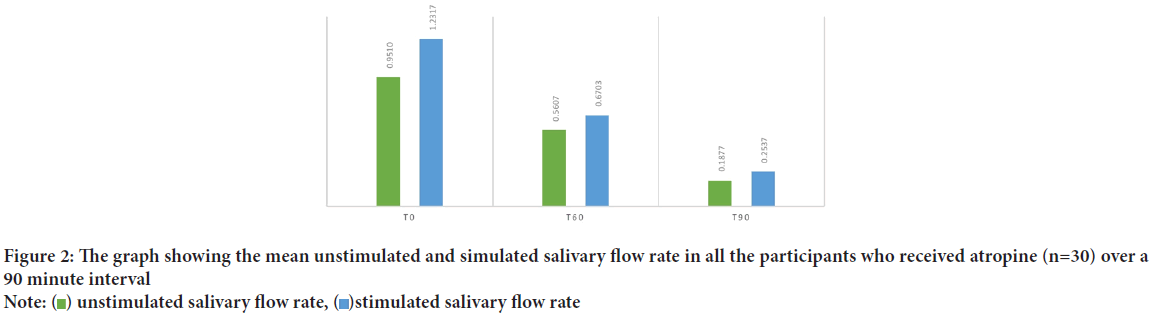
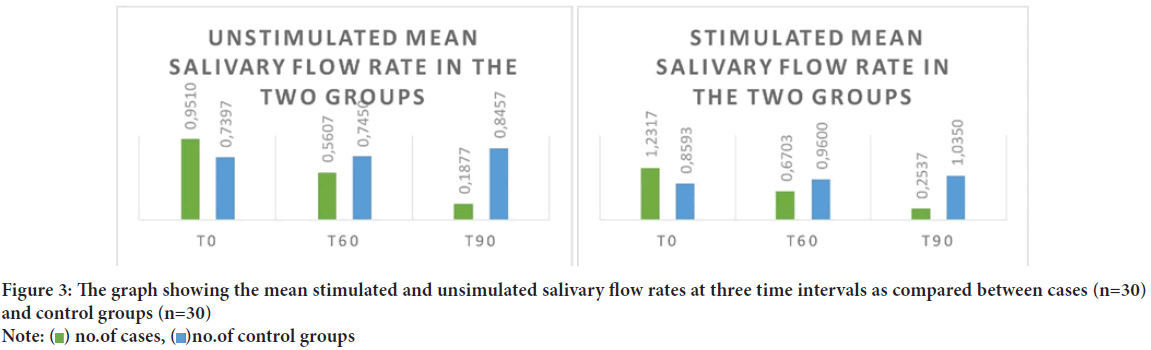
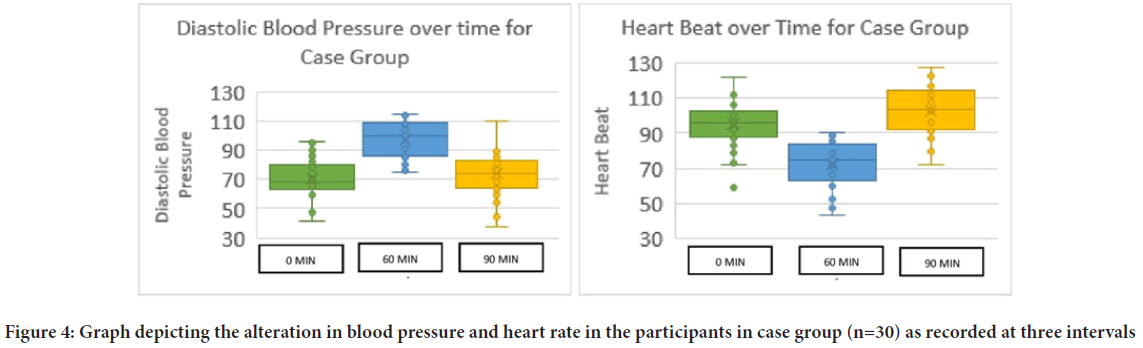
The average age of the participants of the present study was 11.3 years. Once tabulated it was seen that the Unstimulated Saliva Flow (USFR) at 60 minutes after administration of atropine was statistically significant to the age of the child (r=0.40). It was found that it is the Stimulated Saliva Flow Rate (SSFR) at t0 (start of the study) seemed to have statistically significant relation with the age of the patient (r=0.34) at 95% confidence interval (Table 1). The average weight of the patients included in the study was 40.9 kg ranging from 14.3 kg to 99 kg. When the USFR was analyzed with respect to the case group it was seen that weight of the participant was significant statistically in the t90 time interval (r=0.44) whereas in the control group weight strongly associated with the USFR at all the time intervals at 95% confidence interval (Table 2). On analyzing the average salivary flow rate (Figure 2) in the participants who received atropine, and applying one way ANOVA test, to the compare USFR at various time intervals (t0, t60 and t90) it was seen that the F value was extremely high (F=25.22) depicting a varied value among the group and was statistically significant. The same was true when one way ANOVA was applied on SSFR after atropine administration where the mean square value was high at 7.23 and the F value was 26.95. On plotting the graph of mean unstimulated salivary flow rate and stimulated salivary flow rate in the two groups and comparing them, the stark difference in salivation reduction can be appreciated (Figure 3). The effect of atropine sulphate administered sublingually was studied on the participant’s blood pressure and heart rate at various time intervals and was found to produce an increase in diastolic BP at 60-minute post administration. Also, there was seen to be a decrease in the heart rate of the participants who received atropine at 60-minute interval (Figure 4).
| Unstimulated saliva flow rate (ml/min) | Stimulated saliva flow rate (ml/min) | |||||
|---|---|---|---|---|---|---|
| Time | 0 min | 60 min | 90 min | 0 min | 60 min | 90 min |
| controls (n=30) | 0.26 | 0.2 | 0.18 | 0.37* | 0.11 | 0.13 |
| Cases (n=30) | 0.26 | 0.40* | -0.05 | 0.28 | 0.34 | 0.28 |
Note: *=statistically significant
Table 1: Depicting the correlation of the age of the participants and their stimulated and unstimulated salivary flow over a period of three time intervals
| Unstimulated salivary flow rate (ml/min) | Stimulated Salivary flow rate (ml/min) | |||||
|---|---|---|---|---|---|---|
| Time | 0 min | 60 min | 90 min | 0 min | 60 min | 90 min |
| Cases (n=30) | 0.24 | 0.25 | 0.44* | 0.27 | 0.19 | 0.29 |
| Control (n=30) | 0.48* | 0.51* | 0.1 | 0.41* | 0.44* | 0.44* |
Note: *=statistically significant
Table 2: Tabulated correlation of the weight of the participants and the stimulated and unstimulated salivary flow rate over three time intervals
Figure 2: The graph showing the mean unstimulated and simulated salivary flow rate in all the participants who received atropine (n=30) over a
90 minute interval
Note:  unstimulated salivary flow rate,
unstimulated salivary flow rate,  stimulated salivary flow rate
stimulated salivary flow rate
Figure 3: The graph showing the mean stimulated and unsimulated salivary flow rates at three time intervals as compared between cases (n=30)
and control groups (n=30)
Note:  no.of cases,
no.of cases,  no.of control groups
no.of control groups
Figure 4: Graph depicting the alteration in blood pressure and heart rate in the participants in case group (n=30) as recorded at three intervals
Discussion
The successful management of saliva and a good isolated operating area is a pre-requisite for majority of modern intraoral procedures. The challenge is amplified in paediatric practice due to limitations of communication and often comprehension from young patients. Herewith, the present study which was aimed to assess the use of sublingual atropine drops in children to decrease their salivary flow reversibly and assist the dental procedure requiring saliva control, shows promising results.
The use of ophthalmic eye drops with 1% w/v atropine sulphate, which is a commercially available over-the-counter drug, was done based on previously published works, most of which have demonstrated the effectiveness and success of this solution. Hyson HC, et al., 2002 reported the use of ophthalmic atropine drops in a brief report justifying the usage due to its ready availability, low cost, and no requirement of specialized skill while its administration. A similar argument was made in the favour by Meningaud JP, et al., 2006 stating its reversible effect as an added benefit. Other studies like the ones performed by Sockalingam S, et al., 2007, Rapoport A, 2010, Mustafa FA, et al., 2013, Norderyd J, et al., 2017 have also used the same rationale for administering atropine viaophthalmic drops.
The route of administration of drug in the present study was sublingual. This was chosen to avoid unnecessary invasive procedures and to eliminate the first pass metabolism of drug unavoidable viathe oral route. This guarantees that the drug enters the circulation faster than intramuscular or oral routes and potentiates higher absorption due to increased perfusion (Rajpal S, et al., 2010). Also, this route has been the most used in relation with ophthalmic atropine drops in previous studies where their safety has been established, including in the paediatric population (Nuhoglu Y, et al., 2007; Penagos M, et al., 2008).
Correlation of atropine sulphate and age and weight of the participant
It has previously been demonstrated by Rashid MU and Bateman DN, 1990 that age affects the salivary response to atropine. The results of the present study show that the average unstimulated salivary flow rate of the participants of the study (n=60) aged 7-18 was 0.84 ml/min at baseline. This is within range and comparable to previous works done on the subject by Forcella L, et al., 2018, where the mean USFR was found to be 0.76 ml/ min and other researchers all of whom have reported similar unstimulated flow rates in their studies in similar age groups (Gutman D and Aryeh HB, 1974; Rotteveel LJC, et al., 2004; Psoter WJ,et al., 2008; Moreira AR, et al., 2009). It was shown in the present study that the atropine case group showed significant correlation to the ages of the participants at 60-minutes (Table 1).
It has been recommended by the American Academy of Paediatrics (AAP), since 1950 that calculation of atropine dosage be made based on body weight (Unna KR, et al., 1950). The dose range of atropine in the present study ranged from 0.14 mg-0.99 mg. In the current study it was seen that salivary flow rate was statistically correlated to the weight of the child in the control group (Table 2). Body weight is a known factor affecting the salivary flow rate, especially in children. Increased weight of the child has been proven to reduce the salivary flow rate. These children show lower salivary flow rates as compared to children with normal range weight (Pannunzio E, et al., 2010). This could also be a reflection of developmental changes which encompass both height and weight and maturation of salivary glands affecting salivation (Bretz WA, et al., 2001).
Correlation of atropine sulphate and gender of the participant
The mean salivary flow rate estimated by both stimulated and unstimulated methods showed that males had a higher salivary flow rates as compared to females at the baseline (Figure 1). This observation is in accordance with previous works by Anderson R, 1972, Heintze U, et al., 1983 and Percival RS, et al., 1994 who concluded that at various age groups, females had lower mean salivary flow rates than males. A possible explanation for the gender disparity has been proposed by associating salivary gland size with secretory capacity (Leonor SP, et al., 2009). In the present study, at the subsequent readings at various time intervals, no difference was seen based on gender while computing the saliva quantitatively. Hence, atropine exerted similar antisialogogue effect on both girls and boys and showed no gender predilection on its pharmacokinetics.
Decrease in salivation after atropine
It has long been proven that salivary secretion is extremely sensitive to inhibition by antimuscarinic agents (Goodman LS, 1996). In the current study it was recorded to have a stark drop in both USFR and SSFR over a period of 90 minutes after administration of atropine drops (Figure 2). The unstimulated salivary flow rates in the children receiving atropine sulphate dropped by 80.3% in 90 minutes whereas stimulated salivary flow rate decreased by 79.4%. This seemed to echo the findings by Volz-Zang C, et al., 1995 who reported the decline of 84.3% salivation after oral atropine administration in their subjects. This also substantiates the already established fact of the antisialogogue activity of the drug as demonstrated by Bowman WC and Rand MJ, 1980 and Iliopoulou A, et al., 1981 at the dosage and route chosen (Rapoport A, 2010; Norderyd J, et al., 2017) within a span of 90 minutes.
Comparing the effectiveness of atropine drops on salivation between the two study groups
Quantitative analysis: In our study, the USFR saw significant correlation between the two study groups at 90 minutes interval. This difference was highly statistically significant, which goes on to show that atropine produced maximum quantitative effect on salivation at the interval of 90 minutes (Figure 3). When analyzing the SSFR in the study group, it was seen that the cases and control groups showed statistically significant correlation across all three time intervals. This finding of the steep decline of the SSFR in all the children who received atropine signifies the anticholinergic action of atropine that inhibits the action of M3 receptors causing the salivary gland hypofunction (Kubota C, et al., 2017). When given the same dose as the present study, administered sublingually using insulin injectors, authors achieved similar results in children of 3-78 months of age (Azapağası E, et al., 2020).
Time interval: The findings of the current study go on to show that the salivary flow rates progressively regress over the period of 90 minutes when final readings were taken. The USFR declined from 0.95 ml/min at start to a mere 0.19 ml/min at the end of 90 minutes. This shows that although the action of atropine starts to take effect before one hour, the peak action is evidenced only after 90 minutes. A similar decline could be viewed for SSFR where the reduction in salivary flow was drastic at 90 minutes at 0.25 ml/min from 1.23 ml/min at the start (Figure 3). The present findings been documented at 2 hours (6.7 nmol/l) that decreased the salivation until this time in children with a mean age of 5.1 years (Saarnivaara L, et al., 1985). Kazen DH and Dille JM, 1963 concluded a similar 90-minute period for maximum effect of AS when given orally, which then persisted for another two and a half hours.
As per the present study design, seem to echo that of Mirakhur RK, 1978, where oral atropine produced significant oral mucosal dryness at 90 minutes. This could be explained as the peak serum concentration of AS when given the same oral dose as the present study, has the time of observation was found to be inadequate to chart the reversal of the salivary flow in the participants. The only conclusion that could be drawn from the present study was that the antimuscarinic effect of atropine persists even after 90 minutes of the drug. This is a substantiated claim by Brion N, et al., 1988, according to whom saliva volume did not revert up until 7 hrs of 1 mg atropine tablet.
Effect of atropine sulphate on vitals
The administration of atropine sulphate in the dosages of 1.5 mg orally has been known to cause change in diastolic blood pressure (Lönnerholm G and Widerlöv E, 1975) something that was demonstrated by the present study where there was an increase in diastolic Blood Pressure (BP) in children who received atropine drops within 60 minutes of intake. This increase in the diastolic BP was found to be transient and had stabilized to its normal range by the final reading at 90 minutes (Figure 4).
A decrease in heart rate was observed in all the children receiving atropine drug sublingually at 60 minutes interval in the current study. The heart rate was found to revert to the normal range within the span of the study. This transient decrease of heart rate was expected as is known to occur associated with low doses of atropine and thought to be caused due to central vagal effect either on the nucleus or a direct effect on the heart (Baker JP, 1959; Hayes Jr AH, et al., 1971; McGuigan H, 1921). In this context, the most accepted explanation has been that it’s the peripheral effects that contribute more as compared to CNS effects when causing bradycardia in children (Lönnerholm G and Widerlöv E, 1975). Recent studies also report a decrease in standing pulse rate echoing the results of the present study (Mubaslat O and Lambert T, 2020).
Conclusion
The present study leads to the following inferences in its wake-
• The chronological age and body weight of the child were found to have appreciable statistical correlation to the salivary flow rate of the child.
• The mean salivary flow rate at baseline was higher in males as compared to females. Although atropine sulphate did not seem to have any gender predilection on its pharmacokinetics.
• The dose administered in the present study (0.01 mg/kg) was found to be effective in eliciting the antisialogogue action of atropine sulphate via sublingual oral route.
• The unstimulated salivary flow rates in the children receiving atropine sulphate dropped by 80.3% in 90 minutes whereas stimulated salivary flow rate decreased by 79.4%.
• The average time of onset of action of atropine sulphate via sublingual route is around 60 minutes.
The study also evidenced an increase in diastolic blood pressure 60 minutes after atropine administration in children along with simultaneous decrease in heart rate at the same time interval.
Limitations
The present study had certain confinements in the design wherein the span of observation of salivary flow was limited to 90 minutes so as so accommodate the routine dental procedures, this proved insufficient to assess the reversal of salivary flow in participants who received atropine. Also, the bad taste of the eyedrops was a major cause of concern for participants of the study.
Author Contributions
Both the authors contributed to this work. S. Samuel conceived the study, designed, and implemented the trial, collected and analyzed the data, and prepared the manuscript. S. Masih designed the study, collected, and analyzed the data and contributed to the manuscript. Both the authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
For the purpose of this study a written consent was obtained from the parents/ guardians in the language understood by them. The consent form clearly outlined the type of research intervention, voluntary participation, information on atropine and procedures and protocol among other information.
References
- Sapkos SW. The use of antisialogogues in periodontal and restorative dentistry. Int J Periodontics Restorative Dent. 1984; 4(4): 42-49.
[Google scholar] [Pubmed]
- Joseph MC, Vale RJ. Premedication with atropine by mouth. Lancet. 1960; 276(7159): 1060-1061.
- Mushin WW, Galloon S, Lewis FE. Anti-sialagogue and other effects of atropine mucate. Br Med J. 1953; 2(4837): 652-655.
[Crossref] [Google scholar] [Pubmed]
- Hyson HC, Johnson AM, Jog MS. Sublingual atropine for sialorrhea secondary to parkinsonism: A pilot study. Mov Disord. 2002; 17(6): 1318-1320.
[Crossref] [Google scholar] [Pubmed]
- Meningaud JP, Pitak-Arnnop P, Chikhani L, Bertrand JC. Drooling of saliva: A review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101(1): 48-57.
[Crossref] [Google scholar] [Pubmed]
- Sockalingam S, Shammi C, Remington G. Clozapine-induced hypersalivation: A review of treatment strategies. Can J Psychiatry. 2007; 52(6): 377-384.
[Crossref] [Google scholar] [Pubmed]
- Rapoport A. Sublingual atropine drops for the treatment of paediatric sialorrhea. J Pain Symptom Manage. 2010; 40(5): 783-788.
[Crossref] [Google scholar] [Pubmed]
- Mustafa FA, Khan A, Burke J, Cox M, Sherif S. Sublingual atropine for the treatment of severe and hyoscine-resistant clozapine-induced sialorrhea. Afr J Psychiatry (Johannesbg). 2013; 16(4): 242.
[Crossref] [Google scholar] [Pubmed]
- Norderyd J, Graf J, Marcusson A, Nilsson K, Sjöstrand E, Steinwall G, et al. Sublingual administration of atropine eyedrops in children with excessive drooling-a pilot study. Int J Paediatr Dent. 2017; 27(1): 22-29.
[Crossref] [Google scholar] [Pubmed]
- Rajpal S, Ali R, Bhatnagar A, Bhandari SK, Mittal G. Clinical and bioavailability studies of sublingually administered atropine sulfate. Am J Emerg Med. 2010; 28(2): 143-150.
[Crossref] [Google scholar] [Pubmed]
- Nuhoglu Y, Ozumut SS, Ozdemir C, Ozdemir M, Nuhoglu C, Erguven M. Sublingual immunotherapy to house dust mite in pediatric patients with allergic rhinitis and asthma: A retrospective analysis of clinical course over a 3-year follow-up period. J Investig Allergol Clin Immunol. 2007; 17(6): 375-378.
[Google scholar] [Pubmed]
- Penagos M, Passalacqua G, Compalati E, Baena-Cagnani CE, Orozco S, Pedroza A, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008; 133(3): 599-609.
[Crossref] [Google scholar] [Pubmed]
- Rashid MU, Bateman DN. Effect of intravenous atropine on gastric emptying, paracetamol absorption, salivary flow and heart rate in young and fit elderly volunteers. Br J Clin Pharmacol. 1990; 30(1): 25-34.
[Crossref] [Google scholar] [Pubmed]
- Forcella L, Filippi C, Waltimo T, Filippi A. Measurement of unstimulated salivary flow rate in healthy children aged 6 to 15 years. Swiss Dent J. 2018; 128(12): 962-967.
[Google scholar] [Pubmed]
- Gutman D, Aryeh HB. The influence of age on salivary content and rate of flow. Int J Oral Surg. 1974; 3(5): 314-317.
[Crossref] [Google scholar] [Pubmed]
- Rotteveel LJC, Jongerius PH, van Limbeek J, van den Hoogen FJA. Salivation in healthy schoolchildren. Int J Pediatr Otorhinolaryngol. 2004; 68(6): 767-774.
[Crossref] [Google scholar] [Pubmed]
- Psoter WJ, Spielman AL, Gebrian B, St Jean R, Katz RV. Effect of childhood malnutrition on salivary flow and pH. Arch Oral Biol. 2008; 53(3): 231-237.
[Crossref] [Google scholar] [Pubmed]
- Moreira AR, Passos IA, Sampaio FC, Soares MS, Oliveira RJ. Flow rate, pH and calcium concentration of saliva of children and adolescents with type 1 diabetes mellitus. Braz J Med Biol Res. 2009; 42: 707-711.
[Crossref] [Google scholar] [Pubmed]
- Unna KR, Glaser K, Lipton E, Patterson PR. Dosage of drugs in infants and children: I. Atropine. Pediatrics. 1950; 6(2): 197-207.
[Google scholar] [Pubmed]
- Pannunzio E, Amancio OM, Vitalle MS, Souza DN, Mendes FM, Nicolau J. Analysis of the stimulated whole saliva in overweight and obese school children. Revista da Associação Médica Brasileira. 2010; 56: 32-36.
[Crossref] [Google scholar] [Pubmed]
- Bretz WA, do Valle EV, Jacobson JJ, Marchi F, Mendes S, Nor JE, et al. Unstimulated salivary flow rates of young children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91(5): 541-545.
[Crossref] [Google scholar] [Pubmed]
- Andersson R. The flow rate, pH and buffer effect of mixed saliva in schoolchildren. Odontol Revy. 1972; 23(4): 421-428. [Crossref]
[Google scholar] [Pubmed]
- Heintze U, Birkhed D, Björn H. Secretion rate and buffer effect of resting and stimulated whole saliva as a function of age and sex. Swed Dent J. 1983; 7(6): 227-238.
[Google scholar] [Pubmed]
- Percival RS, Challacombe SJ, Marsh PD. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994; 73(8): 1416-1420.
[Crossref] [Google scholar] [Pubmed]
- Leonor SP, Laura SM, Esther IC, Marco ZZ, Enrique AG, Ignacio MR. Stimulated saliva flow rate patterns in children: A six-year longitudinal study. Arch Oral Biol. 2009; 54(10): 970-975.
[Crossref] [Google scholar] [Pubmed]
- Goodman LS. Goodman and Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill. 1996.
- Volz-Zang C, Waldhäuser T, Palm D, Schulte B. Comparison of the effects of atropine in vivo and ex vivo (radioreceptor assay) after oral and intramuscular administration to man. Eur J Clin Pharmacol. 1995; 49(1): 45-49.
[Crossref] [Google scholar] [Pubmed]
- Bowman WC, Rand MJ. Textbook of pharmacology. Blackwell Scientific Publications. 1980.
- Iliopoulou A, Kaspi T, Blackett A, Turner P. Clinical pharmacological studies with LM 5008, a new antidepressant. Pharmatherapeutica. 1981; 2(9): 613-621. [Crossref]
[Google scholar] [Pubmed]
- Kubota C, Kanazawa M, Hama Y, Komagamine Y, Minakuchi S. Association between chewing-stimulated salivary flow under the effects of atropine and mixing ability assessed using a color-changeable chewing gum. J Prosthodont Res. 2017; 61(4): 387-392.
[Crossref] [Google scholar] [Pubmed]
- Azapağası E, Kendirli T, Perk O, Kutluk G, Tunçer GÖ, Teber S, et al. Sublingual atropine sulfate use for sialorrhea in pediatric patients. J Pediatr Intensive Care. 2020; 9(3): 196-200.
[Crossref] [Google scholar] [Pubmed]
- Mirakhur RK. Comparative study of the effects of oral and IM atropine and hyoscine in volunteers. Br J Anaesth. 1978; 50(6): 591-598.
[Crossref] [Google scholar] [Pubmed]
- Saarnivaara L, Kautto UM, Iisalo E, Pihlajamäki K. Comparison of pharmacokinetic and pharmacodynamic parameters following oral or intramuscular atropine in children: Atropine overdose in two small children. Acta anaesthesiologica scandinavica. 1985; 29(5): 529-536.
[Crossref] [Google scholar] [Pubmed]
- Kazen DH, Dille JM. An evaluation of atropine as an antisialogogue in dentistry. Oral Surg Oral Med Oral Pathol. 1963; 16(8): 919-925.
[Crossref] [Google scholar] [Pubmed]
- Brion N, Beaumont D, Advenier C. Evaluation of the antimuscarinic activity of atropine, terfenadine and mequitazine in healthy volunteers. Br J Clin Pharmacol. 1988; 25(1): 27-32.
[Crossref] [Google scholar] [Pubmed]
- Lönnerholm G, Widerlöv E. Effect of intravenous atropine and methylatropine on heart rate and secretion of saliva in man. Eur J Clin Pharmacol. 1975; 8(3): 233-240.
[Crossref] [Google scholar] [Pubmed]
- Baker JP. Effect of atropine on the heart-rate. The Lancet. 1959; 273(7064): 150.
[Crossref] [Google scholar] [Pubmed]
- Hayes Jr AH, Copelan HW, Ketchum JS. Effects of large intramuscular doses of atropine on cardiac rhythm. Clin Pharmacol Therap. 1971; 12(3): 482-486.
[Crossref] [Google scholar] [Pubmed]
- McGuigan H. The effect of small doses of atropine on the heart rate. J Amer med Ass. 1921; 76: 1338-1340. [Crossref]
[Google scholar] [Pubmed]
- Mubaslat O, Lambert T. The effect of sublingual atropine sulfate on clozapine-induced hypersalivation: A multicentre, randomised placebo-controlled trial. Psychopharmacology. 2020; 237(10): 2905-2915.
[Crossref] [Google scholar] [Pubmed]
Author Info
Shannon Samuel* and Shaila MasihCitation: Samuel S: The Impact of Sublingual Atropine Eyedrops on Salivary Flow in 7-17 Year Old Children during Routine Dental Procedures: A Randomized Controlled Trial
Received: 15-Sep-2022 Accepted: 10-Oct-2022 Published: 17-Oct-2022, DOI: 10.31858/0975-8453.13.10.672-677
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3