Research Article - (2021) Volume 12, Issue 12
Why Pharmacovigilance and Adverse Drug Reaction Reporting Practices Lacks in Developing Countries: A Study from Pakistan
Muhammad Aiyaz Sharif*, Afaq Ahmed Kazi and Maria Angelica RweyemamuAbstract
Objective: Pharmacovigilance (PV) has the topmost significance in the world, but unluckily, it is not focused in the developing countries like Pakistan. The purpose for this research study is to measure the knowledge, attitude and practice of adverse drug reactions (ADR) reporting and explore the causes of poor ADR reporting behavior among Health Care Professionals (HCPs) and patients.
Material and methods: A Cross sectional research carried out in January-March 2021 in major cities of Pakistan. The separate questionnaires were adopted for HCPs and patients. Regression analysis using Statistical Package for Social Sciences (SPSS) was performed.
Results: 362 questionnaires were circulated and response rate was 83.4% (n=302). The results revealed that, only 18% of HCPs were aware of the term pharmacovigilance and understand its basic function. However more than 50% of the HCPs mentioned that ADR reporting is neither essential and nor compulsory in their clinical setups while 82.4% of them were not aware where to report ADR in their hospital.
90% of the patients did not have any knowledge and mentioned that their physicians never guided them about ADR reporting. Only 10% patients were aware about ADR but did not know how to report.
Conclusion: Above empirical findings suggest that HCPs have inadequate ADR reporting knowledge, practice and lack appropriate behavior, norm and realization of its importance in patient safety. However HCPs and patients have positive attitude towards ADR reporting and need awareness training, simplification of reporting mechanism and appropriate regulatory control. Awareness drive needs to be created in general population to sensitize them on their responsibility towards side effects reporting.
Keywords
Adverse Drug Reactions (ADRs), Pharmacovigilance (PV), Health Care Professionals (HCPs), Knowledge Attitude and Practice (KAP)
Introduction
Background of the study
Pharmacovigilance (PV) is one of the supreme vital disciplines across the world to safeguard patient and the proper usage of therapeutic agents. World health organization explains PV as “the skill and actions linking to the uncovering, valuation, understanding and avoidance of adverse drug reactions or any additional medicine related hazard” (WHO, 2018). These definitions could bound PV agendas to adverse drug reactions or develop them to embrace medicine related blunders, unsuitable usage, counterfeit drugs, and other quality related issues and deficiency of usefulness. Causativeness is being a serious issue; (WHO, 2002) compared diverse techniques of assessment. The actual definition of the concept of pharmacovigilance cast-off by different authors into their article are comprehensive or thin versions of the WHO published definition for ADRs and ADEs (Adverse Drug Events).
There are multiple barriers in successful implementation of pharmacovigilance in developing countries and amongst them top barriers are related with weak policies and infrastructure of pharmacovigilance, less capacity and limited sustenance towards application of pharmacovigilance actions and lethargic attitude of health care professionals towards pharmacovigilance (Ergün Y, et al., 2019). Subsequently there is a dire need of professional training on pharmacovigilance to address lack of knowledge and to improve their attitude towards spontaneous ADR reporting. This is evident in countries where there is a well-developed healthcare system, their reason for improvement was also proper training and they have improved both quality and quantity of adverse drug reporting.
Pharmacovigilance is considered as the top most priority in health-care across the globe, but unluckily this is nearly missing in Pakistan. Now it is universally accepted that health hazard or illness related mortality is rarely inescapable, but adverse drug reaction related death is now objectionable. Lack of proper faith of patient into health-care set ups upsurges the cost of any ADR twofold. There is now an imperative prerequisite to scheme PV programs for paramount care of patients and device the perception of medication carefulness within the health infrastructure system of Pakistan by connecting key stake-holders (Hussain R, et al., 2020).
Underreporting of ADRs by HCPs is being a big problem in Pakistan. Exploration of works with respect to KAP of health professionals about adverse drug reaction reporting in the research areas bring in no practical outcomes, which is supposed to improve pharmacovigilance practices (Jusot V, et al., 2020); Hence, it is decisive time to take such steps to minimize the gaps on ADR reporting. On the other hand there is a dire need to address all barriers which are responsible for inadequate practice of pharmacovigilance (Zou M, et al., 2021; Al Hail M, et al., 2018).
It is evident that implementation of proper pharmacovigilance system in healthcare setup significantly rise access to safe medicines and healthcare, but its integration in current public health- care remains a challenge in our country. The main barrier to pharmacovigilance integration is recognized as extraordinary patient load and inadequate capacities (Hussain, R, et al., 2021).
Study purpose
Pharmacovigilance is considered as the top most priority in health-care across the globe, but unluckily this is nearly missing in Pakistan. Now it is universally accepted that health hazard or illness related mortality is rarely inescapable, but adverse drug reaction related death is now objectionable. Lack of proper faith of patient into health-care set ups upsurges the cost of any ADR twofold. There is now an imperative prerequisite to scheme PV programs for paramount care of patients and device the perception of medication carefulness within the health infrastructure system of Pakistan by connecting key stake-holders.
Research gap
There are numerous work have been done to identify the knowledge and attitude of HCPs towards ADR but there is no single study available which has focused towards patients and general public, that is why there is a big need of increasing awareness of patients and general public together. Because we sense that, the first contact point of ADR is patient and if patient have proper awareness about how to report and where to report ADR so we may be able to reduce incidence of ADR in our community.
To the best of my information, there is no such research study available, which has focused towards HCPs who are dealing with chronic diseases, because their patients are supposed to take treatment for the long term and their patients are more prone towards adverse drug reactions.
Problem statement
PV is the discipline devoted to safety of medicines which are using in medical practice, grounded on practices from the medical practice, consequently producing understanding on unsafe effects of medications, equally at the individual patient and overall population level, that would ultimately be applied in the medical practice and as a result lead to a harmless use of medicines.
Literature suggest that within a resource scarce country like Pakistan patient’s did not have sufficient knowledge about how to report an ADR and there is lack of proper awareness at both level (HCPs and patients) regarding ADR reporting which causes serious consequences towards patient safety (Hussain R, et al., 2020).
A number of studies had been conducted globally to examine the causes of underreporting of adverse drug reaction (Kopciuch D, et al., 2019). However, in Pakistan there is no empirical study available which highlights the causes of underreporting of ADR. So, it’s very much vital to perform a research study to explore about the barriers towards ADR reporting and to pinpoint the causes responsible for insufficient knowledge towards pharmacovigilance specifically in demographics of Pakistan.
Research questions
• Finding the knowledge of healthcare professionals in Pakistan about pharmacovigilance.
• Finding the attitude of HCPs in Pakistan towards spontaneous reporting of ADRs.
• Finding the practicing behavior and perception of HCPs in Pakistan towards spontaneous reporting of ADRs.
• Finding the knowledge and awareness of patients and general public in Pakistan about ADR reporting
Research significance
This study will be helpful in creating awareness about spontaneous reporting of ADRs for HCPs. Furthermore, the findings of this study will be helpful in creating awareness amongst patients about adverse drug reaction reporting.
Therefore the basic purpose of this research study is to gage the degree and pattern of PV structure flow and its main barriers in our health system. Identification of these factors will certainly help the regulators to improve the overall healthcare system and will improve current ADR reporting system in Pakistan to have significant reduction in health hazard.
Based on the knowledge from variety of literatures, we will develop a pharmacovigilance model for Pakistani context.
Knowledge and attitude of HCPs
Gidey K, et al. investigated the knowledge of healthcare professionals towards ADRs reporting. They have found that majority of HCPs working in a tertiary care teaching hospital in Ethiopia possessed poor knowledge and poor practice of ADRs reporting but has very positive attitude for adverse drug reaction reporting.
Toklu et al. conducted an study on healthcare professionals in Northern Cyprus and according to their findings healthcare professionals lack proper knowledge on pharmacovigilance. Their findings were very much worthy because their sample size was covering majority of healthcare professionals of the country, based on their results they strongly endorsed that healthcare professionals should understand their responsibility for successful implementation of pharmacovigilance practices in the healthcare system.
Belete KA and Tessema TB conducted a study focusing on the health care professionals in working in hospitals of Northeast Ethiopia. They have noticed in the study that health care professionals has been associated with improper knowledge on ADRs reporting and they have very compromised documentation infrastructure for spontaneous adverse drug reaction reporting practices These behaviors ultimately responsible for upsurge in underreporting behavior in health setups.
Hussain R conducted a qualitative study in Pakistan where they have found that most of the HCPs possessed sufficient knowledge about adverse drug reaction reporting. Surprisingly majority of them were totally unaware about existence of national pharmacovigilance center of Pakistan and that is why they were unaware about how to report and where to report adverse drug reaction that is obviously showing communication gaps in between hospital administration and health care staff.
Fornasier et al. in their study represent a comprehensive summary of pharmacovigilance history, whereas Baldo et al. proposes the direction to enhance the understanding terminology (Baldo P, et al., 2018).
Haines, et al. concluded in their empirical finding that there is a serious gap in adverse drug reaction reporting, because evident gaps have been identified in shape of under reporting of ADRs with noticeable deficiencies in knowledge attitude and perception towards adverse drug reaction reporting (Haines HM, et al. 2020). Numerous causes influenced adversely on the inclination towards adverse drug reaction reporting some of the factors were “Did not have idea how to report, they do not have idea about where to report ADRs and when to report adverse drug reaction were the most common amongst all participant.
Patient’s awareness and attitude towards ADR reporting
Most of the healthcare professionals are quite well aware of the term PV but showing lack of proper level of knowledge regarding adverse drug reaction reporting and pharmacovigilance perception, with having positive attitude towards ADR reporting practice. On the other hands Patients generally have found to have very less level of understanding towards PV and ADRs reporting, the frequency of adverse drug reaction reporting by patients itself was less than one fifth of the total population. This imaginably stresses a necessity for systematic compulsory training and educational activity on adverse drug reaction and pharmacovigilance idea amongst all level of healthcare workers, whereas regular public education and consciousness drive on proper and quick reporting of adverse drug reaction is encouraged to improve ADR reporting rate.
Sufficient knowledge and prescribing habits on rational basis simply could reduce adverse drug reaction rate. And so, healthcare professional prescribing habits must be grounded on rational pharmacotherapy procedures, including choice of a suitable medication, at an ideal dose and length of usage, amongst the actual and harmless treatment substitutes, and updating patients regarding the diagnosis, and treatment, could be a key impact on optimizing the risk versus benefit ratio of medications. As a vital phase in the rational pharmacotherapy procedure, provision of sufficient info to the patients about their treatment (i.e. dose, usage directions, cautions, side effects, etc.) might avoid some of the medication related complications. Furthermore, knowledgeable patients are more likely to pursue instruction from their doctors to seek guidance for adverse drug reaction.”
Training need and clinical exposure to report ADR
Improper knowledge and insufficient clinical understanding and absence of proper training pounced as the predictors of poor adverse drug reaction reporting (Singh J, et al., 2018). They have highlighted that there is a need for developing such strategies which may improve the knowledge and practices of HCPs for adverse drug reaction reporting. Therefor there is a dire need of arranging proper training of healthcare professionals particularly those who had never been to such trainings and has less clinical experience.
Medina E, et al. highlighted in their findings that there is a dire need of professional training on pharmacovigilance to address lack of knowledge and to improve their attitude towards ADRs reporting. On the other hand, it is evident in countries where there is a well-developed healthcare system, their reason for improvement was also proper training and they have improved both quality and quantity of adverse drug reporting.
We have also identified lack of health care professional knowledge as another critical barrier and the basic reason for this barrier was no training of pharmacovigilance during undergraduate training and of course this could be overcome if we make pharmacovigilance as a compulsory part of curriculum and training.
Güner MD and Ekmekci PE, 2019 were more focused on the need of structured training and education of health care professionals to reinforce the knowledge of HCPs towards ADR reporting to further expand a pharmacovigilance system in Pakistan.
Haines HM, et al., 2020 concluded that we have already observed significant evolution in overall pharmacovigilance infrastructure in recent years alongside with significant growth in data generation which is most important for automation and improvement in pharmacovigilance process.
Based on their findings it has been endorsed that there is dire need of adding pharmacovigilance education and training in the curriculum of health care professionals in South Africa further more adverse drug reaction reporting should be seen as an essential area of undergraduate level clinical training of all ambulatory care health care professionals.
HCPs awareness about spontaneous reporting
Ergun Y, et al., 2019 concluded in an observational cross sectional study that majority of the Turkish healthcare professionals were very well aware of the concept of pharmacovigilance but surprisingly there was lack of awareness about Turkish national pharmacovigilance center.
According to the findings of (Opadeyi AO, 2019) most of study respondents were unaware of the PV reporting contact place into their hospital setting. As it is universally accepted that spontaneous reporting of ADRs has a pivotal part in patient safety. Highlighted factor during the study was serious unawareness about Turkish national pharmacovigilance system. Based on their study findings they have concluded that there is a serious gap in timely reporting of adverse drug reaction due to multiple factors, and obviously unawareness of national pharmacovigilance center was top of all.
Ali Saleh, (2016) concluded that another barrier which has been identified was the attitude of senior faculty members towards adverse drug reaction because senior colleagues usually do not bother to report any adverse drug reaction and we know that physicians follows their seniors because they got training from them. Based on these findings there is a need to develop training programs for senior faculty members in order to provoke intension to report adverse drug reactions and we know that physicians intensions to report adverse drug reaction mostly influenced by attitude and subjective norms established by their seniors.
Haines HM, et al., 2019 also find that regardless of the fact that most of the Health Care Professionals has shown very much positive attitude towards Adverse Drug Reaction reporting. Based on their findings they have strongly suggested that there is a dire need of creating awareness on pharmacovigilance via arranging regular training programs for healthcare professionals and this will definitely create sensitization and knowledge improvement. They have also endorsed that the timely feedback on adverse drug reaction is much more necessary to improve spontaneous adverse drug reaction reporting which will eventually effects the establishment of excellence in patient care.
Sultana J, et al., 2019 emphasized that the latest European health infrastructure regulatory body continuously trying to improve the volume of spontaneous adverse drug reporting. This can be further improved if we could allow patient directly to send his or her adverse drug reaction to the increase the volume of ADR reporting by allowing patients to send their ADR reports directly to the NCA (National Competent Authorities).
Need for technological and regulatory system advancement
Barry A, et al., 2020 concluded that continuous technological advancement in the field of healthcare will definitely change the current regulations which are applicable to pharmacovigilance systems. They believe that pharmacovigilance system might definitely be more improved by recent technological advancement through quick automation.
Some of the researchers identified that one of the reason for under reporting of ADRs in health care setups were a fear of legal liability because majority of health care professionals belief that adverse drug reaction reporting may cause downfall of their career. The basic reason identified behind this myth was unawareness about online adverse drug reaction reporting form which has been introduced by drug regulatory authority of Pakistan. Researchers focus on spontaneous reporting of adverse drug reaction and that could be done successfully by the effective implementation of information and of course the proper application all guidelines, which are published by the authorities.
Pharmacist role in successful adaptation of pharmacovigilance
Researchers also noticed that those secondary care hospitals in South Africa which have adopted the clinical pharmacist guided enhancement strategy to improve adverse drug reaction were very much successful in reducing drug related noxious effects. Based on their empirical findings they have suggested that similar strategy might be successful model if applied to primary health care hospitals as well.
It is very much encouraging to that our current healthcare system has started to realized the increasing role of clinical and community pharmacist and that they now starting preferring pharmacist to be a part of the health-care team. Because we know that pharmacist was very much underutilized professionals in the past now their role has been expanding encouragingly have endorsed on the basis of evidences from developed countries that a successful pharmacovigilance system contributes greatly in patient safety and addressed other adverse drug reaction related problems very much successfully.
Researchers suggested that drug regulatory authority of Pakistan should advertise national pharmacovigilance center along with their activities between all levels of healthcare physicians.
Barriers which have huge impact in poor ADR reporting
Hussain R, et al., 2020 also recognized multiple barriers to spontaneous adverse drug reporting. The most common barrier discussed was the high-level workload on healthcare physicians. Healthcare professionals had also recognized it in many other similar researches as an obstacle to the unprompted reporting of adverse drug reaction.
Alshammari TM, et al., 2019 observe in a study conducted in Arabic countries and according to their findings that there are multiple variations in pharmacovigilance system of most of the Arabic countries. They main reason may be variation in government support and an independent regulatory body was the highlighted factors facilitating the development of mature pharmacovigilance system. There were some more barriers highlighted, including law and order and war, absence of government backing, and low salaries, these factors delayed the formation of a good PV system in different Arabic states.
Impact of PV on mortality and morbidity
Delays in spontaneous reporting of ADRs is considered as the major reason of drug related morbidity and mortality and accountable to the incidence of adverse drug related hazards, leading to amplified health-care costs. According to the findings of (Gidey K, et al., 2020) spontaneous reporting of adverse drug reaction have turn into a key public health problem in under developed countries.
Theory
The theory of planned behavior: “The behavior of an individual is governed by three main predictors, including the attitude, subjective norm, and perceived control of behavior. These three items create intent to accomplish a particular behavior. Theory of Planned Behavior (TPB) endorse that a positive attitude, favorable social norm, and high level of perceived behavioral control are the top interpreters for the intentions to perform a certain behavior. The probability of a particular behavior reduces if any of the three predictors are disparaging. Thus, intentions of physicians to report an ADR are greatly influenced by the physicians’ attitudes, subjective norms set by their senior or colleagues, and the ability to exhibit a certain behavior toward the reporting of an ADR.” (Melo JRR, et al., 2020).
Theoretical model: Theoretical model developed via using theory of planned behavior (Figure 1).
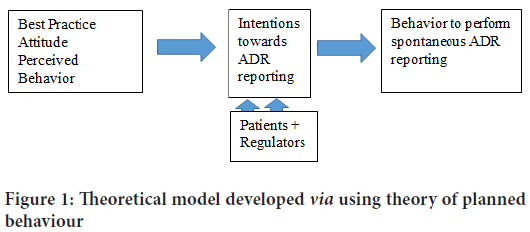
Figure 1: Theoretical model developed via using theory of planned behaviour
Conceptual framework: The results of pharmacovigilance could be comprehended by addressing three major features: Knowledge, attitude, and practice, as mentioned below.
Pharmacovigilance framework: Knowledge of healthcare professional about ADRs reporting (Gidey K, et al., 2020) (Figure 2). Attitude and behavior of HCPs about ADR reporting (Gidey K, et al., 2020). Patients Knowledge and Awareness about adverse drug reaction reporting (Sultana J, et al., 2019). Regulators sensitization towards adverse drug reaction reporting (Sultana J, et al., 2019). Impact of spontaneous ADR reporting on mortality and morbidity (Gidey K, et al., 2020).
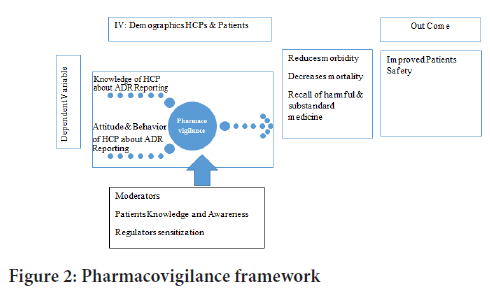
Figure 2: Pharmacovigilance framework
Hypothesis
Following are the directional hypotheses constructed on the basis of literature review:
H1: HCPs in Pakistan has inadequate knowledge about pharmacovigilance.
H2: HCPs in Pakistan has an inappropriate attitude towards ADRs reporting.
H3: HCPs in Pakistan has a very casual practicing behavior towards spontaneous reporting of ADRs.
H4: Patients and public in Pakistan have improper knowledge and awareness about ADR reporting
Methods
Study area, design, and period
Quantitative cross-sectional study will be conducted, as cross sectional studies are recommended for such type of researches because it’s observational in its nature and also known as descriptive research because these studies are not for causal relationship. In such studies the investigator only record the information’s which are present in a particular population, but researcher does not manipulate variables.
Cross section study most often used to label the particular features that prevail in a certain community, but not to conclude cause and effect associations between different variables. Cross section study is mostly used to draw interpretations about probable connections or to gather preliminary data to support further research and experimentation.
The participants for this study will be nurses, physicians who are treating chronic disease like cardiologists and diabetologist and pharmacists working in Pakistan during the study periods along with patients who are using long-term medications. HCPs that will refuse or did not wish to participate in the study will be excluded.
Variables
Dependent variables:
(a) Overall knowledge of HCPs about ADR reporting
(b) Overall attitude and behavior of HCPs towards ADR reporting
(c) Overall knowledge and awareness of patients towards ADR reporting
Independent variables: Age, sex, profession, level of education, years of experience, and attending training on ADR reporting and type of hospital (primary or general).
Information availability
The Data Collection Tool will be a questionnaire that will be adopted from similar previous studies (Gidey K, et al., 2020) on:
(a) The knowledge, attitudes and practices of HCPs on ADR reporting
(b) The perception of patients towards ADR reporting
The questionnaire will contain four different sections. The first section will contain demographic information. The second section will consist of those questions used to measure the knowledge of HCPs related to ADR reporting. The third section will be consisting of such questions, which assessed participants’ attitudes towards ADR reporting. The fourth section will be about the practice of ADR reporting.
Time constrain
We will contact at least 362 HCPs including physicians, nurses and pharmacists within 1 month of questionnaire approval (Gidey K, et al., 2020).
Results and Discussion
Demographic characteristics
The study was conducted in metro cities of Pakistan among 183 Health care professionals to measure the knowledge attitude and perception towards adverse drug reaction reporting. Among 183 respondents, 51 (28%) and 131 (72%) were males and females, respectively. The age of most of the respondents was (96, 52.5%) were in the age range of 35 years and above. Most of the respondents (77, 42. 0%) were pharmacists followed by physicians (55, 30.1%), nursing staff (7, 3.8%), health officers (9, 4.9%), and others (35, 19.1%). Most respondents did not take training on ADR reporting (Table 1).
| Gender | Frequency(%) |
|---|---|
| Female | 131(72) |
| Male | 51(28) |
| Age distribution (in years) | |
| <25 | 26(14.2) |
| 25-34 | 61(33.3) |
| ≥35 | 96(52.5) |
| Professional status | |
| Doctors | 55(30.1) |
| Pharmacist | 77(42.1) |
| Nurses | 7(3.8) |
| Health officer | 9(4.9) |
| Others | 35(19.1) |
Table 1: Demographic details of the healthcare professionals (n=183)
Knowledge and general awareness of HCPs on ADR reporting
Eleven different questions were asked from different HCPs to measure their knowledge on ADR reporting. Only 18% of respondents were aware of the term pharmacovigilance and were able to understand its basic function. Similarly, 49.2% respondents were only aware about the existence of national reporting system and ADR reporting form. Around half of respondents 57.9% knew that ADR reporting is a professional obligation for them. Moreover, significant proportion of the respondents 52.4% replied that ADRs should be reported only when they are serious and life threatening and severe and cause disability, respectively (Table 2).
| Knowledge general awareness‑related questions | Correct response (%) |
Incorrect response (%) |
|---|---|---|
| Define pharmacovigilance | 18 | 82 |
| The most important purpose of pharmacovigilance is | 16.4 | 83.6 |
| Do you think ADR reporting is professional obligation for you? | 57.9 | 42.1 |
| Healthcare professionals responsible for reporting ADRs in a hospital are | 53.1 | 46.9 |
| Monitoring of adverse drug reactions in Pakistan is carried out by | 61.7 | 38.3 |
| Prior to this survey, did you know that an adverse drug reaction could be reported directly to the Drug Regulatory Authority of Pakistan (DRAP) | 49.2 | 51.8 |
| Any problems related to newly marketed drugs | 42.1 | 57.9 |
| Any problems related to generic drugs | 42.1 | 57.9 |
| Only serious problems (death, hospitalization, disability) | 52.4 | 47.6 |
| Only unexpected adverse effects | 54.1 | 47.9 |
| Expected adverse effects | 91.2 | 8.8 |
Table 2: Knowledge and general awareness related questions and percentage of correct and incorrect responses
Attitude of HCPs towards ADR reporting
Regarding the attitude of HCPs towards ADR reporting, 55.8% respondents mentioned that ADR reporting is not necessary in their clinical setups and 57.4% reported that ADR reporting is not compulsory in their healthcare setups and 82.4% agreed that they even do not know where to report ADR in their hospital. Besides, most respondents 78.6% and 70.6% agreed that ADR reporting forms provided by mostly pharmaceutical firms are quite complicated and they have also agreed that due to over burden of patient influx they even did not have sufficient time to report any ADR (Table 3).
| Attitude‑related questions | Correct response(%) | Incorrect response(%) |
|---|---|---|
| ADR reporting is not necessary | 55.8 | 44.2 |
| ADR reporting is not mandatory | 57.4 | 42.6 |
| Don’t know where/how to report ADR | 82.4 | 17.6 |
| ADR reporting forms are too complicated | 78.6 | 21.4 |
| I don’t have enough time to report | 70.6 | 29.4 |
Table 3: Attitude of HCPs towards ADR reporting and percentage of correct and incorrect responses
Practice of HCPs regarding identification, recording, and reporting of ADRs
The present study had found that only a small number of respondents 39.3% do not have enough clinical knowledge to practice ADR reporting. In addition, 74.9% HCPs have endorsed that ADR reporting is the prime responsibility of physician. Almost majority of the participants i.e. 96.1% have agreed that successful implementation of ADR reporting needs proper and comprehensive training of HCPs, further 96.7% have also endorsed that ADR reporting needs to be make compulsory in all healthcare setups. 91.8% of the HCPs agreed that there should be close coordination of HCPs with drug poison and control department, and 94.5% have endorsed that they should have proper feedback on their ADR reports from concern department. 98.9% have agreed that ADR reporting should be promoted in our healthcare systems (Table 4).
| Practice‑related questions | Correct response(%) | Incorrect response(%) |
|---|---|---|
| Don’t have enough clinical knowledge about it | 39.3 | 60.7 |
| ADR reporting is the responsibility of the prescriber | 74.9 | 25.1 |
| I avoid the professional liability | 20.2 | 79.8 |
| ADR reporting training of HCPs | 96.1 | 3.9 |
| Make ADR reporting mandatory | 96.7 | 3.3 |
| Improve communication of HCPs with drug poison and control department | 91.8 | 8.2 |
| We should have proper feedback on our ADR reports from concern department | 94.5 | 5.5 |
| ADR reporting should be promoted in our healthcare systems | 98.9 | 1.1 |
Table 4: Practice‑related questions and percentage of response
Demographic details of the patients and general public
119 participants were the participants having aged 30 years and above and selected by convenience sampling from clients who were dispensed with prescription or over-the-counter and during free screening camps. 92% of the patients were male and most of them were highly educated either graduate 49% and 61% post graduate (Table 5).
| Gender | Frequency | |
|---|---|---|
| Female | 27(22.7) | |
| Male | 92(77.3) | |
| Age distribution(in years) | ||
| >30 | 28(23.7) | |
| 30-39 | 53(44.9) | |
| ≥40 | 37(31.4) | |
| Educational status | ||
| Under graduate | 9(07.6) | |
| Graduate | 49(41.2) | |
| Masters | 61(51.3) | |
Table 5: Demographic details of the patients (n=119)
Patients’ reasons for not reporting adverse drug reactions (ADRs)
Majority of the participants 87.3% confirmed that they have never guided by the HCPs about ADR reporting, simultaneously 53.4% of patients consider that it is not necessary to report any adverse drug reaction by patients (Table 6). Interestingly 53.4% of patients knew the term adverse drug reaction however on 89% of patients did not have any knowledge about how to report adverse drug reaction. One more fact, which we have observed that 56.3 of patients did not have correct understanding about how to comprehend any ADR. Above table summarizes about patients opinion on adverse drug reaction reporting (Tables 7 and 8). Majority of patients 85.7% agreed that timely reporting of any observed side effect can prevent harm to other people. Whereas 66.4% patients felt responsible for reporting side effects. Almost all 94.1% of patients agreed that if we report side effect it will definitely improves drug safety and hence reduce mortality rate via research and development contribution by patient’s data. But majority of patients were lacking in knowledge about which side effect to report and seems confused almost 65.6%. Further 57.1% and 63% were having incorrect information about unexpected and serious side effects reporting. But majority of the patients showed positive attitude towards adverse drug reaction reporting because 94.1% agreed that they will report possible side effects in future.
| Knowledge‑related questions | Correct response (%) |
Incorrect response (%) |
|---|---|---|
| Knowledge about the term pharmacovigilance | 53.4 | 46.6 |
| Guidance on adverse drug reaction reporting by HCPs | 12.7 | 87.3 |
| Patients response on any adverse drug reaction | 71.4 | 28.6 |
| Adverse drug reaction was not serious | 53.8 | 46.2 |
| Not necessary to report adverse drug reaction | 46.6 | 53.4 |
| Knowledge about how to report adverse drug reaction | 11 | 89 |
| Ever realized adverse drug reaction due to medicine | 43.7 | 56.3 |
Table 6: Patients’ reasons for not reporting Adverse Drug Reactions (ADRs)
| Attitude‑related questions | Correct response (%) |
Incorrect response (%) |
|---|---|---|
| Reporting a side effect can prevent harm to other people | 85.7 | 14.3 |
| Felt responsible for reporting the side effect | 66.4 | 33.6 |
| Reporting a side effect contributes to improvement of drug safety | 94.1 | 5.9 |
| Reporting a side effect contributes to research and knowledge | 89.1 | 10.9 |
| Ever benefited from reporting the side effect | 42 | 58 |
| Reporting a side effect that is already mentioned in the patient information leaflet is useless | 71.5 | 28.5 |
| Reporting a side effect if it is not mentioned in the patient information leaflet | 34.4 | 65.6 |
| Reporting a side effect if it is unexpected | 42.9 | 57.1 |
| Only reporting a side effect if it is serious | 37 | 63 |
| In the future, I will report possible side effects | 94.1 | 5.9 |
Table 7: Patients’ perspectives or opinion on ADR reporting
| Hypotheses statement | Results |
|---|---|
| Healthcare professionals in Pakistan have inadequate knowledge about pharmacovigilance | Supported |
| Healthcare professionals in Pakistan have an inappropriate attitude towards adverse drug reaction reporting | Not supported |
| Healthcare professionals in Pakistan have a very casual practicing behavior towards spontaneous reporting of adverse drug reaction | Not supported |
| Patients and public in Pakistan have improper knowledge and awareness about ADR reporting | Supported |
Table 8: Hypotheses summary
Conclusion
Our findings support hypothesis 1 (H1). Specifically, we found that a relatively high percentage of study respondents were lacking sufficient knowledge about pharmacovigilance. Our empirical findings did not support our hypothesis 2 (H2) and 3 (H3) because most of the HCPs showing very positive attitude and behavior towards ADR reporting because most of the HCPS endorsed that ADR reporting should be an integral part of the health care process. Simultaneously our study findings support our hypothesis 4 (H4) because 89% of the patients did not have any knowledge about how to report adverse drug reaction.
Underreporting of adverse drug reaction at all level of our health care system is still being a great challenge for all the stake holders. Search of past literature with regard to Knowledge, Attitude, and Practice (KAP) of HCPs towards ADR reporting in the study areas yielded no practical results which is supposed to improve pharmacovigilance practices; hence, it is found important to develop a comprehensive framework for practical implementation of pharmacovigilance system in Pakistan, this framework might be in shape of ADR registry or adaptation of ADR reporting system in our health care institutions. There is dire need of developing a comprehensive training program from healthcare teaching institutions to address the barrier of inadequate knowledge.
Recommendation
Based on our empirical findings we could recommend that there is a high degree need for the training of HCPs on ADR reporting because most of the HCPs missed proper knowledge about how to and where to report ADR but possess positive attitude and behavior which is a quite positive sign in patient overall safety and it could definitely reduce mortality rate. There is also a need to create awareness drive in general population to sensitize them on their responsibility towards side effects reporting.
Limitation
The first limitation which we are foreseeing is COVID-19 and working restrictions in health set ups. Our study will be conducted in a single city and may not be generalizable to HCPs across Pakistan.
References
- World Health Organization. Development of the roadmap on access to medicines and vaccines 2019-2023. 2018.
- World Health Organization. The importance of pharmacovigilance-safety monitoring of medicinal products. Geneva: World Health Organisation (WHO), 2002.
- Ergün Y, Ergün BT, Toker E, Ünal E, Akben M. Knowledge attitude and practice of Turkish health professionals towards pharmacovigilance in a university hospital. International Health. 2019; 11(3): 177-184.
- Hussain R, Hassali MA, ur Rehman A, Muneswarao J, Hashmi F. Physicians’ understanding and practices of pharmacovigilance: qualitative experience from a lower middle-income country. Int J Environ Res Public Health. 2020; 17: 2209.
- Brosch S, de Ferran AM, Newbould V, Farkas D, Lengsavath M, Tregunno P. Establishing a framework for the use of social media in pharmacovigilance in Europe. Drug Saf. 2019; 42: 921-930.
- Jusot V, Chimimba F, Dzabala N, Menang O, Cole J, Gardiner G, et al. Enhancing pharmacovigilance in sub-Saharan Africa through training and mentoring: A GSK pilot initiative in Malawi. Drug Saf. 2020; 43: 583-593.
- Zou M, Barmaz Y, Preovolos M, Popko L, Menard T. Using statistical modeling for enhanced and flexible pharmacovigilance audit risk assessment and planning. Ther Innov Regul Sci. 2021; 55: 190-196.
- Al Hail M, Elkassem W, Hamad A, Abdulrouf P, Thomas B, Stewart D. Overview of pharmacovigilance practices at the largest academic healthcare system in the state of Qatar. Int J Clin Pharm. 2018; 40: 769-774.
- Hussain R, Hassali MA, Hashmi F, Akram T. Exploring healthcare professionals’ knowledge, attitude, and practices towards pharmacovigilance: A cross-sectional survey. J Pharm Policy Pract. 2021; 14(1): 1-3.
- Kopciuch D, ZaprutkoT, Paczkowska A, Ratajczak P, Tomczak LZ, Kus K, et al. Safety of medicines-pharmacists' knowledge, practice,and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Pharmacoepidemiol Drug Saf. 2019; 28(12): 1-9.
- Baldo P, Francescon S, Fornasier G. Pharmacovigilance workflow in Europe and Italy and pharmacovigilance terminology. Int J Clin Pharm. 2018; 40: 748-753.
- Singh J, Singh H, Rohilla R, Kumar R, Gautam CS. Lack of awareness of pharmacovigilance among young health-care professionals in India: An issue requiring urgent intervention. Int J Appl Basic Med Res. 2018; 8(3): 158- 163.
- Güner MD, Ekmekci PE. Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. J Drug Assess. 2019; 8(1): 13-20.
- Haines HM, Meyer JC, Summers RS, Godman BB. Knowledge, attitudes and practices of health care professionals towards adverse drug reaction reporting in public sector primary health care facilities in a South African district. Eur J Clin Pharmacol. 2020; 76: 991-1001.
- Barry A, Olsson S, Minzi O, Bienvenu E, Makonnen E, Kamuhabwa A, et al. Comparative assessment of the national pharmacovigilance systems in East Africa: Ethiopia, Kenya, Rwanda and Tanzania. Drug Saf. 2020; 43(4): 339-350.
- Alshammari TM, Mendi N, Alenzi KA. Pharmacovigilance systems in Arab countries: Overview of 22 Arab countries. Drug Saf. 2019; 42(7): 849-868.
- Melo JRR, Duarte EC, Ferreira KA, Gonçalves YS, Moraes MV, Arrais PSD. Assessment of knowledge, attitude and practice of pharmacovigilance among healthcare professionals in Brazil. J Young Pharm. 2020; 12(3): 255-260.
- Sultana J, Zaccaria C, de Lisa R, Rossi F, Capuano A, Ferrajolo C. Good pharmacovigilance practice in paediatrics: An overview of the updated european medicines agency guidelines. Pediatr Drugs. 2019; 21: 317-321.
- Gidey K, Seifu M, Hailu BY, Asgedom SW, Niriayo YL. Healthcare professionals knowledge, attitude and practice of adverse drug reactions reporting in Ethiopia: A cross-sectional study. BMJ Open. 2020; 10(2): e034553.
Author Info
Muhammad Aiyaz Sharif*, Afaq Ahmed Kazi and Maria Angelica RweyemamuReceived: 25-Jun-2021 Accepted: 09-Jul-2021 Published: 16-Jul-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






