Review Article - (2023) Volume 14, Issue 6
Abstract
Brain aging is a neurodegenerative disorder, whose prevalence has increased worldwide. According to World Health Organization (WHO) guidelines, dementia due to neurodegenerative disorder is 22%, i.e., ≥ 80 million people among the world’s population are affected by aging. According to a survey in 2010, 8% of the Indian population was affected by aging, which may reach 19% by 2050. Neurodegenerative damage contributes to persistent diseases such as Alzheimer’s disease, Parkinson’s disease and stroke in the aging brain. Functional impairment in cognition is mainly due to the formation of Amyloid beta (Aβ) plaques and neurofibrillary tangles in the brain. The main causes of brain aging are the generation and accumulation of free radicals, i.e., Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), gene regulation, mitochondrial dysfunction, apoptosis, and telomere shortening. A recent approach to overcome aging is to identify novel biomarkers, such as urinary and molecular biomarkers.
In the present review, we emphasize different theories and biomarkers of ageing that lead to neurodegeneration, which helps in identifying the severity and progression of brain aging in healthy and diseased people. From this review, we learn about the long-term goal of identifying new therapeutic targets in drug discovery that can reduce the prevalence rate of neurodegeneration.
Keywords
Amyloid beta (Aβ) plaque, Dementia, Theories of aging, Molecular biomarkers, Neurodegeneration
Abbreviations
AchE: Acetylcholinesterase Enzyme; AD: Alzheimer’s Disease; APP: Amyloid Precursor Protein; CSF: Cerebrospinal Fluid; DNA: Deoxyribonucleic Acid; GABA: Gamma Amino Butyric Acid; HbA1C: Glycated Heamoglobin or Heamoglobin A1c; HPA: Hypothalamic Pituitary Adrenal Axis; IGF-1: Insulin like Growth Factor 1; NMDA: N-Methyl D-Aspartate; RNS: Reactive Nitrogen Species; ROS: Reactive Oxygen Species; TACE: Tumor necrosis factor Alpha Converting Enzyme; TNF: Tumor Necrosis Factor
Introduction
Brain aging is a neurodegenerative disorder that is associated with cognitive decline due to pathophysiological processes in the brain (Morel GR, et al., 2017). Alzheimer’s disease is also a neurodegenerative disease. These two are the leading causes of dementia/cognitive impairment (Shiel Jr WC, 2016). Recent statistics state that almost 46.8 million people are affected by these diseases worldwide at the age of greater than or equal to ≥ 65 years (Gleerup HS, et al., 2019). This prevalence will increase to approximately 88 million people by 2050 (Alzheimer’s Association, 2019). Aging is the major cause of Alzheimer’s disease. Aging is mainly due to the generation of reactive oxygen species, which affect the organelles of mitochondria (Yankner BA, et al., 2008). Aging is mainly due to increased free radical generation, which affects mitochondria. These reactive oxygen species damage mitochondrial DNA, which causes a loss of mitochondrial function, and interferes with the production of Adenosine Triphosphate (ATP) and the metabolism of energy (Nikhra V, 2017). Another cause of neurodegeneration due to aging is the formation of amyloid-beta plaques (Gleerup HS, et al., 2019; Swerdlow RH, 2011) and tangles by tau phosphorylation (Gleerup HS, et al., 2019). Neuronal cell death leads to neurodegeneration and includes Alzheimer’s disease, Parkinson’s disease, and mild cognitive impairment (Nikhra V, 2017). Cognitive decline is mainly due to the degeneration of the temporal lobe, frontal lobe, parietal lobe, visual cortex and hippo-campus (Nikhra V, 2017; Panizzutti R, et al., 2014).
Neuronal changes with aging
Not all brain regions are affected to the same extent (Panizzutti R, et al., 2014). The frontal and temporal lobes are affected more than the occipital and parietal lobes (Gleerup HS, et al., 2019; Lockhart S, et al., 2014). Shrinkage of gray matter and loss of white matter are expressed with age (Panizzutti R, et al., 2014; Lockhart S, et al., 2014). Recent studies state that synaptic dysfunction leads to aging (Azpurua J and Eaton BA, 2015). Strong evidence of aging and Alzheimer’s Disease (AD) is due to the complete loss of synapses, which was found in recent studies (Azpurua J and Eaton BA, 2015).
Role of brain neurotransmitters
Alterations of neurotransmitters and their receptors in different regions of the brain take place during the aging process (Nikhra V, 2017). Excitatory and inhibitory amino acids play a role at synapse glutamate, and Gamma-Aminobutyric Acid type A (GABAA) shows excitatory and inhibitory action at the synapse (Rissman RA, et al., 2007).
Dopamine plays a role in cognitive control and the reward pathway. A decrease in dopamine levels takes place from adulthood. Therefore, decreased dopamine levels with age lead to cognitive and neurological decline (Nikhra V, 2017). N-methyl-D-aspartate (NMDA) receptors, which are excitatory in function, act on learning and memory. A recent review states that cognitive impairment in ageing is due to decreased NMDA receptors (Panizzutti R, et al., 2014). Acetylcholinesterase breaks down the acetylcholine into acetate and choline molecules. AchE enzyme levels are altered/raised during aging (Sirviö J and Riekkinen PJ, 1992).
Literature Review
Theories of aging
Many theories have explained the aging process (Davidovic M, et al., 2010). Theories of aging are broadly categorized into two categories: Programmed and error theories (Sergiev PV, et al., 2015). Three subdivisions exist in programmed theory and they are a) programmed longevity, b) neuroendocrine theory and c) immune theory (Jin K, 2010). The error theory contains (a) wear and tear theory, b) theory of free radicals, c) rate of living theory, d) cross-linking theory, e) gene regulation, f) somatic mutation theory, g) apoptosis and h) cellular senescence/telomere theory (Jin K, 2010; Weinert BT and Timiras PS, 2003) (Figure 1).
Figure 1: Different theories of aging
Programmed theory
Program of longevity: This theory says that people who face moderate stress during the starting stage of life, have a long life. Moderate stress includes environmental variations and dietary habits (high-calorie diet) (Davidovic M, et al., 2010; Jin K, 2010; Kahn A and Olsen A, 2010). Therefore, consuming a low caloric diet delays the aging process.
Neuroendocrine theory: Central Nervous System (CNS) and ductless glands together called neuroendocrine systems. The endocrine system is a part of the cerebrum, i.e., the hypothalamus acts as a control centre, which regulates several functions by secreting some hormones. Hypothalamic-Pituitary-Adrenal (HPA) plays a major role in the response to stress (Jin K, 2010; Weinert BT and Timiras PS, 2003; van Heemst D, 2010). After several studies on primates, we gained information about the overactivity of the HPA axis (Aguilera G, 2011). HPA axis overactivity leads to neuronal degeneration associated with aging.
Immune theory: According to this theory, the immune system progressively increases in puberty and then gradually declines its function. Due to a decrease in immunity, there is decreased protection against infectious diseases. Therefore, the immune system plays a role in aging (Jin K, 2010; Weinert BT and Timiras PS, 2003; Fulop T, et al., 2014). Recently, researchers have been working in this view by enhancing immune action in older people, the aging process is delayed (Fulop T, et al., 2014; Park DC and Festini SB, 2017).
Error theory
Error theory includes free radicals, wear and tears, gene regulation, mitochondrial DNA damage, and the cellular senescence/telomere theory. This theory is also known as the damage theory, because it is due to progressive damage to tissues at various levels (Jin K, 2010; Weinert BT and Timiras PS, 2003).
Wear and tear theory: Essential parts of cells and tissues that wear out which leads to aging. The repeated use of body parts leads to wear out. This theory was first explained by Weismann A, a German biologist, in 1882 (Jin K, 2010; Weinert BT and Timiras PS, 2003).
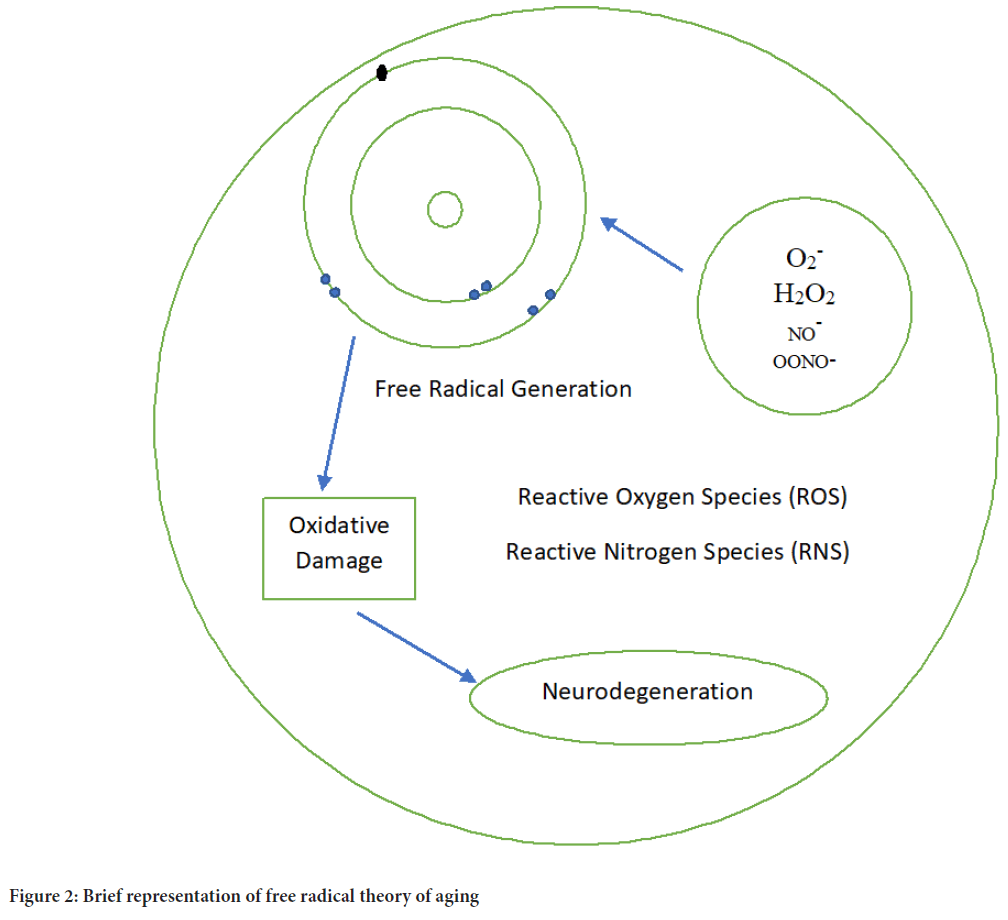
Free radical theory: This is the best theory to explain brain aging. Free radical generation and accumulation cause oxidative damage to macromolecular components of the cell such as DNA, lipids and proteins (Jin K, 2010; Weinert BT and Timiras PS, 2003; Kumar H, et al., 2012). Free radicals consist of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). ROS include hydroxyl ions (OH-), superoxide (O2-) and hydrogen peroxide (H2O2). The generation of ROS and RNS is particularly the cause of neurodegenerative disorders (Kumar H, et al., 2012; Vina J, et al., 2013; Salminen LE and Paul RH, 2014). Free radical generation leads to mitochondrial dysfunction, which damages mitochondrial DNA, and causes cell death. This process leads to cognitive decline because white matter is affected by oxidative species (Vina J, et al., 2013; Salminen LE and Paul RH, 2014) (Figure 2).
Figure 2: Brief representation of free radical theory of aging
Rate of living theory: This theory explains that, based on the metabolic potential of living organisms, assume the life span. Greater the metabolic potential, short term its life span. This theory cannot completely explain the reasons for the greater life span (Jin K, 2010; Weinert BT and Timiras PS, 2003; Vina J, et al., 2013; Brys K, et al., 2007; Hulbert AJ, et al., 2007).
Crosslinking theory: Aging is due to the assembly of cross-linked proteins that impair the functions of cells and tissues (Jin K, 2010; Bjorksten J and Tenhu H, 1990).
Gene regulation: Changes in gene expression lead to aging. Recent research in aging focuses on the Insulin-like Growth Factor-1 (IGF-1) pathway regulates the aging process in rodents (Weinert BT and Timiras PS, 2003; Tatar M, et al., 2003). IGF-1 has more than one action on the brain, such as neuroprotection and the production of neurons.
Somatic mutation theory: According to this theory, impairment of cellular function is due to mutations in somatic cells (Jin K, 2010; Weinert BT and Timiras PS, 2003). One of the causes of mutations in mitochondrial DNA (mtDNA) is increased production of reactive oxygen species, leading to neurodegeneration (Kennedy SR, et al., 2012; Schulz TJ, et al., 2007).
Apoptosis: Generally, it is called cell suicide or cell death. This theory explains that due to extensive damage to DNA or genetic events, aging takes place (Weinert BT and Timiras PS, 2003).
Cellular senescence/telomere theory: This phenomenon was established by Flick H, 1965. Cell senescence is the process that decreases the number of cell divisions compared with normal cells. After a few divisions of cells, cell division stops due to changes in function (Weinert BT and Timiras PS, 2003; Schulz TJ, et al., 2007; Ogrodnik M, et al., 2019). At one particular time, cell division stops permanently. This process is known as replicative senescence (Schulz TJ, et al., 2007; Davalli P, et al., 2016).
Telomere theory explains that the replication of cells stops when the length of the telomere decreases. Due to these cells die. Finally, it causes the death of the organism. The shortening of telomeres is one of the causes of neurodegeneration (Schulz TJ, et al., 2007; Ogrodnik M, et al., 2019; Davalli P, et al., 2016; Ferrón SR, et al., 2009; Strimbu K and Tavel JA, 2010).
Biomarkers of aging
According to National Institute of Health (NIH), biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention (Crimmins E, et al., 2008; WHO, 2001; Butler RN, Sprott RL, 2004; de Gruttola VG, et al., 2001) (Table 1).
| S.No | Biomarker category | Subcategory | Biomarker | Mechanism of aging | Method of estimation | Reference |
|---|---|---|---|---|---|---|
| 1. | CSF biomarkers | β-Amyloid | Aβ-42, Aβ-40 | Aβ senile plaques | ELISA | Blennow K, et al., 2001 |
| Tau | Total tau | Increased tau levels leads to neuronal death | ELISA | Buée L, et al., 2000; Chai X, et al., 2012 | ||
| 2. | Blood based biomarkers | Aβ and APP | Amyloid-β Precursor Protein | Increased APP increases A β which causes neurodegeneration | ELISA | Roher AE, et al., 2018 |
| Diabetes marker | HbA1c | Increased HbA1c leads to diabetes. Diabetes is the cause for cognitive decline | High-Performance Liquid Chromatography (HPLC) | Raval DK, et al., 2011 | ||
| Hormonal marker | NTproBNP | Higher NTproBNP leads to lowest systolic pressure causes cognitive decline | Chemiluminescent Immunoassay | Daniels LB, et al., 2011 | ||
| IGF-1 | IGF-1 production declines with age. Major role of IGF-1 in cell proliferation which regulates aging process | ELISA | Gubbi S, et al., 2019 | |||
| Renal biomarker | Cystatin C | Low serum cystatin C is a biomarker of future risk of AD and cognitive decline | ELISA or Radio Immunoassay (RIA) | Mathews PM and Levy E, 2016 | ||
| Inflammatory marker | IL-6 | Increased IL-6 reduced total brain volume | ELISA | Ridker PM, 2003; Gorelick PB, et al., 2011 | ||
| CRP | Increased CRP reduced total brain volume | Nephlometry and immunoturbidometry method | ||||
| TNF-α | Increased TNF-α leads to apoptosis which causes aging | ELISA and Highly sensitive enzyme amplified lanthanide luminescence immunoassay | ||||
| 3. | Salivary biomarkers | β-amyloid | Aβ-42, Aβ-40 | From CSF these are secreted in to saliva | ELISA | Farah R, et al., 2018 |
| Tau protein | P-tau, T-tau | P-tau, T-tau protein levels increased in neurodegenerative disorder | ELISA | Ashton NJ, et al., 2018 | ||
| Enzyme | AchE | Decreased AchE enzyme, increases the Acetylcholine concentration which leads to Aβ plaque formation | Ellman’s colorimetric method | Inestrosa NC, et al., 1996 | ||
| 4. | Urinary biomarkers | Hopeful biomarkers | Methionine, Desaminotyrosine, 5-hydroxy indole acetic acid, Taurine, N1-acetylspermidine | These proteins are elevated due to oxidative stress, which is the main cause of aging | Nuclear magnetic resonance (NMR) based metabolomics and Liquid Chromatography-Mass Spectrometry (LC-MS) based metabolomics | An M and Gao Y, 2015 |
| Prior to onset of cognitive decline | 3-hydroxykynurenine Homogentisate Allantoin | These proteins are elevated due to oxidative stress, which is the main cause of aging | NMR based metabolomics | Lovestone S, 2010 | ||
| 5. | Molecular biomarkers | DNA and chromosome | Leukocyte telomere length | Telomere length decreases with aging | Neuroimaging | Xia X, et al., 2017 |
| DNA damage | DNA repair | DNA repair slow down by aging and unrepaired DNA causes aging | Gas Chromatography-Mass Spectrometry (GS-MS), RIA, ELISA and electrochemical methods | Maynard S, et al., 2015 | ||
| RNA transcriptome | Transcriptome profiles | Heterogeneity of T-cells decreases or increases with aging | Dynamic Transcriptome Analysis (DTA) method. | Dillman AA, et al., 2017 | ||
| Micro RNAs | mi-34a, miR21, miR-1263P, miR-151a-3P, miR-181a-5P and miR-1248 | miRNAs function post-transcriptionally by inhibiting translation from specific target miRNAs. These small RNA molecules were thought to contribute to ageing or miRNA cause a general reduction of message-specific translational inhibition during ageing | HITS-CLIP (High Throughput Sequencing to Crosslinking Immunoprecipitation) and Northern blot | Grammatikakis I, et al., 2014; Pincus Z, et al., 2017 |
Table 1: An illustrated table showing key biomarkers of aging
Importance of biomarkers
Neurodegeneration is the main cause of brain aging and it occurs due to the presence of senile plaques and by the formation of neurofibrillary tangles (de Gruttola VG, et al., 2001; Rao P, et al., 2013). The importance of biological markers is to predict, diagnose and monitor health problems in the human population. Biomarkers detect the disease, before the onset of symptoms. It is helpful for studying cross-sectional and longitudinal studies in humans (Xia X, et al., 2017; Wiltfang J, et al., 2002). Aging can be predicted by biomarkers. Different categories of biomarkers are discussed below.
Cerebrospinal Fluid (CSF) biomarkers
CSF consists of biomarkers of neurodegenerative disease, in primates. Amyloid beta (Aβ) and Tau proteins that are present in CSF are biomarkers to estimate brain aging.
CSF Aβ: Aβ generally exists in many isoforms, i.e., Aβ1-42. Aβ1-40 is the most abundant isoform (Chen JA, et al., 2018; Masters CL, et al., 1985). Aβ1-42 is less abundant, but it is a major isoform that forms plaques in human brains (Haass C and Selkoe DJ, 1993). Amyloid Precursor Protein (APP) is a precursor and the proteolytic cleavage of APP generates Aβ (Buxbaum JD, et al., 1998). Aβ is cleaved from APP by the enzyme alpha-secretase. Researchers recently identified that ADAM (A Disintegrin and Metalloproteinase), ADAM 10 and Tumor necrosis factor-α-Converting Enzyme (TACE) also have alpha-secretase action (Lammich S, et al., 1999; Jarrett JT, et al., 1993). Aβ-42 aggregates more quickly than Aβ- 40, which forms a senile plaque (Masters CL, et al., 1985; Blennow K, et al., 2001). Reduced levels of Aβ42 in CSF lead to aging. However, the decrease in AB-42 levels is due to the deposition of Aβ-42 as senile plaques (Motter N, et al., 1995; Tamaoka A, et al., 1997). It is little contemporary to find a strong correlation between levels of Aβ-42 and number of plaques. A recent study says that there is no change in CSF Aβ-40, but a marked decrease in Aβ-42 leads to aging, early AD and Mild Cognitive Impairment (MCI) (Fukuyama R, et al., 2000; Mehta PD, et al., 2000; Blennow K, et al., 1995). The most commonly used method to estimate CSF Aβ levels is Enzyme-Linked Immunosorbent Assay (ELISA) (Blennow K, 2004; Buée L, et al., 2000).
CSF tau: Tau protein is present in the axon of neurons. Six isoforms are based on size, i.e., 352 to 441 amino acids. Tau protein hyperphosphorylation leads to the formation of neurofibrillary tangles and senile plaques (Grundke-Iqbal I, et al., 1986; Franz G, et al., 2003).
An increase in CSF total Tau leads to neuronal death (Chai X, et al., 2012; Kohnken R, et al., 2000). It is also estimated by the ELISA method (Zetterberg H and Burnham SC, 2019).
Blood-based biomarkers
Biological markers in blood are present at very minute concentrations because the BBB (Blood Brain Barrier) prevents the entry of molecules between the central nervous system and blood compartments. However, some biomarkers related to neurodegenerative disorders are present in peripheral tissues, and are measured in the blood. Due to the pathological process of brain aging, other biological markers are estimated, i.e., inflammatory biomarkers, IGF-1, NT- and HbA1c (Justice JN, et al., 2018; Balducci C and Forloni G, 2014).
Plasma Aβ and Amyloid β Precursor Protein (APP): Recent studies have reported that the presence of APP (Amyloid β precursor protein) peripherally it increases Aβ. APP is a membrane protein that plays a major role in the growth and repair of neurons.
APP is increased, by the action of secretases. Aβ is capable of causing changes in the pathology of neurodegenerative diseases (Watts JC, et al., 2004; Mormino EC, et al., 2012; Roher AE, et al., 2017; Oh H, et al., 2014; Wagner KH, et al., 2016).
Inflammatory markers: Interleukin-6 (IL-6), CRP and Tumor Necrosis Factor- α (TNF-α) are collectively called inflammation markers (Wyss- Coray T and Rogers J, 2012). Inflammation also contributes to neurodegeneration (Tancredi V, et al., 2000). Neurodegeneration is due to neuronal apoptosis and synaptic plasticity and inhibits hippocampal neurogenesis (Balschun D, et al., 2004; Gu Y, et al., 2017; McCarty MF, 1999). According to Ridker PM and MC Carty, peripheral inflammatory markers cause cardiovascular diseases and these changes affect cerebrovascular pathology (Ridker PM, 2003; Gorelick PB, et al., 2011; Berelowitz M, et al., 1981).
IGF-1: Insulin-like Growth Factor-1 hormone is produced from both endocrine and autocrine cells. IGF-1 hormone production is high at early years of age and at the puberty stage. IGF-1 production declines with age (Yamamoto H, et al., 1991; Tarantini S, et al., 2016). IGF-1 plays a major role in cell proliferation, which regulates the aging process (Gubbi S, et al., 2018; Deak F and Sonntag WE, 2012; Dar B, et al., 2015).
Haemoglobin A1c (HbA1c): The glycated haemoglobin HbA1c test is used to monitor the blood sugar levels in diabetes. A recent study revealed that HbA1c also helps to identify age-accelerating glycation. Increased HbA1c leads to an increase risk of diabetes. Dar B reported that diabetes is one of the causes of cognitive decline and neurodegeneration (Wu L, et al., 2017; Raval DK, et al., 2011; van Vliet P, et al., 2014), which leads to aging.
N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP): It is an inactive form of pro-brain natriuretic peptide hormone. NT-proBNP is a marker of congestive heart failure and MI. However increased NT-proBNP levels are not specific due to CHF in normal people (Daniels LB, et al., 2011).
Daniels LB, et al., 2011; Feinkohl I, et al., 2012 and Marksteiner J, et al., 2014 stated that higher NT-proBNP levels lead to cognitive decline when compared to healthy subjects. Recent studies stated that both higher NT-proBNP and lowest systolic blood pressure lead to cognitive decline when compared with other subjects (Daniels LB, et al., 2011). NT-proBNP reflects cardiac, neurovascular and neurodegenerative etiologies (Filler G, et al., 2005).
Cystatin: Higher serum cystatin C levels indicate Chronic Kidney Disease (CKD), cancer, hypertension, rheumatoid arthritis, cardiovascular disease and neurodegeneration (Sundelöf J, et al., 2008). A recent study by Sundelof J reported that lower serum Cys C leads to an increased risk of AD, which is not dependent on age. This study indicates that low serum Cys C is a biomarker of future risk of AD and cognitive decline (Mathews PM and Levy E, 2016; Mandel ID, 1987).
Salivary biomarkers
Saliva is a physiological fluid that is secreted by salivary glands. It plays a role in the digestion of carbohydrates, antibacterial action, and lubrication.
Saliva collection for the estimation of biomarkers is easy, inexpensive and painless when compared with blood and CSF. CSF proteins such as Aβ-42, Aβ-40, and tau are excreted into saliva (Farah R, et al., 2018). The following proteins are estimated in the saliva:
β-Amyloid: Aβ-42 and Aβ-40 are estimated in saliva. A recent study by Farah R reported that Aβ-42 predicts familial genotype neurodegeneration (Lee M, et al., 2017). Aβ-40 did not show any variation between two-state control subjects (Ashton NJ, et al., 2018). Aβ-42 and Aβ-40 levels were estimated by ELISA.
Tau protein: Phosphorylated tau (p-tau) and total tau (t-tau) were detected in saliva. P-tau and t-tau levels are increased in Alzheimer’s disease. However, there is no significant difference, due to the undefined source that secretes the biomarkers into the saliva (Lau HC, et al., 2015; Whitehouse PJ, et al., 1981).
Acetylcholinesterase (AchE) activity: AchE is an enzyme that degrades the acetylcholine neurotransmitter at the synapse. Recent research reported that the AchE enzyme decreases with age. Decreased AchE leads to an increased acetylcholine concentration, which damages neurons by enhancing Aβ-plaque formation (Inestrosa NC, et al., 1996; Rees T, et al., 2003; Boston PF, et al., 2008). Decreased AchE is a biomarker of brain aging, which was estimated by Ellman’s colorimetric method (van der Strate BW, et al., 2001).
Other biomarkers
Lactoferrin (Huan T, et al., 2018), spinganine-1-phosphate, ornithine and phenyllactic acid (Liang Q, et al., 2015), and inosine-3-dehydrocarnithine and hypoxanthine (Mucke L, 2009) are other biomarkers in saliva.
Urinary biomarkers: Neurodegenerative disorders were diagnosed by the estimation of biomarkers in CSF that take place at the late-stage of the disorder. Therefore, early diagnosis of neurodegenerative disorders can be diagnosed by the estimation of markers in urine.
Urinary biomarkers can be estimated in transgenic animal models (Fukuhara K, et al., 2013; Lovestone S, 2010). Biomarkers before the onset of cognitive decline are hydroxy-kynurenine, homogentisate, and tyrosine (An M and Gao Y, 2015). Other markers in urine are 1-methyl nicotinamide, dimethylamine, trigonelline, dimethylamine, citrate, urea, and 2-oxoglutarate, which are identified at the late stage of the neurodegenerative disorder (An M and Gao Y, 2015; Bratic A and Larsson NG, 2013).
Methionine, desaminotyrosine, taurine and N1-acetylspermidine are promising biomarkers (An M and Gao Y, 2015).
Molecular biomarkers: Alterations at the molecular level lead to aging. Molecular biomarkers predict and monitor the aging process (Kirkwood TB, 2005). Molecular mechanisms contributing to aging include, DNA damage, oxidative stress, changes in RNA expression and telomere shortening (Sedelnikova OA, et al., 2004; Dollé ME, et al., 1997).
Free radical theory is a common cause of DNA damage, mitochondrial dysfunction, and telomere shortening (Hayflick L, 2007).
DNA damage: DNA damage occurs due to free radical generation and accumulation. DNA repair slows down with aging. Unrepaired DNA damage causes genomic instability and aging. Damaged DNA causes mutations that affect neurons (Maynard S, et al., 2015).
Telomere shortening: Telomeres are located at the end of chromosomes, and after each replication, they become shorter. The length of the leukocyte telomere indicates aging. Leukocyte telomere length decreases with age (Nakamura KI, et al., 2007). Recent studies by Nakamura KI, stated that changes in telomere length reported a positive correlation with age in 40 older individuals (Lukens JN, et al., 2009). Longer telomere length is greater than 60 years, and increases life span (Cawthon RM, et al., 2003). Shorter telomere length reflected mortality in humans for less than 60 years (Leal SL and Yassa MA, 2015).
Ribonucleic Acid (RNA) and transcriptome: RNA quality, i.e., the DNA sequence, is one of the markers of brain aging (Dillman AA, et al., 2017). The RNA sequence contains a relatively large number of detected genes. Dillman AA reported that changes in RNA-sequence affect neurotransmitters at synapses, i.e., both excitatory and inhibitory neurotransmitters (Grolleau-Julius A, et al., 2010). Gene expression in the brain was affected by heterogeneous cellularity. RNA sequencing provides good insight into brain aging.
microRNAs (miRNAs): It is a noncoding RNA. Recent studies on miRNAs state that, they can be present in peripheral tissues, which can be used to identify changes in the origin of cells (Hooten NN, et al., 2013). miRNA is considered a significant biomarker of brain aging (Grammatikakis I, et al., 2014).
Two sources of RNA exist peripherally extracellular RNA and Peripheral Mononuclear Blood Cells (PBMCs). Blood, plasma and CSF help to develop miRNA markers for neurodegenerative disorders such as AD, brain aging and other neurological diseases (Pincus Z, et al., 2011).
Different miRNA markers are identified as miRNA-34a, miR-21, miR-126-3p, miR-151a-3p, miR-181a-5p and miR-1248 (Li X, et al., 2011). Recent work on miRNA-34a was reported by Li X, miRNA-34a is a tumor suppressor in the brain, and its absence leads to the development of tumors in the brain (Wagner KH, et al., 2015). However, a sharp increase in miRNA-34a is a noninvasive marker of neurodegeneration and age-dependent brain decline. Therefore, miRNA-34a is considered an early biomarker for changes in the brain (Pincus Z, et al., 2011; Wagner KH, et al., 2015).
Novel biomarkers
Recently, some biomarkers have been identified. Bilirubin is a novel marker for aging (Simm A, et al., 2015). Advanced Glycation End products (AGEs) a marker for age-related diseases, including neurodegeneration (Sharma S, et al., 2013). Metallothioneins act as free radical scavengers. It plays a role as a neuroprotector of the aging brain (Cenini G, et al., 2019).
Discussion and Conclusion
Multiple theories of aging have been proposed in the present review, including biomarkers of aging. Current evidence suggests that the free radical theory of aging may be associated with neurodegenerative diseases. Apoptosis and mitochondrial dysfunction due to the generation and accumulation of free radicals mainly increase oxidative phosphorylation in the body, which affects the normal aging process. Kumar H reported that as age increases, oxidative stress increases, which is one of the causes of many people prone to neurodegenerative disorders. Cenini G also stated that free radical accumulation leads to early neurodegeneration at normal age, which causes cognitive decline. Novel biomarkers at the molecular level are mentioned in the form of a concise table, which provides information about different markers that help in predicting human aging. From this review, the long term goal is to identify new therapeutic targets in drug discovery, that can reduce the prevalence rate of neurodegeneration.
References
- Morel GR, León ML, Uriarte M, Reggiani PC, Goya RG. Therapeutic potential of IGF-I on hippocampal neurogenesis and function during aging. Neurogenesis. 2017; 4(1): e1259709.
[Crossref] [Google Scholar] [Pubmed]
- Shiel Jr WC. Dementia. Alzheimer’s and Aging Brains. 2016.
- Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer’s disease in saliva: A systematic review. Dis Markers. 2019.
[Crossref] [Google Scholar] [Pubmed]
- Alzheimer's Association. 2019 Alzheimer's disease facts and figures. Alzheimers Dement. 2019; 15(3): 321-387.
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol Mech Dis. 2008; 3: 41-66.
[Crossref] [Google Scholar] [Pubmed]
- Nikhra V. The aging brain: Recent research and concepts. Gerontol Geriatr Stud. 2017; 1: 1-11.
- Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta. 2011; 1812(12): 1630-1639.
[Crossref] [Google Scholar] [Pubmed]
- Panizzutti R, Scoriels L, Avellar M. The co-agonist site of NMDA-glutamate receptors: A novel therapeutic target for age-related cognitive decline. Curr Pharm Des. 2014; 20(32): 5160-5168.
[Crossref] [Google Scholar] [Pubmed]
- Lockhart S, de Carli C, Fama R. Neuroimaging of the aging brain: Introduction to the special issue of neuropsychology review. Neuropsychol Rev. 2014; 24: 267-270.
[Crossref] [Google Scholar] [Pubmed]
- Azpurua J, Eaton BA. Neuronal epigenetics and the aging synapse. Front Cell Neurosci. 2015; 9: 208.
[Crossref] [Google Scholar] [Pubmed]
- Rissman RA, de Blas AL, Armstrong DM. GABAA receptors in aging and Alzheimer’s disease. J Neurochem. 2007; 103(4): 1285-1292.
[Crossref] [Google Scholar] [Pubmed]
- Sirviö J, Riekkinen PJ. Brain and cerebrospinal fluid cholinesterases in Alzheimer's disease, parkinson's disease and aging. A critical review of clinical and experimental studies. J Neural Transm Park Dis Dement Sect. 1992; 4: 337-358.
[Crossref] [Google Scholar] [Pubmed]
- Davidovic M, Sevo G, Svorcan P, Milosevic DP, Despotovic N, Erceg P. Old age as a privilege of the “selfish ones”. Aging Dis. 2010; 1(2): 139-146.
[Google Scholar] [Pubmed]
- Sergiev PV, Dontsova OA, Berezkin GV. Theories of aging: An ever-evolving field. Acta Naturae. 2015; 7(1): 9-18.
[Crossref] [Google Scholar] [Pubmed]
- Jin K. Modern biological theories of aging. Aging Dis. 2010; 1(2): 72.
[Google Scholar] [Pubmed]
- Weinert BT, Timiras PS. Invited review: Theories of aging. J Appl Physiol. 2003; 95(4): 1706-1716.
[Crossref] [Google Scholar] [Pubmed]
- Kahn A, Olsen A. Stress to the rescue: Is hormesis a ‘cure’for aging? Dose Response. 2010; 8(1): 48-52.
[Crossref] [Google Scholar] [Pubmed]
- van Heemst D. Insulin, IGF-1 and longevity. Aging Dis. 2010; 1(2): 147-157.
[Google Scholar] [Pubmed]
- Aguilera G. HPA axis responsiveness to stress: Implications for healthy aging. Exp Gerontol. 2011; 46(2-3): 90-95.
[Crossref] [Google Scholar] [Pubmed]
- Fulop T, Witkowski JM, Pawelec G, Alan C, Larbi A. On the immunological theory of aging. Aging. 2014; 39: 163-176.
[Crossref] [Google Scholar] [Pubmed]
- Park DC, Festini SB. Theories of memory and aging: A look at the past and a glimpse of the future. J Gerontol B Psychol Sci Soc Sci. 2017; 72(1): 82-90.
[Crossref] [Google Scholar] [Pubmed]
- Kumar H, Lim HW, More SV, Kim BW, Koppula S, Kim IS, et al. The role of free radicals in the aging brain and Parkinson’s disease: Convergence and parallelism. Int J Mol Sci. 2012; 13(8): 10478-10504.
[Crossref] [Google Scholar] [Pubmed]
- Vina J, Borras C, Abdelaziz KM, Garcia-Valles R, Gomez-Cabrera MC. The free radical theory of aging revisited: The cell signaling disruption theory of aging. Antioxid Redox Signal. 2013; 19(8): 779-787.
[Crossref] [Google Scholar] [Pubmed]
- Salminen LE, Paul RH. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: A theoretical review. Rev Neurosci. 2014; 25(6): 805-819.
[Crossref] [Google Scholar] [Pubmed]
- Brys K, Vanfleteren JR, Braeckman BP. Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol. 2007; 42(9): 845-851.
[Crossref] [Google Scholar] [Pubmed]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007; 87(4): 1175-1213.
[Crossref] [Google Scholar] [Pubmed]
- Bjorksten J, Tenhu H. The crosslinking theory of aging-added evidence. Exp Gerontol. 1990; 25(2): 91-95.
[Crossref] [Google Scholar] [Pubmed]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003; 299(5611): 1346-1351.
[Crossref] [Google Scholar] [Pubmed]
- Kennedy SR, Loeb LA, Herr AJ. Somatic mutations in aging, cancer and neurodegeneration. Mech Ageing Dev. 2012; 133(4): 118-126.
[Crossref] [Google Scholar] [Pubmed]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007; 6(4): 280-293.
[Crossref] [Google Scholar] [Pubmed]
- Ogrodnik M, Salmonowicz H, Gladyshev VN. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell. 2019; 18(1): e12841.
[Crossref] [Google Scholar] [Pubmed]
- Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016.
[Crossref] [Google Scholar] [Pubmed]
- Ferrón SR, Marqués-Torrejón MÁ, Mira H, Flores I, Taylor K, Blasco MA, et al. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci. 2009; 29(46): 14394-14407.
[Crossref] [Google Scholar] [Pubmed]
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010; 5(6): 463.
[Crossref] [Google Scholar] [Pubmed]
- Crimmins E, Vasunilashorn S, Kim JK, Alley D. Biomarkers related to aging in human populations. Adv Clin Chem. 2008; 46: 161-216.
[Crossref] [Google Scholar] [Pubmed]
- WHO. WHO international programme on chemical safety biomarkers in risk assessment: validity and validations. Environmental Health Criteria 222. 2001.
- Butler RN, Sprott RL. Biomarkers of aging: From primitive organisms to man. J Gerontol A Biol Sci Med Sci. 2004; 59(6): 560-567.
[Crossref] [Google Scholar] [Pubmed]
- de Gruttola VG, Clax P, deMets DL, Downing GJ, Ellenberg SS, Friedman L, et al. Considerations in the evaluation of surrogate endpoints in clinical trials: Summary of a National Institutes of Health workshop. Control Clin Trials. 2001; 22(5): 485-502.
[Crossref] [Google Scholar] [Pubmed]
- Rao P, Benito E, Fischer A. MicroRNAs as biomarkers for CNS disease. Front Mol Neurosci. 2013; 6: 39.
[Crossref] [Google Scholar] [Pubmed]
- Xia X, Chen W, McDermott J, Han JD. Molecular and phenotypic biomarkers of aging. F1000Res. 2017; 6: 860.
[Crossref] [Google Scholar] [Pubmed]
- Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, Paul S, et al. Highly conserved and disease‐specific patterns of carboxyterminally truncated Aβ peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer's disease and in patients with chronic neuroinflammation. J Neurochem. 2002; 81(3): 481-496.
[Crossref] [Google Scholar] [Pubmed]
- Chen JA, Fears SC, Jasinska AJ, Huang A, Al‐Sharif NB, Scheibel KE, et al. Neurodegenerative disease biomarkers Aβ1-40, Aβ1-42, tau, and p‐tau181 in the vervet monkey cerebrospinal fluid: Relation to normal aging, genetic influences, and cerebral amyloid angiopathy. Brain Behav. 2018; 8(2): e00903.
[Crossref] [Google Scholar] [Pubmed]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985; 82(12): 4245-4249.
[Crossref] [Google Scholar] [Pubmed]
- Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993; 75(6): 1039-1042.
[Crossref] [Google Scholar] [Pubmed]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, et al. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998; 273(43): 27765-27767.
[Crossref] [Google Scholar] [Pubmed]
- Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, et al. Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA. 1999; 96(7): 3922-3927.
[Crossref] [Google Scholar] [Pubmed]
- Jarrett JT, Berger EP, Lansbury Jr PT. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993; 32(18): 4693-4697.
[Crossref] [Google Scholar] [Pubmed]
- Blennow K, Vanmechelen E, Hampel H. CSF total tau, Aβ42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Mol Neurobiol. 2001; 24: 87-97.
[Crossref] [Google Scholar] [Pubmed]
- Motter N, Vigo‐Pelfrey C, Kholodenko D, Barbour R, Johnson‐Wood K, Galasko D, et al. Reduction of β‐amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995; 38(4): 643-648.
[Crossref] [Google Scholar] [Pubmed]
- Tamaoka A, Sawamura N, Fukushima T, Matsubara E, Shoji M, Hirai S, et al. Amyloid β protein 42 (43) in cerebrospinal fluid of patients with Alzheimer's disease. J Neurol Sci. 1997; 148(1): 41-45.
[Crossref] [Google Scholar] [Pubmed]
- Fukuyama R, Mizuno T, Mizuno T, Mori S, Nakajima K, Fushiki S, et al. Age-dependent change in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer’s disease patients. Eur Neurol. 2000; 43(3): 155-160.
[Crossref] [Google Scholar] [Pubmed]
- Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid β proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000; 57(1): 100-105.
[Crossref] [Google Scholar] [Pubmed]
- Blennow K, Wallin A, Ågren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995; 26: 231-245.
[Crossref] [Google Scholar] [Pubmed]
- Blennow K. CSF biomarkers for mild cognitive impairment. J Intern Med. 2004; 256(3): 224-234.
[Crossref] [Google Scholar] [Pubmed]
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000; 33(1): 95-130.
[Crossref] [Google Scholar] [Pubmed]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986; 83(13): 4913-4917.
[Crossref] [Google Scholar] [Pubmed]
- Franz G, Beer R, Kampfl A, Engelhardt K, Schmutzhard E, Ulmer H, et al. Amyloid beta 1-42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003; 60(9): 1457-1461.
[Crossref] [Google Scholar] [Pubmed]
- Chai X, Dage JL, Citron M. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis. 2012; 48(3): 356-366.
[Crossref] [Google Scholar] [Pubmed]
- Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, deBernardis J, et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer's disease patients. Neurosci Lett. 2000; 287(3): 187-190.
[Crossref] [Google Scholar] [Pubmed]
- Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain. 2019; 12(1): 1-7.
[Crossref] [Google Scholar] [Pubmed]
- Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. Geroscience. 2018; 40(5-6): 419-436.
[Crossref] [Google Scholar] [Pubmed]
- Balducci C, Forloni G. In vivo application of beta amyloid oligomers: A simple tool to evaluate mechanisms of action and new therapeutic approaches. Curr Pharm Des. 2014; 20(15): 2491-2505.
[Crossref] [Google Scholar] [Pubmed]
- Watts JC, Condello C, Stöhr J, Oehler A, Lee J, deArmond SJ, et al. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2014; 111(28): 10323-10328.
[Crossref] [Google Scholar] [Pubmed]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Aβ deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb Cortex. 2012; 22(8): 1813-1823.
[Crossref] [Google Scholar] [Pubmed]
- Roher AE, Kokjohn TA, Clarke SG, Sierks MR, Maarouf CL, Serrano GE, et al. APP/Aβ structural diversity and Alzheimer's disease pathogenesis. Neurochem Int. 2017; 110: 1-3.
[Crossref] [Google Scholar] [Pubmed]
- Oh H, Madison C, Villeneuve S, Markley C, Jagust WJ. Association of gray matter atrophy with age, β-amyloid, and cognition in aging. Cereb Cortex. 2014; 24(6): 1609-1618.
[Crossref] [Google Scholar] [Pubmed]
- Wagner KH, Cameron-Smith D, Wessner B, Franzke B. Biomarkers of aging: From function to molecular biology. Nutrients. 2016; 8(6): 338.
[Crossref] [Google Scholar] [Pubmed]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012; 2(1).
[Crossref] [Google Scholar] [Pubmed]
- Tancredi V, D'Antuono M, Cafè C, Giovedì S, Buè MC, D'Arcangelo G, et al. The inhibitory effects of interleukin‐6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen‐activated protein kinase ERK. J Neurochem. 2000; 75(2): 634-643.
[Crossref] [Google Scholar] [Pubmed]
- Balschun D, Wetzel W, del Rey A, Pitossi F, Schneider H, Zuschratter W, et al. Interleukin-6: A cytokine to forget. FASEB J. 2004; 18(12): 1788-1790.
[Crossref] [Google Scholar] [Pubmed]
- Gu Y, Vorburger R, Scarmeas N, Luchsinger JA, Manly JJ, Schupf N, et al. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav Immun. 2017; 65: 150-160.
[Crossref] [Google Scholar] [Pubmed]
- McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: Down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 1999; 52(5): 465-477.
[Crossref] [Google Scholar] [Pubmed]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003; 107(3): 363-369.
[Crossref] [Google Scholar] [Pubmed]
- Gorelick PB, Scuteri A, Black SE, deCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2011; 42(9): 2672-2713.
[Crossref] [Google Scholar] [Pubmed]
- Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981; 212(4500): 1279-1281.
[Crossref] [Google Scholar] [Pubmed]
- Yamamoto H, Sohmiya M, Oka N, Kato Y. Effects of aging and sex on plasma Insulin-like Growth Factor I (IGF-I) levels in normal adults. Acta Endocrinol. 1991; 124(5): 497-500.
[Crossref] [Google Scholar] [Pubmed]
- Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, et al. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: Implications for the developmental origins of health and disease hypothesis. Age. 2016; 38(4): 239-258.
[Crossref] [Google Scholar] [Pubmed]
- Gubbi S, Quipildor GF, Barzilai N, Huffman DM, Milman S. 40 years of IGF1: IGF1: The Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018; 61(1): T171-185.
[Crossref] [Google Scholar] [Pubmed]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012; 67(6): 611-625.
[Crossref] [Google Scholar] [Pubmed]
- Dar B, Dar M, Bashir S, Nazir S, Bashir S, Bashir Y. Glycosylated Hemoglobin (HbA1c): A biomarker of anti-aging. Int J Biol Med Res. 2015; 6(3): 5084-5086.
- Wu L, Lin H, Gao J, Li X, Xia M, Wang D, et al. Effect of age on the diagnostic efficiency of HbA1c for diabetes in a Chinese middle-aged and elderly population: The Shanghai Changfeng Study. PLoS One. 2017; 12(9): e0184607.
[Crossref] [Google Scholar] [Pubmed]
- Raval DK, Shah HK, Meghani NM, Bhut VG. Hemoglobin A1C: Biomarker for diabetes prediction. Int J Clin Pharmacol Ther. 2011; 1: 1-2.
- van Vliet P, Sabayan B, Wijsman LW, Poortvliet RK, Mooijaart SP, de Ruijter W, et al. NT-proBNP, blood pressure, and cognitive decline in the oldest old: The Leiden 85-plus Study. Neurology. 2014; 83(13): 1192-1199.
[Crossref] [Google Scholar] [Pubmed]
- Daniels LB, Laughlin GA, Kritz-Silverstein D, Clopton P, Chen WC, Maisel AS, et al. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. Am J Med. 2011; 124(7): 670-671.
[Crossref] [Google Scholar] [Pubmed]
- Feinkohl I, Sattar N, Welsh P, Reynolds RM, Deary IJ, Strachan MW, et al. Association of N-terminal pro-brain natriuretic peptide with cognitive function and depression in elderly people with type 2 diabetes. PLoS One. 2012; 7: e44569.
[Crossref] [Google Scholar] [Pubmed]
- Marksteiner J, Imarhiagbe D, Defrancesco M, Deisenhammer EA, Kemmler G, Humpel C. Analysis of 27 vascular-related proteins reveals that NT-proBNP is a potential biomarker for Alzheimer's disease and mild cognitive impairment: A pilot-study. Exp Gerontol. 2014; 50: 114-121.
[Crossref] [Google Scholar] [Pubmed]
- Filler G, Bökenkamp A, Hofmann W, le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR-history, indications, and future research. Clin Biochem. 2005; 38(1): 1-8.
[Crossref] [Google Scholar] [Pubmed]
- Sundelöf J, Ärnlöv J, Ingelsson E, Sundström J, Basu S, Zethelius B, et al. Serum cystatin C and the risk of Alzheimer disease in elderly men. Neurology. 2008; 71(14): 1072-1079.
[Crossref] [Google Scholar] [Pubmed]
- Mathews PM, Levy E. Cystatin C in aging and in Alzheimer’s disease. Ageing Res Rev. 2016; 32: 38-50.
[Crossref] [Google Scholar] [Pubmed]
- Mandel ID. The functions of saliva. J Dent Res. 1987; 66(2): 623-627.
[Crossref] [Google Scholar] [Pubmed]
- Farah R, Haraty H, Salame Z, Fares Y, Ojcius DM, Sadier NS. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed J. 2018; 41(2): 63-87.
[Crossref] [Google Scholar] [Pubmed]
- Lee M, Guo JP, Kennedy K, McGeer EG, McGeer PL. A method for diagnosing Alzheimer’s disease based on salivary amyloid-β protein 42 levels. J Alzheimers Dis. 2017; 55(3): 1175-1182.
[Crossref] [Google Scholar] [Pubmed]
- Ashton NJ, Ide M, Schöll M, Blennow K, Lovestone S, Hye A, et al. No association of salivary total tau concentration with Alzheimer's disease. Neurobiol Aging. 2018; 70: 125-127.
[Crossref] [Google Scholar] [Pubmed]
- Lau HC, Lee IK, Ko PW, Lee HW, Huh JS, Cho WJ, et al. Non-invasive screening for Alzheimer’s disease by sensing salivary sugar using Drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EG-ISFET) biosensor. PLoS One. 2015; 10(2): e0117810.
[Crossref] [Google Scholar] [Pubmed]
- Whitehouse PJ, Price DL, Clark AW, Coyle JT, deLong MR. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981; 10(2): 122-126.
[Crossref] [Google Scholar] [Pubmed]
- Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, et al. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer's fibrils: Possible role of the peripheral site of the enzyme. Neuron. 1996; 16(4): 881-891.
[Crossref] [Google Scholar] [Pubmed]
- Rees T, Hammond PI, Soreq H, Younkin S, Brimijoin S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol Aging. 2003; 24(6): 777-787.
[Crossref] [Google Scholar] [Pubmed]
- Boston PF, Gopalkaje K, Manning L, Middleton L, Loxley M. Developing a simple laboratory test for Alzheimer's disease: Measuring acetylcholinesterase in saliva-a pilot study. Int J Geriatr Psychiatry. 2008; 23(4): 439-440.
[Crossref] [Google Scholar] [Pubmed]
- van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK. Antiviral activities of lactoferrin. Antiviral Res. 2001; 52(3): 225-239.
[Crossref] [Google Scholar] [Pubmed]
- Huan T, Tran T, Zheng J, Sapkota S, MacDonald SW, Camicioli R, et al. Metabolomics analyses of saliva detect novel biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2018; 65(4): 1401-1416.
[Crossref] [Google Scholar] [Pubmed]
- Liang Q, Liu H, Zhang T, Jiang Y, Xing H, Zhang AH. Metabolomics-based screening of salivary biomarkers for early diagnosis of Alzheimer's disease. Rsc Adv. 2015; 5(116): 96074-96079.
- Mucke L. Alzheimer's disease. Nature. 2009; 461(7266): 895-897.
[Crossref] [Google Scholar] [Pubmed]
- Fukuhara K, Ohno A, Ota Y, Senoo Y, Maekawa K, Okuda H, et al. NMR-based metabolomics of urine in a mouse model of Alzheimer’s disease: Identification of oxidative stress biomarkers. J Clin Biochem Nutr. 2013; 52(2): 133-138.
[Crossref] [Google Scholar] [Pubmed]
- Lovestone S. Searching for biomarkers in neurodegeneration. Nat Med. 2010; 16(12): 1371-1372.
[Crossref] [Google Scholar] [Pubmed]
- An M, Gao Y. Urinary biomarkers of brain diseases. Genomics Proteomics Bioinformatics. 2015; 13(6): 345-354.
[Crossref] [Google Scholar] [Pubmed]
- Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013; 123(3): 951-957.
[Crossref] [Google Scholar] [Pubmed]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005; 120(4): 437-447.
[Crossref] [Google Scholar] [Pubmed]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004; 6(2): 168-170.
[Crossref] [Google Scholar] [Pubmed]
- Dollé ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997; 17(4): 431-434.
[Crossref] [Google Scholar] [Pubmed]
- Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007; 1100(1): 1-3.
[Crossref] [Google Scholar] [Pubmed]
- Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL, Bohr VA. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb Perspect Med. 2015; 5(10).
[Crossref] [Google Scholar] [Pubmed]
- Nakamura KI, Takubo K, Izumiyama-Shimomura N, Sawabe M, Arai T, Kishimoto H, et al. Telomeric DNA length in cerebral gray and white matter is associated with longevity in individuals aged 70 years or older. Exp Gerontol. 2007; 42(10): 944-950.
[Crossref] [Google Scholar] [Pubmed]
- Lukens JN, van Deerlin V, Clark CM, Xie SX, Johnson FB. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer's disease. Alzheimers Dement. 2009; 5(6): 463-469.
[Crossref] [Google Scholar] [Pubmed]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003; 361(9355): 393-395.
[Crossref] [Google Scholar] [Pubmed]
- Leal SL, Yassa MA. Neurocognitive aging and the hippocampus across species. Trends Neurosci. 2015; 38(12): 800-812.
[Crossref] [Google Scholar] [Pubmed]
- Dillman AA, Majounie E, Ding J, Gibbs JR, Hernandez D, Arepalli S, et al. Transcriptomic profiling of the human brain reveals that altered synaptic gene expression is associated with chronological aging. Sci Rep. 2017; 7(1): 16890.
[Crossref] [Google Scholar] [Pubmed]
- Grolleau-Julius A, Ray D, Yung RL. The role of epigenetics in aging and autoimmunity. Clin Rev Allergy Immunol. 2010; 39(1): 42-50.
[Crossref] [Google Scholar] [Pubmed]
- Hooten NN, Fitzpatrick M, Wood WH, de S, Ejiogu N, Zhang Y, et al. Age-related changes in microRNA levels in serum. Aging (Albany NY). 2013; 5(10): 725.
[Crossref] [Google Scholar] [Pubmed]
- Grammatikakis I, Panda AC, Abdelmohsen K, Gorospe M. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY). 2014; 6(12): 992.
[Crossref] [Google Scholar] [Pubmed]
- Pincus Z, Smith-Vikos T, Slack FJ. MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS Genet. 2011; 7(9): e1002306.
[Crossref] [Google Scholar] [Pubmed]
- Li X, Khanna A, Li N, Wang E. Circulatory miR-34a as an RNA-based, noninvasive biomarker for brain aging. Aging (Albany NY). 2011; 3(10): 985.
[Crossref] [Google Scholar] [Pubmed]
- Wagner KH, Wallner M, Mölzer C, Gazzin S, Bulmer AC, Tiribelli C, et al. Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci. 2015; 129(1): 1-25.
[Crossref] [Google Scholar] [Pubmed]
- Simm A, Müller B, Nass N, Hofmann B, Bushnaq H, Silber RE, et al. Protein glycation-between tissue aging and protection. Exp Gerontol. 2015; 68: 71-75.
[Crossref] [Google Scholar] [Pubmed]
- Sharma S, Rais A, Sandhu R, Nel W, Ebadi M. Clinical significance of metallothioneins in cell therapy and nanomedicine. Int J Nanomedicine. 2013;8:1477-88.
[Crossref] [Google Scholar] [Pubmed]
- Cenini G, Lloret A, Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid Med Cell Longev. 2019.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Swathi Nalla1,2* and Ganta Suhasin22Department of Pharmacology, GITAM School of Pharmacy, GITAM Deemed University, Visakhapatnam, India
Citation: Nalla S: An Overview on Biomarkers of Neurodegenerative Disease: Brain Aging
Received: 29-May-2023 Accepted: 23-Jun-2023 Published: 30-Jun-2023, DOI: 10.31858/0975-8453.14.6.383-392
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3