Research Article - (2023) Volume 14, Issue 1
Abstract
Depression is a neurological disorder that can affect any individual and manifests symptoms like prolonged periods of low moods and loss of appetite. The high incidence and suicide rates of depression pose a threat to daily life and social development. Researchers have identified the defective synthesis of serotonin (5-HT) as a critical risk factor for depression. Extant literature revealed several mechanisms affecting 5-HT levels, of which the Tryptophan Hydroxylase 2 ( TPH2 ) isozyme and tryptophan pathways are closely linked to depressive-like behaviors. In previous studies, patients taking the current antidepressants that target the serotonergic system experience many side effects and have high relapse rates after medicine withdrawal, suggesting that the current knowledge of the serotonergic system is insufficient. Mutations on the gene encoding the TPH2 enzyme, the first and rate-limiting factor on endogenous 5-HT synthesis, can lead to abnormal levels of synthesized 5-HT. TPH2 enzymatic activity depends on tryptophan, which also participates in the kynurenine pathway to form various neuroactive metabolites that can decrease 5-HT levels by drawing away tryptophan concentrations for 5-HT synthesis and destroying 5-HT both indirectly and directly. Potential treatments targeting these two mechanisms may increase 5-HT levels in patients with depression. However, current data is inadequate to confirm the feasibility of TPH2-and tryptophan pathway-based treatments, so more experiments are desired. Further investigation on TPH2 and tryptophan pathways in relation to depression may lead to a breakthrough in treatment development for depression by augmenting current medications.
Keywords
Depression, Serotonin (5-HT), Tryptophan hydroxylase 2 ( TPH2 ), Tryptophan (Trp)
Introduction
Depression, one of the most common mental disorders and the leading global cause of disability, has been reported to affect more than 264 million people worldwide (WHO, 2021). People with depression are 20 times more likely to commit suicide, suffering from loss of interest in life, low self-evaluation, and other symptoms (Roser M and Ritchie H, 2018). A combination of biological, environmental, and psychological factors contributes to depression. One of the biological factors is serotonin (5-hydroxytryptamine, 5-HT), a neurotransmitter that plays critical regulatory roles in humans. Many of its functions are linked to the symptoms of depression. Recent studies in mice showed that low 5-HT levels are linked to loss of appetite and low moods, some of the main symptoms of depression. The current mainstream treatment for depression combines psychotherapy with medications to alleviate such 5-HT-induced symptoms.
Most medications, such as Selective Serotonin Reuptake Inhibitors (SSRIs), are antidepressants that reduce symptoms of depression primarily by regulating 5-HT levels in the brain. Although effective in treating depression, many medications, including SSRIs, mostly influence 5-HT levels only by interfering with the 5-HT reuptake, which may explain the low response and remission rates after treatment. Patients with depression who take antidepressants still have a recurrence rate of 85% within a decade (Baldessarini R, 2013), and up to 30% of these patients are treatment-resistant (Jaffe DH, et al., 2019), indicating that current medical treatments are limited by their similar and specific function on modulating 5-HT levels. Nevertheless, though limited, these medications can help improve symptoms of depression. Therefore, it is cardinal to study multiple aspects of the 5-HT pathway besides 5-HT reuptake that are involved in depression to develop a better and more holistic medical treatment method. In this review, we will analyze 5-HT synthesis as a potential biological cause of depression and a mechanism to target to cure patients.
TPH2 is a critical rate-limiting enzyme in serotonin formation, responsible for converting Tryptophan (Trp) into 5-HTP, the precursor for serotonin. Trp serves as the raw material for several pathways, including 5-HT synthesis. Research on Trp in relation to depression is relatively recent and in progress to understanding the biological basis of depression. This paper highlights the relationship between TPH2 and Trp and 5-HT levels in depres sion. There is insufficient data and application in a clinical setting supporting a direct and clear connection between the two factors and depression, but existing knowledge outlines a potential intersection of TPH2 and Trp and depression. Therefore, more research conducted on these two particular aspects may aid current 5-HT-related medical treatments for depression.
Materials and Methods
TRP and TPH2 regulate 5-HT levels in depression
5-HT is both a hormone and monoaminergic neurotransmitter that can be found in the enterochromaffin cells of the gastrointestinal tract, Central Nervous System (CNS), and platelets in the human body (Kling A, 2013). It plays several regulatory roles that are critical to the normal functions of the human body. Abnormalities in 5-HT synthesis in CNS are linked to several neurological diseases, especially depression, in many aspects, and this relationship is extensively studied to understand the causes of depression. Related studies have shown that low 5-HT levels due to a deficiency in 5-HT formation can give rise to depression-associated symptoms (Cowen PJ, Browning M, 2015). In addition, statistics show that women have a higher incidence of depression than men, which may correlate to the finding that the rate of 5-HT formation in healthy women is about 52% lower than that in healthy men (Nishizawa S, et al., 1997). Moreover, many of the symptoms of depression are signs of a dysfunctional 5-HT pathway. For example, 5-HT is responsible for synthesizing melatonin, which regulates circadian rhythms and controls appetite (Höglund E, et al., 2019). Patients with depression usually have irregular sleep cycles and a loss of appetite, corresponding to malfunctions in 5-HT pathways. Therefore, the connections between 5-HT pathways, particularly its synthesis, and depression are worth further investigation to understanding depression. Polymorphisms of the TPH2 gene and Trp are two crucial factors in the 5-HT synthesis pathway that influence 5-HT levels and are discussed in this review.
TPH2 mutations result in impaired 5-HT synthesis
Tryptophan hydroxylase 2 ( TPH2 ), an indispensable rate-limiting isozyme in the process of 5-HT synthesis, is mainly expressed in the serotonergic neurons in the brain. The gene that encodes TPH2 is located on chromosome 12 in humans and chromosome 10 in mice. Existing studies showed that mice with mutations on TPH2 exhibit significantly lower levels of 5-HT synthesis and increased depression-like behaviors (Belmaker RH, et al., 2008). Due to the randomness and uncontrollability of mutations, the genetic causes of abnormal TPH2 functions are also diverse and illustrated in Figure 1. Besides supervising the 5-HT levels directly in patients, quantifying the expression level of TPH2 can also serve as an indirect indicator when studying the relationship between 5-HT levels and depression. Lower levels of expression of the enzyme can also cause a serotonergic deficiency.
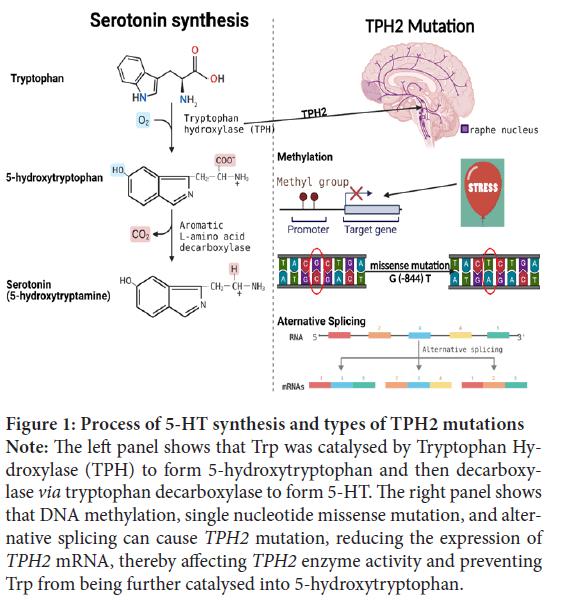
Figure 1: Process of 5-HT synthesis and types of TPH2 mutations
Note: The left panel shows that Trp was catalysed by Tryptophan Hydroxylase (TPH) to form 5-hydroxytryptophan and then decarboxylase via tryptophan decarboxylase to form 5-HT. The right panel shows
that DNA methylation, single nucleotide missense mutation, and alternative splicing can cause TPH2 mutation, reducing the expression of TPH2 mRNA, thereby affecting TPH2 enzyme activity and preventing
Trp from being further catalysed into 5-hydroxytryptophan.
TPH2 promoter methylation causes a decrease in TPH2 expression
Klengel T, et al., 2014 conjecture that TPH2 promoter methylation leads to a decrease in TPH2 expression. To verify this hypothesis, the methylation status of the TPH2 promoter region in controls and patients with depression was detected by methylation-specific polymerase chain reaction. The results showed that compared with normal people, depressed patients had a higher methylation proportion of the THP2 promoter and thus a lower TPH2 mRNA expression level (Zhang Y, et al., 2015). Chen Y, et al., 2017 compared 5-HT levels between healthy and stress-treated rats using Enzyme-Linked Immunosorbent Assay and found that less 5-HT formed in the brains of stress-treated rats. Using western blot and immunohisto-chemistry, Chen Y, et al., 2017 also documented that the TPH2 expression levels were significantly lower in stress-treated rats than those of healthy rats, with only 30% of the expression of TPH2 mRNA in healthy rats. Further studies using MSP-PCR assay explain this phenomenon: The higher methylation probability in the TPH2 promoter region in stress-treated rats results in decreased TPH2 expression (Chen Y, et al., 2017). Consequently, stress can cause methylation in the TPH2 promoter region as an environmental factor. The causes of TPH2 promoter methylation are manifold, but the data proves that a decrease in TPH2 expression caused by mutations will indirectly induce depression since insufficient TPH2 lowers 5-HT levels, giving rise to depression symptoms such as loss of attention and interest.
Alternative splicing in the intron regions of TPH2 affects its enzymatic activity
Single Nucleotide Polymorphisms (SNPs) located in the intron regions of the TPH2 can also affect the synthesis of 5-HT, decreasing TPH2 enzymatic activity via alternative splicing. The wild-type human TPH2 gene has 11 exons, while the truncated transcript Q8N1X9, formed by alternatively spliced SNP, contains only 6 exons (Ota T, et al., 2004). The first five exons of Q8N1X9 are the same as those in the normal TPH2 gene, but the sixth exon of Q8N1X9 is within the range of the fifth intron on the normal TPH2 gene. Hence, the Q8N1X9 mutant is possibly caused by alternative splicing due to a mutation on the fifth intron of the normal TPH2 gene (Ota T, et al., 2004). Another experiment measured the effect of alternative splicing on TPH2 activity by co-transfecting full-length cDNA of Q8N1X9 (truncated TPH2 ) with normal TPH2 by monitoring 5-HT production (Zhang X, et al., 2004). Experimental results show that the truncated TPH2 alone loses its enzymatic function in the cell and becomes incapable of catalyzing 5-HT synthesis, negatively impacting the catalytic efficiency of TPH2 .
SNPs exist not only on the intron of the TPH2 gene but also in other regions of the TPH2 gene, but not every SNP is associated with depression. One study that focused on analyzing SNP located on exon 7 of the TPH2 gene found that an allele was more likely to mutate to G in patients with depression (Ke L, et al., 2006). The SNP located on exon 7 in the TPH2 gene may be related to other neurological diseases other than depression, so further polymorphism studies on the TPH2 regulatory regions and adjacent genes may add to understanding other mental disorders.
TPH2 mutation affects amygdala activity resulting in low 5-HT levels
Previously described mutations in the TPH2 influence 5-HT levels by causing functional changes in TPH2 enzymatic activity through DNA methylation, alternatively splicing, and missense mutations. However, it has been demonstrated that SNP (G (-844) T) on the proximal promoter region of the TPH2 regulates 5-HT levels by influencing the amygdala’s reactivity, which is a part of the brain to modulate emotions and is entirely innervated by 5-HT projections. Data show that the amygdala activity of TPH2 T allele carriers is more potent than that of G allele homozygotes (Brown SM, et al., 2005). Combined with another 5-HTTLPR study (Hariri AR, et al., 2005), which confirmed that the increase in 5-HT levels is related to the decrease in 5-HT reuptake caused by the increased activity of the amygdala, these experiments together show that the mutation of the TPH2 enhances the amygdala activity, TPH2 expression, and the subsequent 5-HT synthesis. Meanwhile, the density of serotonergic fibers in the dorsal area of the amygdala is extremely high, which indirectly corroborates the influence of amygdala activity on 5-HT levels (Smith HR, et al., 1999).
In short, different types of TPH2 mutations mentioned above are all related to the decline of 5-HT levels from environmental and biological perspectives. Nevertheless, more data are required to clarify the linkage between TPH2 expression and the pathogenesis of depression, and further studies on this connection can provide insights for treatment development. Combined with existing drugs, new medications targeting TPH2 can help improve the therapeutic effect of the treatments for depression.
Intersections between TRP pathways and 5-HT pathways
TRP availability affects 5-HT formation: Tryptophan (Trp) is an essential and the least abundant amino acid in the human body that can be obtained only through dietary means and about 90%-95% of Trp is found in the plasma (Badawy AA, 2009; Li Y, et al., 2017). TRP is the precursor in both the 5-HT and the Kynurenine Pathway (KP), and it can cross the blood-brain barrier to enter these pathways (Gostner JM, et al., 2020). Less than 5% of the Trp not used for protein synthesis is involved in the endogenous 5-HT synthesis. TRP availability in the brain is strongly and positively correlated with 5-HT synthesis but only in hindbrain raphe areas with neurons encoding the TPH2 gene for vertebrates. The first and rate-limiting step of 5-HT synthesis involves both the TPH2 isozyme and TRP where the TPH enzyme converts Trp into 5-HTP, which is immediately decarboxylated into 5-HT (Figure 2). The enzymatic activity of TPH depends largely on the Trp availability, for the enzymes are unsaturated with substrates (Höglund E, et al., 2019; Badawy AA, 2009). Hence, TRP availability, along with TPH2 function, plays a critical role in brain 5-HT formation, and increasing Trp availability may help raise brain 5-HT levels. However, excessive TRP availability may also decrease 5-HT levels by inhibiting the TPH2 isozyme. Increasing doses of the Trp in vivoand in vitroled to lower 5-HT levels (Badawy AA, 2009). Therefore, there may be an optimum Trp concentration for a maximum 5-HT formation. Investigating this quantitative relationship between Trp and 5-HT may help in designing treatments for depression.
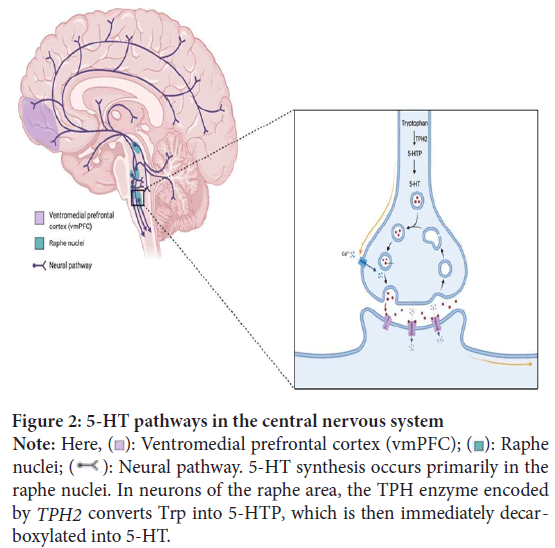
Figure 2: 2: 5-HT pathways in the central nervous system
Note: Here,  : Ventromedial prefrontal cortex (vmPFC);
: Ventromedial prefrontal cortex (vmPFC);  : Raphe
nuclei;
: Raphe
nuclei;  : Neural pathway. 5-HT synthesis occurs primarily in the
raphe nuclei. In neurons of the raphe area, the TPH enzyme encoded by TPH2 converts Trp into 5-HTP, which is then immediately decarboxylated into 5-HT.
: Neural pathway. 5-HT synthesis occurs primarily in the
raphe nuclei. In neurons of the raphe area, the TPH enzyme encoded by TPH2 converts Trp into 5-HTP, which is then immediately decarboxylated into 5-HT.
Stimulus initiating the kynurenine pathway: Brain Trp availability also depends on the KP. About 95% of the dietary Trp enters the KP to produce neurotrophic and neurotoxic acids. This pathway (Figure 3) occurs primarily in the liver and is initiated when hepatic and extrahepatic enzymes-Tryptophan 2,3-Dioxygenase (TDO) and Indoleamine 2,3-Dioxygenase (IDO), respectively-are activated (Höglund E, et al., 2019). IDO and TDO are activated by different internal and external stimuli. Most TDO is located in the liver and primarily responds to hormonal signals. TDO can be activated by glucocorticoids, cortisol, glucagon, and Trp and be inhibited by NAD+, the product of the KP (Badawy AA, 2009; Remus JL and Dantzer R, 2016). Höglund E, et al., 2019, found that acute stress, inflammation, and infection can induce the secretion of glucocorticoids, which then initiates the KP (Höglund E, et al., 2019). TDO may play a role in this process by responding to the glucocorticoids and then starting Trp breakdown. In contrast, IDO starts the KP via immune activation by interferons (Badawy AA, 2009). The activation of IDO seems to be involved in both inflammation and neurological diseases. When activated, IDO depletes Trp concentrations and consequently reduces 5-HT and melatonin production, potentially inducing depression and influencing moods (Mellor Al, et al., 2017). Moreover, tests on mice with chronic inflammatory diseases show that IDO suppresses adaptive and innate immunity to improve tolerance. Consequently, IDO may also play a role in diseases related to inflammation. Patients with Inflammatory Bowel Disease (IBD) show a higher incidence rate of depression, which may represent the link between IDO and depression with 5-HT as an intermediary point (Chen LM, et al., 2021). Since IDO relates to both depression and inflammation diseases, studies on IDO and the link between inflammation and depression may help patients with inflammation diseases as a risk factor for depression. In particular, indoleamine 2,3-dioxygenase-1 (IDO-1) is the main enzyme associated with starting the Trp catabolism. IDO-1 not only binds to Trp but also 5-HT and can damage both (Gostner JM, et al., 2020). Therefore, inhibiting the IDO-1 enzyme can potentially elevate 5-HT and melatonin levels and reduce symptoms of depression like irregular sleep cycles.
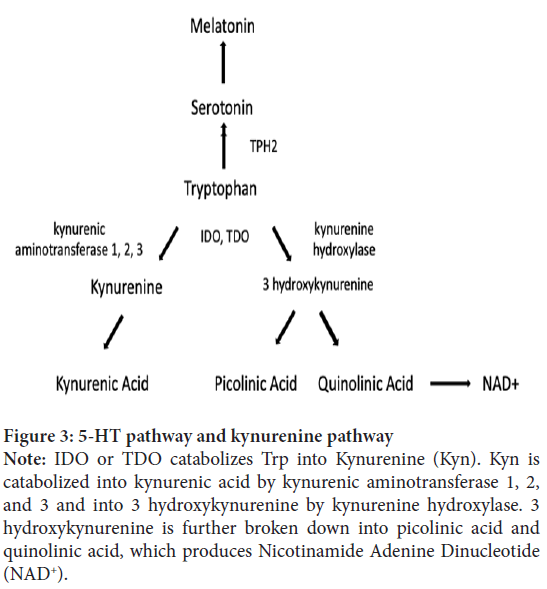
Figure 3: 5-HT pathway and kynurenine pathway
Note: IDO or TDO catabolizes Trp into Kynurenine (Kyn). Kyn is
catabolized into kynurenic acid by kynurenic aminotransferase 1, 2,
and 3 and into 3 hydroxykynurenine by kynurenine hydroxylase. 3
hydroxykynurenine is further broken down into picolinic acid and
quinolinic acid, which produces Nicotinamide Adenine Dinucleotide
(NAD+).
A low level of 5-HT, acute and chronic stress and inflammation can all cause Trp to be shunted to the KP via activation of IDO or TDO and decrease tryptophan availability for brain 5-HT synthesis (Höglund E, et al., 2019). Once IDO and TDO are activated, several neuroactive metabolites are produced, of which quinolinic acid and kynurenic acid is also linked to depression.
Products of the kynurenine pathway involved in neuroprogression and depression: Quinolinic acid (QUIN) is a neurotoxic acid primarily produced in microglia and an agonist on the N-Methyl-D-Aspartate (NMDA) receptor. When bound to the NMDA receptor, QUIN promotes the release of glutamate, a major excitatory neurotransmitter (Remus JL, Dantzer R, 2016). Further breakdown of QUIN produces neurotoxic compounds that are neurodegenerative and neurotoxic. Mellor AL, et al., 2017 have shown that these neurotoxic compounds cause brain dysfunctions in depressive patients (Höglund E, et al., 2019). Also, patients with depression have lower Kynurenic Acid (KYNA) to QUIN ratios, suggesting that an imbalance of KYNA and QUIN concentrations may have connections with depressive-like behaviors (Remus JL, Dantzer R, 2016; Erabi H, et al., 2020). Depressive patients who committed suicide showed increased QUIN levels in microglial cells of the subgenual Anterior Cingulate Cortex (sACC) and anterior Midcingulate Cortex (aMCC) (Kruse JL, et al., 2019). Moreover, increased QUIN levels are linked to several depression symptoms, including learning difficulties (Müller N and Schwarz MJ, 2007). As a result, inhibiting the synthesis of QUIN can potentially alleviate symptoms of depression and increase response rates. In addition, QUIN increases the release of glutamate, which can have excitotoxic effects that cause the neuroprogression of depression (Bansal Y, et al., 2019). Extant research shows that patients with depression have elevated glutamate levels in the Cerebrospinal Fluid (CSF) and the plasma, corresponding to the positive correlation between QUIN and glutamate levels. However, some studies indicate that treatment-resistant patients have reduced glutamate levels in the CSF (Moriguchi S, et al., 2019). More studies on the connection between glutamate levels and depression can help establish consistency. Glutamatergic hyperfunction may affect serotonergic activity via excitotoxicity or other mechanisms, but existing research is insufficient to confirm this relationship right now (Müller N and Schwarz MJ, 2007). Exploring the serotonin-glutamine interaction may further clarify the connections between Trp and 5-HT and between 5-HT and depression.
Kynurenic acid (KYNA) is one of the metabolites in the KP primarily produced in astrocytes, and it binds to the NMDA receptor at the glycine site as a neuroprotective antagonist, acting oppositely from QUIN. NMDA receptor antagonists can help raise extracellular 5-HT levels and promote 5-HT neurotransmission; therefore, increasing Trp usage for KYNA synthesis in the KP not only averts QUIN formation but may also alleviate symptoms of depression by increasing 5-HT concentrations (Müller N and Schwarz MJ, 2007). Moreover, when bound to the NMDA receptor, KYNA decreases the release of glutamate (Remus JL, Dantzer R, 2016; Rossi F, et al., 2019). Astrocyte, the site of KYNA synthesis, can reabsorb excessive glutamate to prevent excitotoxicity (Remus JL, Dantzer R, 2016), but it excludes the reuptake of glial cells-induced glutamate release. Hence, astrocytes may be ineffective in countering the QUIN-induced glutamate release. However, increasing KYNA synthesis can decrease glutamate levels and may counteract the overproduction of glutamate by QUIN and NMDA receptors.
Results and Discussion
Current and potential treatments for depression
Depression is not a new mental disorder that has just emerged in recent years. Many specific psychotherapy and antidepressants have been developed to treat depressive patients and achieved varying degrees of efficacy (Table 1). Many patients are resistant to antidepressants and suffer from several side effects. Levkovitz Y, et al., 2011 showed that antidepressants are effective to an extent, with 54% of depressive patients showing a reduction in their symptoms after being treated. However, a study from the same year proposed that 25%-40% of patients who recovered from depression will relapse within two years (Richards D, 2011). Moreover, 38% of depression patients taking antidepressants reported side effects such as sleepiness and weight gain (Cascade E, et al., 2011). When patients develop antidepressant resistance, psychotherapies and brain stimulation therapies work as adjunctive therapies to alleviating symptoms of depression. Since current medications for depression have several limitations and downsides, such as high relapse rates and severe side effects, developing medicine from new aspects is crucial to provide better treatment for depressive patients. TPH2 enzyme and Trp, which are related to 5-HT synthesis and depression, are two potential mechanisms that can be targeted to complement current treatments.
| Medication | Mechanism | Side Effects | Contraindication |
|---|---|---|---|
| Selective Serotonin Reuptake Inhibitors (SSRIs) | Block 5-HT reuptake | Nausea, anxiety, headache, dry mouth | Bleeding disorder, kidney disease, diabetes |
| Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) | Block NE and 5-HT reuptake | Nausea, anxiety, headache, insomnia | Heart disease and high blood pressure |
| Tricyclic Antidepressants (TCAs) | Block NE and 5-HT reuptake | Confusion, numbness, dizziness | Heart and liver disease |
| Monoamine Oxidase Inhibitors (MAOIs) | Inhibit 5-HT metabolites production | Nausea, dry mouth, constipation, diarrhea | Cardiovascular, liver, kidney diseases |
Note: NE: Norepinephrine
Table 1: Four mainstream categories for antidepressants and their corresponding caveats
Regulation of TPH2 activity and expression increases 5-HT synthesis
Since low 5-HT levels can induce depression, maintaining or increasing 5-HT production in the brain is the key to treating depression. Selective Serotonin Reuptake Inhibitor (SSRI) is a first-line antidepressant, boosting 5-HT levels in the brain through increasing synaptic 5HT transmission and inhibiting reuptake of 5-HT via selective receptors (Khushboo SB and Sharma BJ, 2007). Given the role of TPH2 in regulating 5-HT levels, increasing TPH2 activity and inducing TPH2 expression in patients can elevate 5-HT levels as a promising therapeutic proposal for depression.
Phosphorylation at serine (Ser) in the TPH2 improves its enzymatic activity
Post-Translational Modifications (PTMs) have been verified to change protein functions; thus, TPH2 enzymatic activity can also be regulated through PTMs. Studies have shown that phosphorylation at Ser19 and Ser104 in the TPH2 regulatory region enhances its stability and activity. Notably, phosphorylation at Ser58 within TPH1, an isoform of TPH2 , has been shown to increase 5-HT levels. Phosphorylation at Ser on TPH2 can produce similar results. Moreover, Tyrosine Hydroxylase (TH) has highly homologous catalytic domains with TPH2 sharing similar catalytic mechanisms; phosphorylated Ser19 in TH also increases its enzymatic activity (Torrente MP, et al., 2012). Based on the study of increased enzyme activity resulting from serine phosphorylation of TPH1 and TH, PTMS-phosphorylation may regulate the stability and activity of TPH2 to increase the synthesis of 5-HT in the brain for the treatment of depression.
Natural metabolites of Vitamin D and pomegranate increase TPH2 expression
TPH2 expression increases not only the TPH2 gene modification but also some natural metabolites induction. A recent study showed that the expression of TPH2 mRNA in brain cells doubled at 10 nM 1,25-dihydroxy vitamin D3 (1,25 D), which is a metabolite of vitamin D3. Furthermore, Livingston S, et al., 2020 enhanced 1,25 D, increasing TPH2 mRNA expression up to 2.5 fold by adding 10 µM urolithin A, a pomegranate metabolite. More surprisingly, adding urolithin A increased 3.5 fold 5-HT levels. Different from 1,25 D3, which regulates VDRE mediated gene expression through VDR, urolithin A regulates that by Adenosine Monophosphate-Activated Protein Kinase (AMPK). Interestingly, unlike 1,25 D3 and urolithin A, which increase TPH2 mRNA expression, short-term lithium treatment resulted in a 45% and 35% decrease in TPH2 mRNA and TPH2 protein expression, respectively. Although the addition of lithium resulted in reduced TPH2 expression, it did not impair 5-HT release. One possible explanation is that deficient TPH2 prompts Trp uptake in the synaptosomes, increasing 5-HT synthesis, which is a compensatory response. Following long-term lithium treatment, TPH2 mRNA expression was compensated, and 5-HT levels remained elevated (Scheuch K, et al., 2010). It seems that both long-term and short-term lithium treatments do not affect 5-HT levels increase in the brain, although there are some negative regulations of TPH2 expression in the early stage.
In conclusion, new technologies such as high-end proteomic methods can regulate TPH2 in vivo, increasing TPH2 activity and 5-HT levels in the brain to treat depression. Simultaneously, patients can take antidepressants with vitamin D and pomegranate, which contain natural metabolites that may enhance the therapeutic effect. In other words, the direct addition of metabolites such as 1,25 D3, urolithin A, and lithium when producing the antidepressants also enhances these drugs’ ability to increase 5-HT levels, preventing recurrence of depression.
Increasing brain Trp availability elevates 5-HT levels
Brain Trp availability for 5-HT synthesis is a predominant factor of endogenous 5-HT levels. This availability is largely affected by the Trp-Kyn pathway, and one way to maximize it is to increase Trp influx to the 5-HT pathway. Since Trp is not a human-made protein and can be only obtained via dietary intake, nutrition is a critical aspect to modulating Trp concentration in the human body and easing symptoms of depression. Lindseth G, et al., 2015 found that individuals with a high Trp diet had more positive moods than others with a low Trp diet in their experiment involving 25 healthy individuals over four days. Additionally, according to Zung’s Self-Rating Depression Scale (SDS), the group that consumed lower Trp reached the threshold for depression while the other group did not (Lindseth G, et al., 2015). Furthermore, study discovered that after taking doses of hydrolysate, an egg protein rich in Trp, participants with low or high chronic stress demonstrated better mood and performance in the face of acute stress (Friedman M, 2018). Hence, existing literatures suggests that incorporating more Trp in daily meals or as a dietary supplement, like hydrolysate, may increase plasma Trp concentration and its influx to the brain and thus alleviate symptoms. Researchers also identified a significant improvement in mood and increased Trp transport to the brain in healthy participants who consumed hydrolyzed Trp rather than pure Trp. Research regarding dietary Trp and depression is inadequate. More experiments on the correlation between the different forms of dietary Trp and 5-HT deficiency-induced depression symptoms on MDD patients can propel the progress on various treatments for depression.
Besides directly targeting the increase of brain Trp availability, decreasing Trp’s participation in the Trp-Kyn pathway can make Trp available for brain 5-HT synthesis. TDO and IDO are enzymes responsible for initiating the KP; thus, inhibition of the enzymes may redirect plasma Trp to flow to the brain instead. As mentioned previously, IDO-1 is primarily responsible for the initiation of the KP and its activity. For its harmful effects on 5-HT and Trp levels, IDO-1, when inhibited, may increase 5-HT levels not only by increasing brain Trp availability but also by preventing IDO-1 from binding to 5-HT and destroying it. Several IDO-1 inhibitors such as 1-MT, coenzyme Q10, and desipramine were effective in improving depression symptoms. 1-MT is structurally like Trp and can halt the KP by substrate inhibition of IDO-1. Coenzyme Q10 helps regulate the Trp flow to the KP and 5-HT pathway, and desipramine can decrease expression of IDO-1 enzyme and interferons, in particular IFN-γ, which are activating factors of IDO-1 activity. All of these inhibitors have been proved to reduce depressive-like behaviors. Since IDO relates to inflammation signals, additional research and long-term application of IDO inhibitors may help with inflammation-associated depression. On the other hand, TDO inhibitors can also have therapeutic effects. Anti-glucocorticoid RU846 binds to the TDO in place of glucocorticoids, inhibiting TDO. At the same time, the expression of glucocorticoid receptors can lower cortisol concentrations and avoid TDO activation because of negative feedback. Currently, selective TDO inhibitors have been investigated but have not been used in a clinical setting (Qin Y, et al., 2018). Developing selective TDO inhibitors can focus both on inhibiting TDO and enhancing the expression of glucocorticoid receptors to drive future clinical applications.
Conclusion
Limited by its specificity on targeting one aspect of 5-HT levels and its side effects, current first-line medications like SSRIs have low efficacy in treating patients with depression. Also, after withdrawing from antidepressants, patients may revert to a deficient brain 5-HT synthesis and at risk of high relapse rates, giving rise to aggravated symptoms. The activity and expression levels of the catalytic isozyme TPH2 and the amino acid tryptophan can impact 5-HT synthesis and potentially be used to mediate it. Methylation, alternative splicing, and single nucleotide missense mutation of the TPH2 gene can lead to an 80% reduction in the functional activity of TPH2 , thus affecting 5-HT synthesis and inducing depression. Moreover, decreased Trp availability for brain 5-HT synthesis as a result of the kynurenine pathway correlates to depressive-like behaviors. Developing treatment that alters the tryptophan-kynurenine pathway and restores TPH2 enzymatic activity can aid current treatments and better treat depressive patients. More pre-clinical research is needed to verify the relationships between TPH2 and the KP and depression. Further research on these two links is vital to propel the progress of treatment development and understanding of depression to help cure patients.
References
- Depression. World Health Organization (WHO). 2021.
- Roser M, Ritchie H. Mental health. Our World in Data. 2018.
- Baldessarini R. Chemotherapy in psychiatry. Springer. 2013.
- Jaffe DH, Rive B, Denee TR. The humanistic and economic burden of treatment-resistant depression in Europe: A cross-sectional study. BMC psychiatry. 2019; 19(1): 1.
[Crossref] [Google scholar] [Pubmed]
- Kling A. 5-HT2A: A serotonin receptor with a possible role in joint diseases. DIVA. 2013.
- Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015; 14(2): 158.
[Crossref] [Google scholar] [Pubmed]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza SD, de Montigny C, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci USA. 1997; 94(10): 5308-5313.
[Crossref] [Google scholar] [Pubmed]
- Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front Endocrinol. 2019: 158.
[Crossref] [Google scholar] [Pubmed]
- Belmaker RH, Agam G, Bersudsky Y. Role of GSK3β in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008; 105(20): E23.
[Crossref] [Google scholar] [Pubmed]
- Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014; 80: 115-132.
[Crossref] [Google scholar] [Pubmed]
- Zhang Y, Chang Z, Chen J, Ling Y, Liu X, Feng Z, et al. Methylation of the tryptophan hydroxylase‑2 gene is associated with mRNA expression in patients with major depression with suicide attempts. Mol Med Rep. 2015; 12(2): 3184-3890.
[Crossref] [Google scholar] [Pubmed]
- Chen Y, Xu H, Zhu M, Liu K, Lin B, Luo R, et al. Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget. 2017; 8(38): 63247.
[Crossref] [Google scholar] [Pubmed]
- Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat genet. 2004; 36(1): 40-45.
[Crossref] [Google scholar] [Pubmed]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004; 305(5681): 217.
[Crossref] [Google scholar] [Pubmed]
- Ke L, Qi ZY, Ping Y, Ren CY. Effect of SNP at position 40237 in exon 7 of the TPH2 gene on susceptibility to suicide. Brain res. 2006; 1122(1): 24-26.
[Crossref] [Google scholar] [Pubmed]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, et al. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol psychiatry. 2005; 10(9): 884-888.
[Crossref] [Google scholar] [Pubmed]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005; 62(2): 146-152.
[Crossref] [Google scholar] [Pubmed]
- Smith HR, Daunais JB, Nader MA, Porrino LJ. Distribution of [3H] citalopram binding sites in the nonhuman primate brain. Ann N Y Acad Sci. 1999; 877: 700-702.
[Crossref] [Google scholar] [Pubmed]
- Badawy AA. Tryptophan metabolism: A versatile area providing multiple targets for pharmacological intervention. Egypt J Basic Clin Pharmacol. 2019; 9.
[Crossref] [Google scholar] [Pubmed]
- Li Y, Hu N, Yang D, Oxenkrug G, Yang Q. Regulating the balance between the kynurenine and serotonin pathways of tryptophan metabolism. FEBS J. 2017; 284(6): 948-966.
[Crossref] [Google scholar] [Pubmed]
- Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D. Tryptophan metabolism and related pathways in psychoneuroimmunology: The impact of nutrition and lifestyle. Neuropsychobiology. 2020; 79(1-2): 89-99. [Crossref]
[Google scholar] [Pubmed]
- Remus JL, Dantzer R. Inflammation models of depression in rodents: Relevance to psychotropic drug discovery. Int J Neuropsychopharmacol. 2016; 19(9).
[Crossref] [Google scholar] [Pubmed]
- Mellor AL, Lemos H, Huang L. Indoleamine 2, 3-dioxygenase and tolerance: where are we now? Front immunol. 2017; 8: 1360.
[Crossref] [Google scholar] [Pubmed]
- Chen LM, Bao CH, Wu Y, Liang SH, Wang D, Wu LY, et al. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J Neuroinflammation. 2021; 18(1): 1-3.
[Crossref] [Google scholar] [Pubmed]
- Erabi H, Okada G, Shibasaki C, Setoyama D, Kang D, Takamura M, et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Scientific reports. 2020; 10(1): 1-8.
[Crossref] [Google scholar] [Pubmed]
- Kruse JL, Cho JH, Olmstead R, Hwang L, Faull K, Eisenberger NI, et al. Kynurenine metabolism and inflammation-induced depressed mood: A human experimental study. Psychoneuroendocrinology. 2019; 109: 104371.
[Crossref] [Google scholar] [Pubmed]
- Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol psychiatry. 2007; 12(11): 988-1000.
[Crossref] [Google scholar] [Pubmed]
- Bansal Y, Singh R, Parhar I, Kuhad A, Soga T. Quinolinic acid and nuclear factor erythroid 2-related factor 2 in depression: Role in neuroprogression. Front pharmacol. 2019; 10: 452.
[Crossref] [Google scholar] [Pubmed]
- Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol psychiatry. 2019; 24(7): 952-964.
[Crossref] [Google scholar] [Pubmed]
- Rossi F, Miggiano R, Ferraris DM, Rizzi M. The synthesis of kynurenic acid in mammals: an updated kynurenine aminotransferase structural katalogue. Front Mol Biosci. 2019; 6: 7.
[Crossref] [Google scholar] [Pubmed]
- Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: A meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011; 72(4): 5964.
[Crossref] [Google scholar] [Pubmed]
- Richards D. Prevalence and clinical course of depression: A review. Clinical psychology review. 2011; 31(7): 1117-1125.
[Crossref] [Google scholar] [Pubmed]
- Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont). 2009; 6(2): 16.
[Google scholar] [Pubmed]
- Khushboo SB, Sharma BJ. Antidepressants: Mechanism of action, toxicity and possible amelioration. J Appl Biotechnol Bioeng. 2017; 3(5): 437-448.
- Torrente MP, Gelenberg AJ, Vrana KE. Boosting serotonin in the brain: is it time to revamp the treatment of depression? J Psychopharmacol. 2012; 26(5): 629-635.
[Crossref] [Google scholar] [Pubmed]
- Livingston S, Mallick S, Lucas DA, Sabir MS, Sabir ZL, Purdin H, et al. Pomegranate derivative urolithin A enhances vitamin D receptor signaling to amplify serotonin-related gene induction by 1, 25-dihydroxyvitamin D. Biochem Biophys Rep. 2020; 24: 100825.
[Crossref] [Google scholar] [Pubmed]
- Scheuch K, Höltje M, Budde H, Lautenschlager M, Heinz A, Ahnert-Hilger G, et al. Lithium modulates tryptophan hydroxylase 2 gene expression and serotonin release in primary cultures of serotonergic raphe neurons. Brain res. 2010; 1307: 14-21.
[Crossref] [Google scholar] [Pubmed]
- Lindseth G, Helland B, Caspers J. The effects of dietary tryptophan on affective disorders. Arch Psychiatr Nurs. 2015; 29(2): 102-107.
[Crossref] [Google scholar] [Pubmed]
- Friedman M. Analysis, nutrition, and health benefits of tryptophan. Int J Tryptophan Res. 2018.
[Crossref] [Google scholar] [Pubmed]
- Qin Y, Wang N, Zhang X, Han X, Zhai X, Lu Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab Brain Dis. 2018; 33(6): 1787-1800.
[Crossref] [Google scholar] [Pubmed]
Author Info
Qiyu Bian1* and Jiamei Wang22Department of Infection Biology, Annie Wright Schools, Tacoma, United States
Citation: Bian Q: Tryptophan Hydroxylase 2 and Tryptophan Mediate Depression by Regulating Serotonin Levels
Received: 02-Dec-2022 Accepted: 27-Dec-2022 Published: 03-Jan-2023, DOI: 10.31858/0975-8453.14.1.52-57
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






