Review Article - (2023) Volume 14, Issue 5
Abstract
Hepatitis C is a rapidly spreading illness in India and a major cause of liver damage. The significant likelihood of chronicity associated with this blood-borne infection, as well as its link to hepatocellular carcinoma, highlight its public health significance. Two avoidable modes of hepatitis C infection dissemination include blood transfusion and improper therapeutic procedures with contaminated needles. Furthermore, risk factor modification, such as lowering the number of intravenous drug users, will aid in decreasing the incidence of this illness. The scope, nature, and consequences of this relatively novel pathogen in causing illness in India are summarized in this article.
Keywords
Hepatitis, HCV, Blood, Transfusion, Infection
Introduction
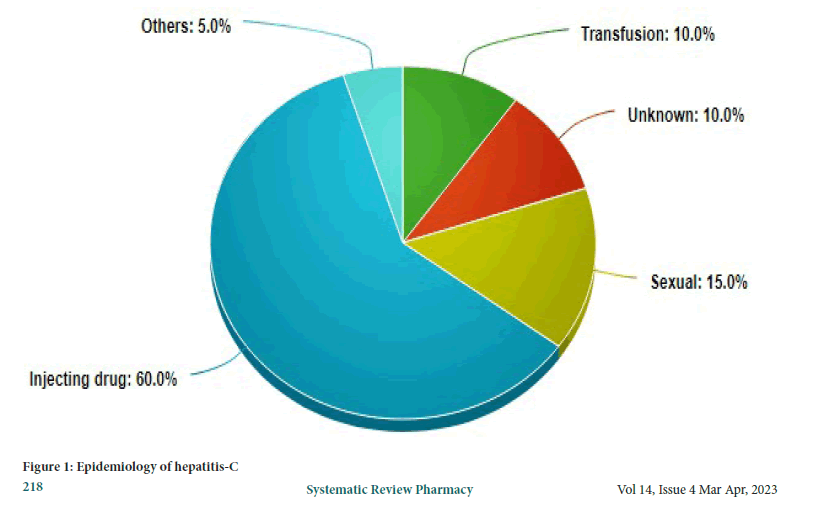
After thorough testing of serum from experimentally infected animals, HCV was first discovered in 1989 utilizing molecular biology methods. It was eventually identified as an RNA virus from the Flaviviridae family and the name Hepacivirus (Memon MI and Memon MA, 2002). Since its discovery, it has been obvious that this virus is the leading cause of acute hepatitis after a blood transfusion that is not caused by hepatitis A or B. (hence the early name for this disease, non-A, non-B hepatitis) (Bonkovsky HL and Mehta S, 2001). According to estimates, the worldwide prevalence of HCV virus (HCV) infection is roughly 2 percent, with 170 million people chronically infected and 3 to 4 million people being infected each year (Kohli A, et al., 2010). It is now well acknowledged as one of the most prevalent causes of liver cirrhosis. In wealthy nations such as the United States, it is the top cause of liver transplantation and the most frequent chronic blood borne infection (Kamal SM, 2008). In India, the effects of this virus are just now becoming apparent. The blood-banking system in India has severe flaws. Despite the fact that professional blood donation is legal, it continues to thrive. Another problem in our health-care system is the reuse of needles that have not been adequately sanitized. Both of these reasons have the potential to spread HCV in India (Modi AA, Liang TJ, 2008). An epidemiological distribution of HCV is presented in Figure 1.
Figure 1: Epidemiology of hepatitis-C
Literature Review
Social and historical perspectives
As Stephen S. Morse points out, the history of infectious illnesses is primarily a history of bacteria using the many possibilities humanity provided them to live, grow, and multiply. The global HCV pandemic exemplifies this point-HCV’s fast proliferation and global dispersion are due to its effective transmission through blood and blood products transfusions, parenteral medicines, and other invasive medical procedures that were more widely accessible over the twentieth century (Alberti A, et al., 1999). Between the two world wars, when glass syringes and other medical instruments were mass-produced, massive and dangerous injection campaigns began. The unit price per syringe fell by 80 percent between 1920 and 1950, but worldwide output expanded by more than 100-fold. Because injections were utilized for vaccination or prophylactic campaigns, antibiotic delivery, and insulin treatments, they were synonymous with successful therapies (Alter MJ, 1997). Furthermore, blood transfusion became a frequent clinical procedure during and soon after World War II, and fractionated blood derivatives began to be created and delivered. Poorly sterilized syringes, as well as unscreened blood and blood products, were ideal vectors for infectious disease transmission. The formation of the main HCV subtypes in various nations generally correlates with local outbreaks of hazardous parenteral treatments, according to certain research groups that used advanced epidemiological and molecular evolutionary methodologies (Alberti A and Benvegnu L, 2003). Antimony-based parenteral antischistosomal therapy was responsible for the spread of HCV among general populations in Japan and Egypt; the Egyptian epidemic (when oral medicine was introduced to replace schistosomiasis treatment) and resulted in the world’s highest national prevalence of anti-HCV (25 percent) (Westbrook RH and Dusheiko G, 2014).
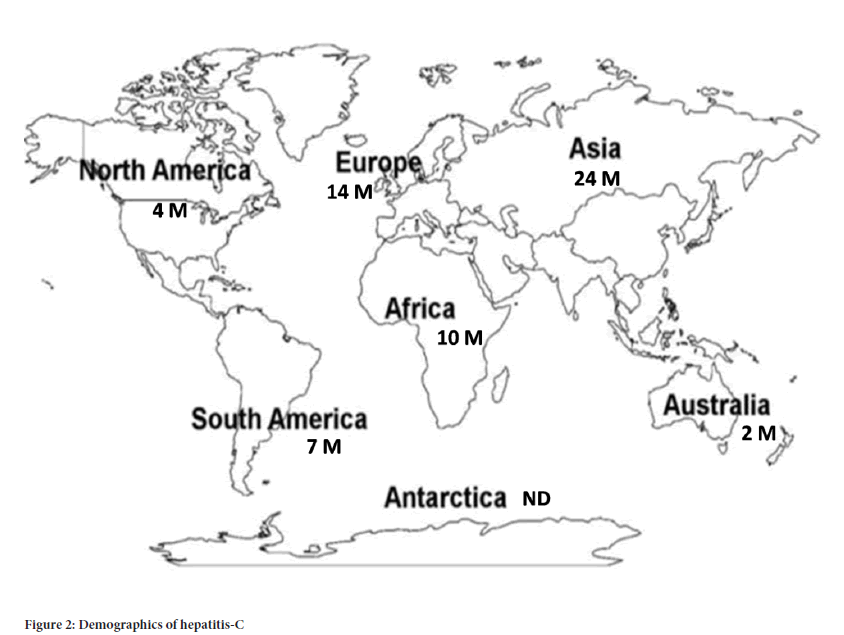
Increasing usage of risky medical operations, especially during wartime conflicts, is likely to have generated and amplified a number of additional local diseases. Blood transfusions, in particular, were a major source of viral transmission: By the end of the 1980s, one out of every 50 blood units in affluent nations had been infected with HCV. As a consequence, chronic hepatitis occurred in almost all chronic transfusion recipients and nearly all patients receiving clotting factor concentrates (Wang LS, et al., 2016). However, because of improved infection control standards, the implementation of effective virus inactivation procedures for blood derivatives, and the introduction of second-generation anti-HCV tests for blood donors, the incidence of HCV infection in resource-rich countries decreased dramatically within a few years. The bulk of new HCV infections in these nations are currently caused by intravenous drug users exchanging needles (Lauer GM and Walker BD, 2001). A demographic distribution of HCV is presented in Figure 2.
Figure 2: Demographics of hepatitis-C
Spread of HCV via blood transfusions
Developed nations: Clinicians in affluent nations are currently dealing with the long-term consequences of previous transfusion-associated HCV outbreaks. Adult cohort studies have shown that roughly 75 percent of patients are positive for HCV RNA 15 years after blood transfusion, and the prevalence of liver cirrhosis is 15%-20%, whereas children and young women have had better results (Seeff LB, 1997). Blood supplies, on the other hand, are today quite safe. Since 1994, there have been no occurrences of HCV transmission owing to the injection of blood derivatives, as has been carefully examined elsewhere. In terms of labile blood components, it has also been shown that the most majority of HCVases now seen in blood recipients are not generated by blood transfusions, but rather by nosocomial transmission (Poynard T, et al., 2003). Because of the limited number of documented transmission events since the introduction of blood donor anti-HCV screening, the residual risk is essentially limited to the units collected during the donors’ serological window period, and it has become so small that it cannot be accurately measured by traditional approaches (i.e. prospective surveys of blood recipients). As a result, only indirect measurements, such as mathematical models including the frequency of infection and the length of the pre-seroconversion window period, may be used to forecast it (Maheshwari A, et al., 2008). As of 2000, estimates were about 1:2,00,000 blood units or fewer, indicating that the risk of getting an HCV-infected transfusion was much lower than the risk of dying following a percutaneous liver biopsy (1:10,000-12,000) and equivalent to the risk of dying while playing football (1:1,50,000) (Rosen HR, 2011).
Infection with the HCV virus in the United States
HCV is the most frequent chronic blood borne infection in the country. Despite the fact that the yearly number of new HCV infections has decreased by more than 80 percent since last decade, the overall prevalence of infection remains 1.8 percent, equal to an estimated 3.9 million Americans afflicted with HCV. Because they are not clinically unwell, the majority of these people is chronically infected and may be unaware of their infection (Thomas DL and Seeff LB, 2005). During the first two or more decades after infection, infected people may spread the virus to others and are at risk of developing chronic liver disease or other HCV-related chronic disorders. In the overall population, 65 percent of HCV infected people are between the ages of 30 and 49. The biggest difference in HCV infection prevalence occurs among those who have various infection risk factors (Strader DB, et al., 2004). Case control studies established the majority of risk variables linked to HCV transmission in the United States. Blood transfusions, IVDU, health-care employment in patient care or laboratory work, exposure to a sexual partner or household member with a history of hepatitis, several sexual partners, and poor socioeconomic status were among the factors. Prospective studies have recently shown that HCV infection may be transmitted from mother to child. There is no link between military duty and exposures from medical, surgical, or dental treatments, tattoos, acupuncture, ear piercing, or international travel. If such exposures cause transmission, the frequency may be too low to detect (Orland JR, et al., 2001).
Over time, the relative impact of the two most prevalent HCV transmission exposures, blood transfusion and IVDU, has shifted. Blood transfusion, which was responsible for a large proportion of HCV infections acquired more than ten years ago, now only accounts for a small percentage of newly acquired infections. IVDU, on the other hand, has continuously responsible for a significant share of HCV infections and now accounts for 60 percent of HCV transmission (Kim WR, 2002). Many people with chronic HCV infection may have acquired the virus as a consequence of limited or infrequent unlawful IVDU 26-30 years ago. In one research of volunteer blood donors, intranasal cocaine usage was shown to be independently linked to HCV infection. Intranasal cocaine use in the absence of IVDU was infrequent among individuals with acute HCV documented (Rehermann B and Nascimbeni M, 2005). Intranasal cocaine usage, at least in the recent past, seems to have had a minor role in transmission. It’s unclear if those with a history of non-IVDU usage alone, such as intranasal cocaine use, are more likely to be infected with HCV until further data is available. Except in the chronic hemodialysis context, nosocomial transmission has been observed in the United States. Health-care workers (HCW) who come into contact with blood in the workplace are at risk of contracting bloodborne infections. HCV infection is no more common among HCWs, such as orthopaedic, general, and oral surgeons, than it is in the general population, with a frequency of l%-2%. A history of unintentional needlestick injury was the only occupational risk factor independently related with HCV infection in the one research that looked at risk variables for infection (Wilkins T, et al., 2010).
According to case-control studies, there is a link between sex contact with a history of hepatitis or exposure to many sex partners and the acquisition of HCV, and up to 20 percent of patients with acute HCV have a history of sexual exposures even if there are no percutaneous risk factors. Two-thirds had an anti-HCV positive sex partner, and one-third had more than two partners in the six months leading up to sickness. In contrast, investigations of long-term spouses of patients with chronic HCV infection who had no other risk factors for infection found a low prevalence (1.5 percent) of HCV infection (McHutchison JG, 2004). Anti-HCV was shown to be prevalent among people with signs of high-risk sexual activities (e.g., patients visiting STD clinics and female prostitutes) who denied a history of IVDU (range l%-10%). Greater numbers of sex partners, a history of past STDs, and failure to use a condom were all related with anti-HCV positive in both heterosexuals and Homosexual males (HS). However, the number of partners linked to infection risk varied across research, ranging from less than one partner in the preceding month to more than 50 in the previous year (Alter MJ, 2002). Only one research has identified a link between HCV infection and HS activity, and the frequency of HCV infection among HS has typically been comparable to that of heterosexuals, at least in STD clinic settings. The low prevalence of HCV infection among HS and long-term steady sex partners of people who have chronic HCV infection has cast doubt on the role of sexual activity in HCV transmission. Unknown percutaneous risk factors may enhance the likelihood of HCV infection in those who engage in high-risk sexual behaviors (Fried MW, 2002).
According to recent research, a possible risk factor may be identified in nearly 90 percent of those infected with HCV in the United States. HCV transmission is caused by IVDU in 60 percent of cases, sexual activity in 20 percent of cases, and other recognized exposures (occupational, hemodialysis, domestic, perinatal) in 10 percent of cases. There is no known source of infection among the remaining 1 percent, despite the fact that the majority of those in this group have a poor socio-economic status. Low socio-economic status has been linked to a variety of infectious illnesses and may serve as a proxy for high-risk exposures; however, since it is non-specific, it is difficult to focus preventative strategies (Mukhopadhya A, 2008).
Discussion
Developing nations
HCV in India: In India, the epidemiology of HCV has not been well investigated. The majority of HCV prevalence studies have been conducted in blood banks, with the idea that blood donors are a proxy for the general population. With the introduction of professional donors, however, this presumption may be incorrect (Seeff LB, 2009). HCV prevalence has been shown to be less than 2 percent in many investigations of voluntary or mixed donors. Alarmingly, the proportion of professional donors was found to be 55.3 percent and 87.3 percent in two separate investigations.
This emphasises the necessity for more strict blood donor screening in India. There are few major community-based studies that look at HCV prevalence in the general population. These studies provide an accurate picture of the country’s HCV health burden (Lavanchy D, 2011). The patients in the two Andhra Pradesh investigations were recruited from a gastroenterology camp and a tribal Lambada community. The prevalence was 1.4 percent in one group and 2.02 percent in the other. A smaller research from Arunachal Pradesh, on the other hand, found a substantially higher HCV prevalence of 7.89 percent (Chander G, et al., 2002). Another Maharashtra rural study of over 1000 villagers revealed an extremely low prevalence rate of HCV infection of about 0.09 percent. The most systematic population-based research was reported from West Bengal, where 3,579 people were chosen from 10,737 people in 9 villages using a 1:3 sample procedure. HCV was found by ELISA in 26 individuals out of 2,973 who agreed to take part in the study. By PCR, a total of 21 cases were found to be true positive (0.71 percent). The highest prevalence (1.5 percent) was found in the elder age group of >60 years, while the lowest prevalence was found in the younger age group of 10 years (0.31 percent) (Paterson BL, et al., 2007).
HCV and liver disease
In India, HCV is progressively being identified as a major etiological cause for liver disease. Acute hepatitis, chronic hepatitis, cirrhosis, and hepatocellular carcinoma are some of the clinical symptoms. The relative contributions of HCV to various diseases are summarised.
Acute hepatitis: None of the thirty-eight patients presenting with acute self-limiting sporadic non-A, non-B hepatitis tested positive for HCV viral antibodies in the first investigation on HCV as a pathogen causing acute hepatitis, which was conducted in Kashmir. Following studies, it was discovered that HCV is a small actor in the vast range of acute hepatitis. In a research from Delhi, HCV was identified in 12.5 percent of 32 individuals with acute hepatitis. In a comparable research from Indore, just 4.85 percent of 103 individuals tested positive for HCV antibodies. According to a Delhi research, the prevalence is 12 percent. The most recent trial by the same group of researchers included the largest number of patients with acute hepatitis, totaling 306 individuals, 20.6 percent of whom had HCV evidence (Thomas DL, 2013). Given the inherent referral bias of hospital-based research, it’s probable that these varying numbers aren’t totally accurate. Acute hepatitis in certain contexts deserves particular attention, with transfusion-associated acute viral HCV being one of them. During surgery, 182 patients were transfused with a total of 818 units of blood, according to a prospective research. Prior to surgery, all of these individuals tested negative for both HBV and HCV. During the course of the study, 14 patients (7.69 percent) developed transfusion-associated hepatitis. 10 of the 14 cases were caused by HCV (71.5 percent). This research confirms what has always been said about the critical need to standardize screening techniques in blood banks (de Francesco R and Migliaccio G, 2005).
Chronic liver disease: HCV is currently one of the most frequent causes of chronic liver disease in the world, and it is also one of the most common reasons for liver transplantation. The prevalence of HCV in chronic liver disease in India has been studied in many researches. HCV prevalence has been reported to vary from 10.8 percent to 48.5 percent. The research with the greatest HCV prevalence also had the highest hepatitis B prevalence of 69.5 percent, indicating that HBV and HCV co-infection is a serious issue in India. A substantially high incidence of co-infection was also reported in the Punjab study, with a rate of 24.7 percent (Lingala S and Ghany MG, 2015).
Fulminant hepatitis: In a Delhi investigation of 167 patients with Fulminant Hepatic Failure (FHF), Sub-Acute Hepatic Failure (SAHF), and acute hepatitis, HCV was first identified as a cause of fulminant liver illness. Anti-HCV positive rates in these individuals were 43 percent, 47 percent, and 42 percent, respectively. In patients with fulminant hepatitis and SAHF, a second investigation from the same hospital found a comparable estimate of 45 percent and 44.6 percent, respectively (Simmonds P, 2013). Only 3 (7.9 percent) of the 38 instances of fulminant hepatic failure admitted to the Government hospital in Aurungabad could be ascribed to HCV, according to another study. The biggest series of fulminant hepatitis cases came from Delhi, where 423 patients with fulminant hepatic failure were hospitalized. A subgroup of 50 individuals with non-A-non-B hepatitis had their HCV RNA analyzed. HCV was found in seven of these samples (19 percent), indicating that HCV is a definite factor in a percentage of fulminant hepatic failure patients in India (Alter MJ, 2007). In an Indore study of 95 patients with fulminant hepatic failure, HCV was not identified in any of the patients. In this situation, more sensitive testing like as PCR may be required to identify HCV, and standard antibody tests may be mistakenly negative. Another research out of Delhi showed that PCR may properly detect more instances than serological testing. HCV was found in 15.5 percent of FHF patients in this study (Shepard CW, et al., 2005). The major issue in the relationship between HCV infection and fulminant hepatic failure has always been whether HCV is a causal factor or only a co-infection. HCV was detected in 7 out of 50 patients (14 percent) in a series from Delhi; however it was invariably in combination with other hepatotropic viruses, most often hepatitis B. Despite the fact that viral hepatitis is the most prevalent cause of FHF in children, HCV has not been linked to FHF in this case. Over the course of a year, a small research from Pune looked at 36 children who presented with FHF and found no evidence of HCV in any of them (Zein NN, 2000).
Hepatocellular carcinoma: HCV’s significance in the development of hepatocellular carcinoma has been extensively established. This link has also been confirmed in a few Indian researches. According to the first data from Delhi, 15.1 percent of patients with hepatocellular carcinoma tested positive for HCV antibodies. In another paper from Delhi, this link was found in just 4 percent of instances with haepatocellular carcinoma, with an additional 8 percent having combined HCV and HBV infection. This was comparable to findings from Chandigarh, where only 4 percent of hepatocellular carcinoma patients tested positive for anti-HCV. HBV infection is still the most prevalent cause of hepatocellular carcinoma in India. However, given the sluggish nature of HCV’s growth, Hepatocellular carcinoma caused by HCV is predicted to become a substantial clinical concern in the next decades (Maasoumy B and Wedemeyer H, 2012).
Hepatitis non-A, non-B/HCV post-transfusion before anti- HCV testing
PTH-NANB (Post-Transfusion Hepatitis Non-A, Non-B) was the most common infectious illness transmitted by blood transfusion in the 1970s and 1980s. PTH-NANB was assigned to 80%-90% of PTH patients, according to estimates. The incidence of PTHNANB among blood recipients was found to be 24 percent in Northern Europe, 618 percent in Southern Europe, and 12 percent in the United States. In the United States, 6 percent of the retrospectively tested recipients of cellular blood products, predominantly hematological and/or oncological patients, were antiHCV positive with RIBA after the introduction of anti-HCV testing, compared to only 1 percent in Europe. Paid donors are more likely to be carriers of parenterally transmitted viruses than non-remunerated donors, which may explain the disparity in HCV frequency between the United States and Europe. “Look-back” programmes were started once anti-HCV screening was implemented (González-Grande R, et al., 2016). In a study of 22 previously given blood products from donors who were reported HCV-RNA positive following anti-HCV screening, 81 percent of the products were shown to be related with HCV in the receiver. Due to repeated steps made by blood banks to prevent HIV and hepatitis transmission, the incidence of PTH-NANB has substantially decreased since then. In Europe, “hemovigilance” programmes have been set up to track unfavorable consequences of blood transfusions in a systematic manner (McGowan CE, Fried MW, et al., 2012).
Hemodialysis patients
Hemodialysis patients have been identified as a high-risk category for HCV infection. In recent studies, seroprevalences in reasonably large patient groups tested using 2nd or 3rd generation antiHCV tests and/or PCR were reported as 4%-24% in Europe, 47%-82% in Brazil, and 22%-56% in Japan. This risk was linked to red cell transfusions to some extent. Hemodialysis patients without a history of transfusions, on the other hand, had higher HCV prevalence, indicating the likelihood of nosocomial transmission. Additional evidence for inter-patient transmission comes from the discovery of similar HCV genotypes and genomic sequences, as well as occasionally even otherwise unusual HCV strains, in numerous infected patients in hemodialysis units (Manns MP, et al., 2017).
Clotting factor preparations
Prior to the mid-1980s, when virus-inactivation of large-pool clotting factor concentrates was introduced, almost all patients who received clotting factor preparations got infected with HCV. In a cohort analysis of 183 haemophilia patients, the development of HCV-related liver disease was assessed. The median follow-up period following exposure to concentrate was 15.1 years, and 40.4 percent of the patients were HIV positive. The probability of hepatic decompensation 20 years after initial exposure to HCV is predicted to be 10.8 percent, and HIV positive individuals are 21 times more likely than HIV negative patients to develop hepatic decompensation (Yu ML and Chuang WL, 2009). Other investigations have corroborated these results. In a recent Dutch study, 99 percent of patients who got large pool concentrates before anti-HCV testing and 66 percent of patients who received small-pool cryoprecipitates before anti-HCV testing were found to be anti-HCV positive, with 81 percent also being HCV RNA positive (Giannini C and Brechot C, 2003).
Inactivated plasma derivatives
Hemophilia patients treated with vapour-heat inactivated factor VIII concentrates have been shown to have anti-HCV seroconversion. Other risk factors may have contributed to HCV infection in one research. However, the batch factor of vapour-heated VIII concentrate was revealed to be HCV-RNA positive in a second research. Plasma products inactivated by modern procedures such as pasteurisation or solvent-detergent inactivation do not transmit HCV in general (SD). This was validated in a follow-up research in the Netherlands of 57 patients who received SD inactivated products between 1989 and 1993 and had no HCV seroconversions (Kato N, 2001).
Immunoglobulin preparations pose a risk of HCV infection
Prior to anti-HCV testing, immunoglobulin preparations were thought to have a good safety record, especially when made using cold ethanol fractionation (Cohn fractionation). However, an accidental outbreak of HCV infection was reported in former Eastern Germany in 1978-1979 after administration of an anti-D immunoglobulin preparation, and recipients of anti-D immunoglobulin were infected with HCV in Ireland by contaminated preparations, samples of which were preserved and recently re-tested for HCV RNA. Since 1977, recipients of anti-D immunoglobulin preparations have been invited to participate in a voluntary HCV screening programme as a result of this discovery. However, ethanol fractionation was not used in the manufacture of any of these anti-D immunoglobulin preparations (Cuthbert JA, 1994).
Anti-HCV antibody seroconversion was also documented in patients who received polyvalent Intravenous Immunoglobulins (IVIG), however such antibodies were also passively transmitted. Although the role of anti-HCV screening in improving the viral safety of IVIG preparations is unclear, a recent transmission episode by a previously safe IVIG preparation suggests that the absence of anti-HCV antibodies in the preparation may affect the partitioning characteristics of HCV virions and result in a loss of neutralizing antibody in the end product. HCV RNA is identified in IVIG batches and plasma pools from compensated plasma donors more often than in pools from unpaid donors. Following the aforesaid discoveries, viral inactivation methods such as SD treatment were used to IVIG products, and HCV nucleic acid amplification technology (NAT) was implemented for quality control of plasma production pools (Li HC and Lo SY, 2015).
IV drug administration
HCV is known to be transmitted via intravenous and percutaneous drug use. This is a major issue in northeast India, and it is likely to be a concern across the nation. The incidence of HCV was alarmingly high at 92 percent among 77 IV drug addicts in the only research that looked at this. HCV is becoming more common in this subset of individuals, according to reports from throughout the globe (Gumber SC and Chopra S, 1995).
Co-infection with HIV and HCV
Because HIV and HCV infection share the same transmission channels, it’s no surprise that the two viruses are often co-infected. The frequency of HCV infection in HIV patients has been quite varied. Co-infection rates of 1.61 percent and 2.2 percent, respectively, were found in two investigations from Lucknow and Chennai. Both of these investigations were carried out on individuals who used IV drugs infrequently (Bertino G, et al., 2016). However, a study from Imphal revealed a relatively high proportion of HIV and HCV co-infection among injecting IV drug users, at 52.4 percent. Another research conducted in an STD clinic found that 21.4 percent of HIV positive people had HCV seroprevalence. The HIV pandemic in India is rapidly growing out of control. In India, it is believed that 2.5 million individuals are infected with HIV. HCV infection is predicted to follow the HIV pandemic into India and become a significant source of morbidity (Dubuisson J, 2007).
Employees in the medical field
Because they come into touch with possibly infected patients, health care professionals are at a greater risk of contracting HCV. The prevalence of HCV was found to be between 0 and 4 percent in this group. HCV infection has been linked to a higher risk of infection in certain occupations. A research indicated that dentists had a considerably high prevalence of HCV, with an estimate of 5.4 percent (Zeuzem S, et al., 2009).
Selection of blood donors
HCV is identified more often in compensated blood donors than in unpaid blood donors. Incentives for blood donations are widely recognised for persuading blood donors not to reveal risky behaviour, with the most apparent explanation being the money required for Intravenous Drug Use (IVDU). Prior to the implementation of systematic anti-HCV screening for blood donors, a significant number of those who tested positive for HCV were admitted to IVDU. Because HCV may cause long-term infection, blood donors who inadvertently injected drugs in the 1970s are still HCV viremic today, according to PCR. According to research on blood donors who were informed of positive HCV test results, 60%-90% reported to having a risk factor for HCV parenteral exposure, depending on the study design and demographic. Because paid donors are discovered to have greater prevalences of transmissible illnesses every time a new blood screening marker is developed, it is preferable to collect blood donations solely from unpaid volunteers. This is in line with EU health-care policy (Houghton M, 2009).
Surrogate blood donor screening
Attempts were undertaken in the 1980s to increase the safety of blood transfusion by screening blood donors for PTH-NANB surrogate indicators of infectivity. Blood donations with increased ALT levels were more often connected with a recipient with PTH-NANB than blood donations with normal ALT levels, according to two independent investigations conducted in US. It was estimated that excluding donor blood with ALT values more than two Standard Deviations (SD) above the logarithmic mean would avoid 30 percent of PTH-NANB cases. This cut-off is close to the “usual upper limit.” Excluding blood donors having antibodies to the hepatitis B core antigen (anti-HBc) as a paradoxical co-variable of infectivity for PTH-NANB would prevent another 20-30 percent of PTH-NANB cases, was subsequently discovered. Surrogate testing was used to screen blood donors for ALT and anti-HBc in order to avoid PTH-NANB (Wieland SF and Chisari FV, 2005).
Three European investigations, on the other hand, found no link between donor anti-HBc and recipient PTH-NANB. Surrogate screening had a number of issues, including high rejection rates of donor blood (up to 3 percent), issues with standardisation of ALT cut-off values, non-specificity of antiHBc test results, and rejection of rejected donors who had to be explained anti-HBc positivity as a sign of passed HBV infection as well as a sign of probable PTH-NANB infectivity. Following the implementation of anti-HCV testing, the question of whether or not to continue surrogate testing of blood donors arose. The incidence of PTH-NANB was not significantly different in two consecutive recipient groups, one receiving blood products that were not surrogate screened and the other receiving blood products that were surrogate screened for the surrogate markers ALT and anti-HBc, according to a study on PTH-NANB in Spain (Thomson BJ and Finch RG, 2005).
A Canadian research found that the HCV-PTH incidence was similar in 1909 receivers of “surrogate tested” blood and 1880 recipients of “surrogate-non tested” blood: 1.6-2.7 per 1000, respectively. There was no added utility for ALT testing for the prevention of PTH, including HCV and hepatitis non-A-C, in a meta-analysis of five prospective PTHNANB trials in Europe, in which the preserved donor samples were retrospectively tested for anti-HCV. The Food and Drug Administration (FDA) in the US never required ALT testing, although it was implemented by blood banks in 1986 in response to AIDS-related difficulties and lawsuits. Residual HBV transmission was not prevented by ALT testing, according to a study of 2.3 million donations and 34 well-documented cases of post-transfusion HBV and HCV. Three cases of HCV window phase donations could theoretically have been traced among 1 million donations, given the HCV incidence among repeat donors in the USA donor population. Based on these findings, as well as the estimates from the two main American studies, an NIH consensus committee in 1995 concluded, and FDA subsequently confirmed, that ALT testing would be phased out. However, ALT testing is still carried out in several European nations (Liaw YF, 1995).
Anti-HCV testing of blood donors
When anti-HCV testing was established, four prospective studies on PTH- NANB found that screening blood donors with ELISA-1 might prevent 50%-90% of PTH-NANB. The ELISA-1 had a severe flaw: It had a poor sensitivity and a lengthy window before antibodies appeared in early infection, often up to a year after transfusion infection. With the development of ELISA-2/3, blood donor screening became more sensitive (Zeisel MB, 2013). In a study comparing first and second generation tests in a population of 12,804 donors in the US, 44 RIBA-positive donors were found with ELISA-1, compared to 61 with ELISA-2, resulting in a 28 percent increase in sensitivity. When ELISA-1 negative blood donors were retested with ELISA-2 and RIBA in another trial in the United States, 39/46 872 (0.08 percent) were discovered positive. Re-analysis of sera from previously conducted prospective trials showed this enhanced sensitivity, as predicted. Anti-HCV positive (infected) donors were tracked in 81 percent vs. 93 percent of instances of HCV transmission to recipients in the US, according to ELISA-1 and ELISA-2 examination of preserved blood samples from donors participating in a multicentre prospective research. Thus, following the adoption of ELISA-1 screening, the residual estimated incidence rate of PTH-C would be 11/1232 (0.9 percent), and after the introduction of ELISA-2 screening, 4/1232 (0.3 percent). In a donor group that had previously tested negative in ELISA-2, ELISA-3 did not discover any more HCV-infected persons, although it did detect HCV antibodies sooner in certain patients with acute HCV infection. Second-generation and third-generation recombinant immunoblot tests were much less sensitive than ELISA-2 and ELISA-3 (Pagliaro L, et al., 1999).
Residual HCV infection
Blood given during the window period, i.e. when a donor who has just acquired HCV infection has not yet established detectable anti-HCV antibodies, poses the greatest risk of HCV transmission through cellular blood products. The average anti-HCV window time with second- and third-generation anti-HCV testing is around 12 weeks, although it may persist up to 27 weeks in certain situations. The residual risk of HCV transmission to recipients of cellular products may be approximated as 1 in 1,000 transfusions based on this window period and the prevalence of new HCV infections among donors who give often (95 percent confidence interval 28,000 to 2,88,000). The number is likely lower in Europe (Mohamed AA, et al., 2015).
Testing plasma pools and minipools for HCV nucleic acid amplification method (NAT)
The discovery that plasma production pools may include HCV RNA as a consequence of donations made within the anti-HCV window period prompted European regulatory organizations to take action. The commission on proprietary and medical goods ordered in 1994 that anti-HCV antibodies be evaluated in all plasma pools used in the manufacture of medicinal products as a quality control tool. However, the relative insensitivity of this anti-HCV testing was questioned due to the dilution impact of pooling 2000-20,000 plasma donations, and CPMP required HCV RNA testing by NAT of the manufacturing plasma pools to be effective. Because cellular blood components such as red cells and platelets are not considered pharmaceutical goods under EU laws, this testing for HCV NAT excludes them. If a manufacturing pool is confirmed to be HCV NAT positive, traceability of the likely infected donor is required; however given the vast number of donors to a plasma manufacturing pool, this is very difficult. If one of the donors is found to be HCV RNA positive, the receivers of the cellular products, who have already been transfused owing to the substantially shorter shelf life, will be notified (Kim WR, 2002).
The notion of HCV NAT testing in minipools was born out of these practical considerations and the possible loss of vast pools of human plasma in the event of an HCV NAT positive result. Before entering the manufacturing pool for fractionation, samples of 48-128 donors are pooled and screened for HCV RNA, depending on the HCV NAT system. It is being discussed, mostly in Germany, to undertake HCV NAT before to the release of cellular goods using minipool testing. HCV NAT can detect 50- 100 Genome Equivalents (Geq) per ml in most cases, while the CPMP needs a NAT test to identify 100 IU/ml, or roughly 270 Geq/ml. Because of the dilution impact of the test in pooled samples, German rules also set the HCV NAT sensitivity per donor at 5000 IU/ml (Suzuki T, et al., 2007). The Paul Erhlich Institute (PEI) will also request the release of HCV NAT-affected cellular products. During the anti-HCV negative window period, HCV RNA viral load may reach 106-l09 RNA copies per ml, however lower viral titers are more likely. HCV infectivity during the window period would be reduced by 72 percent if HCV NAT could be performed on individual undiluted blood donations. Given the low dilutions of the minipool format, the overall sensitivity of HCV NAT of about 104 Geq/ml is likely to be nearly as effective in reality. The cost per avoided case of HCV transmission by cellular materials given within the window period might be up to 2 million Euros in geographical locations with a low prevalence of HCV, such as the Netherlands. These expenditures per case averted may be lower in southern European areas, where the incidence is greater. HCV NAT testing is not as accurate as serological infectious disease testing, which blood banks currently use. The latter uses completely automated methods with extremely low rates of false positives (0.05%-0.2%), while HCV NAT testing is currently done using partially manual systems built for low throughput hospital testing and recognized for its lack of specificity. This makes the introduction of HCV NAT logistically challenging, particularly if it’s employed to release platelets with a 5-day shelf life (Szabó E, et al., 2003).
Conclusion
HCV is a new illness in India, with long-term consequences that will be realized in the next decades. It’s a pathogen that is already accountable for a large percentage of liver illness in India’s varied areas. Sterilization and reuse of needles should be discouraged, and strict blood banking legislation should be enacted. All of this is impossible without a greater public understanding of the scope and consequences of this chronic illness, as well as its route of transmission. Health officials must keep HCV on their radar as a disease that has the potential to cause major morbidity and death in the future.
References
- Memon MI, Memon MA. Hepatitis C: An epidemiological review. J Viral Hepat. 2002; 9(2): 84-100.
[Crossref] [Google scholar] [Pubmed]
- Bonkovsky HL, Mehta S. Hepatitis C: A review and update. J Am Acad Dermatol. 2001; 47(12): 610-647.
[Crossref] [Google scholar] [Pubmed]
- Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: A systematic review. JAMA. 2014; 312(6): 631-640.
[Crossref] [Google scholar] [Pubmed]
- Kamal SM. Acute hepatitis C: A systematic review. Am J Gastroenterol. 2008; 103(5): 1283-1297.
[Crossref] [Google scholar] [Pubmed]
- Modi AA, Liang TJ. Hepatitis C: A clinical review. Oral Dis. 2008; 14(1): 10-14.
[Crossref] [Google scholar] [Pubmed]
- Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999; 31: 17-24.
[Crossref] [Google scholar] [Pubmed]
- Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997; 26(S3): 62S-5S.
[Crossref] [Google scholar] [Pubmed]
- Alberti A, Benvegnu L. Management of hepatitis C. J Hepatol. 2003; 38: 104-118.
[Crossref] [Google scholar] [Pubmed]
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014; 61(1): S58-68.
[Crossref] [Google scholar] [Pubmed]
- Wang LS, D'Souza LS, Jacobson IM. Hepatitis C-a clinical review. J Med Virol. 2016; 88(11): 1844-1855.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001; 345(1): 41-52.
[Crossref] [Google scholar] [Pubmed]
- Seeff LB. Natural history of hepatitis C. Hepatology. 1997; 26(S3): 21S-8S.
[Crossref] [Google scholar] [Pubmed]
- Poynard T, Yuen MF, Ratzin V, Lai CL. Viral hepatitis C. Lancet. 2003; 362(9401): 2095-2100.
[Crossref] [Google scholar] [Pubmed]
- Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008; 372(9635): 321-332.
[Crossref] [Google scholar] [Pubmed]
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011; 364(25): 2429-2438.
[Crossref] [Google scholar] [Pubmed]
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005; 9(3): 383-398.
[Crossref] [Google scholar] [Pubmed]
- Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004; 39(4): 1147-1171.
[Crossref] [Google scholar] [Pubmed]
- Orland JR, Wright TL, Cooper S. Acute hepatitis C. Hepatology. 2001; 33(2): 321-327.
[Crossref] [Google scholar] [Pubmed]
- Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002; 36(S1): S30-S34.
[Crossref] [Google scholar] [Pubmed]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005; 5(3): 215-229.
[Crossref] [Google scholar] [Pubmed]
- Wilkins T, Malcolm JK, Raina D, Schade RR. Hepatitis C: Diagnosis and treatment. Am Fam Physician. 2010; 81(11): 1351-1357.
[Google scholar] [Pubmed]
- McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004; 10(2 Suppl): S21-S29.
[Google scholar] [Pubmed]
- Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002; 36(5B): S93-S98.
[Crossref] [Google scholar] [Pubmed]
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002; 36(S1): S237-S244.
[Crossref] [Google scholar] [Pubmed]
- Mukhopadhya A. Hepatitis C in India. J Biosci. 2008; 33: 465-473.
[Crossref] [Google scholar] [Pubmed]
- Seeff LB. The history of the “natural history” of hepatitis C (1968-2009). Liver Int. 2009; 29: 89-99.
[Crossref] [Google scholar] [Pubmed]
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011; 17(2): 107-115.
[Crossref] [Google scholar] [Pubmed]
- Chander G, Sulkowski MS, Jenckes MW, Torbenson MS, Herlong HF, Bass EB, et al. Treatment of chronic hepatitis C: A systematic review. Hepatology. 2002; 36(5B): S135-S144.
[Crossref] [Google scholar] [Pubmed]
- Paterson BL, Backmund M, Hirsch G, Yim C. The depiction of stigmatization in research about hepatitis C. Int J Drug Policy. 2007; 18(5): 364-373.
[Crossref] [Google scholar] [Pubmed]
- Thomas DL. Global control of hepatitis C: Where challenge meets opportunity. Nat Med. 2013; 19(7): 850-858.
[Crossref] [Google scholar] [Pubmed]
- de Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005; 436(7053): 953-960.
[Crossref] [Google scholar] [Pubmed]
- Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin North Am. 2015; 44(4): 717-734.
[Crossref] [Google scholar] [Pubmed]
- Simmonds P. The origin of hepatitis C virus. Hepatitis C virus: From molecular virology to antiviral therapy. 2013: 1-5.
[Crossref] [Google scholar] [Pubmed]
- Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007; 13(17): 2436.
[Crossref] [Google scholar] [Pubmed]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005; 5(9): 558-567.
[Crossref] [Google scholar] [Pubmed]
- Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000; 13(2): 223-235.
[Crossref] [Google scholar] [Pubmed]
- Maasoumy B, Wedemeyer H. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol. 2012; 26(4): 401-412.
[Crossref] [Google scholar] [Pubmed]
- González-Grande R, Jiménez-Pérez M, Arjona CG, Torres JM. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016; 22(4): 1421. [Crossref]
[Google scholar] [Pubmed]
- McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int. 2012; 32: 151-156.
[Crossref] [Google scholar] [Pubmed]
- Manns MP, Buti M, Gane ED, Pawlotsky JM, Razavi H, Terrault N, et al. Hepatitis C virus infection. Nat Rev Dis Primers. 2017; 3(1): 1-9.
[Crossref] [Google scholar] [Pubmed]
- Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: When East meets West. J Gastroenterol Hepatol. 2009; 24(3): 336-345.
[Crossref] [Google scholar] [Pubmed]
- Giannini C, Brechot C. Hepatitis C virus biology. Cell Death Differ. 2003; 10(1): S27-S38.
[Crossref] [Google scholar] [Pubmed]
- Kato N. Molecular virology of hepatitis C virus. Acta Medica Okayama. 2001; 55(3): 133-160.
[Crossref] [Google scholar] [Pubmed]
- Cuthbert JA. Hepatitis C: Progress and problems. Clin Microbiol Rev. 1994; 7(4): 505-532.
[Crossref] [Google scholar] [Pubmed]
- Li HC, Lo SY. Hepatitis C virus: Virology, diagnosis and treatment. World J Gastroenterol. 2015; 7(10): 1377.
[Crossref] [Google scholar] [Pubmed]
- Gumber SC, Chopra S. Hepatitis C: A multifaceted disease: Review of extrahepatic manifestations. Ann Intern Med. 1995; 123(8): 615-620.
[Crossref] [Google scholar] [Pubmed]
- Bertino G, Ardiri A, Proiti M, Rigano G, Frazzetto E, Demma S, et al. Chronic hepatitis C: This and the new era of treatment. World J Gastroenterol. 2016; 8(2): 92.
[Crossref] [Google scholar] [Pubmed]
- Dubuisson J. Hepatitis C virus proteins. World J Gastroenterol. 2007; 13(17): 2406.
[Crossref] [Google scholar] [Pubmed]
- Zeuzem S, Berg T, Moeller B, Hinrichsen H, Mauss S, Wedemeyer H, et al. Expert opinion on the treatment of patients with chronic hepatitis C. J Viral Hepat. 2009; 16(2): 75-90.
[Crossref] [Google scholar] [Pubmed]
- Houghton M. Discovery of the hepatitis C virus. Liver Int. 2009; 29: 82-88.
[Crossref] [Google scholar] [Pubmed]
- Wieland SF, Chisari FV. Stealth and cunning: Hepatitis B and hepatitis C viruses. J Virol. 2005; 79(15): 9369-9380.
[Crossref] [Google scholar] [Pubmed]
- Thomson BJ, Finch RG. Hepatitis C virus infection. Clin Microbiol Infect. 2005; 11(2): 86-94.
[Crossref] [Google scholar] [Pubmed]
- Liaw YF. Role of hepatitis C virus in dual and triple hepatitis virus infection. Hepatology. 1995; 22(4): 1101-1108.
[Crossref] [Google scholar] [Pubmed]
- Zeisel MB, Felmlee DJ, Baumert TF. Hepatitis C virus entry. Hepatitis C virus: From molecular virology to antiviral therapy. Springer. 2013: 87-112.
[Crossref] [Google scholar] [Pubmed]
- Pagliaro L, Peri V, Linea C, Camma C, Giunta M, Magrin S. Natural history of chronic hepatitis C. Ital J Gastroenterol Hepatol. 1999; 31(1): 28-44.
[Google scholar] [Pubmed]
- Mohamed AA, Elbedewy TA, El-Serafy M, El-Toukhy N, Ahmed W, El Din ZA. Hepatitis C virus: A global view. World J Hepatol. 2015; 7(26): 2676.
[Crossref] [Google scholar] [Pubmed]
- Kim WR. Global epidemiology and burden of hepatitis C. Microbes Infect. 2002; 4(12): 1219-1225.
[Crossref] [Google scholar] [Pubmed]
- Suzuki T, Aizaki H, Murakami K, Shoji I, Wakita T. Molecular biology of hepatitis C virus. J Gastroenterol. 2007; 42: 411-423.
[Crossref] [Google scholar] [Pubmed]
- Szabó E, Lotz G, Páska C, Kiss A, Schaff Z. Viral hepatitis: New data on hepatitis C infection. Pathol Oncol Res. 2003; 9: 215-221.
[Crossref] [Google scholar] [Pubmed]
Author Info
Amulyaratna Behera*Citation: Behera A: Transfusion-Induced Hepatitis-C Virus Infections: Understanding the Current Indian and International Scenario
Received: 27-Apr-2023 Accepted: 22-May-2023 Published: 29-May-2023, DOI: 10.31858/0975-8453.14.5.349-356
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3