Research Article - (2024) Volume 15, Issue 1
Abstract
Medicinal plants have been used in Zambia as folk medicine from time immemorial. Ethnopharmacological studies have indicated that various parts of Paropsia brazzeana have been used to treat various ailments such as Venereal Diseases (VD), gastrointestinal complications, malaria, hernia, and toothaches while also possessing antibacterial, antispasmodic, antiamoebic activity and aphrodisiac potential. In the present study, we report the qualitative phytochemical screening, Gas Chromatography Mass Spectrometry (GC-MS) phytochemical profiling, Global Natural Product Social (GNPS) and (METLIN) Gen 2 Database assisted molecular mining, total phenolic content, total flavonoid content, in vitro antioxidant activity of crude root bark extract, and molecular docking analysis of selected phytochemicals of Paropsia brazzeana. Qualitative phytochemical screening revealed the presence of alkaloids, flavonoids, phenols, carbohydrates, tannins, terpenoids, and steroids. Phytochemical profiling revealed the presence of therapeutic compounds such as d-allose, vanillic acid, 2,5-diphenyloxazole, linoelaidic acid, beta-sitosterol, and many other molecules. Total phenol content was estimated to be 403.7165 ± 6.4771 mg GAE/kg, while total flavonoid content was found to be 587.15 ± 2.4768 mg QE/kg. The root bark extract of P. brazzeana showed strong antioxidative potential with IC50 value of 42.37 mg/l, while for ascorbic acid (standard), IC50 was 23.19 mg/l. Furthermore, the identified compounds could potentially have anti-cancer, anti-diabetic, anti-obesity, anti-fungal, anti-bacterial, anti-inflammatory properties while also having the ability to ease cramps, and thus it can be used as a potential nutraceutical. Molecular docking analysis and its results indicated that beta-sitosterol outperformed the anti-inflammatory drug, ibuprofen when bound to the Cyclooxygenase 2 (COX2) enzyme. With a binding energy of -11.43 kcal/mole and an inhibition constant of 4.18 nM, beta-sitosterol exhibited stronger binding affinity, outperforming Ibuprofen, which displayed a binding energy of -6.88 kcal/mole and an inhibition constant of 9.10 µM. Consequently, beta-sitosterol emerges as a promising candidate for further investigations in anti-inflammatory drug development. The findings of this study are promising for advancing future drug development initiatives.
Keywords
Antioxidant, Paropsia brazzeana, Quercetin, Total flavonoid content, Total phenol content
Introduction
Humankind has used medicinal herbs to treat or prevent diseases for thousands of years (Belhaj MR, et al., 2021). These herbs contain pharmacologically active secondary metabolites, which benefit humans and animals (Kamboj VP, 2000). Extensive in vivo and in vitro studies have demonstrated the potential of phytochemicals like alkaloids, flavonoids, steroids, tannins, terpenoids, and others found in medicinal plants to treat various health issues and relieve pain (Sharma A, et al., 2008). For example, animal experiments have shown that naturally occurring monoterpenoids in plants can influence heart rate, blood vessels (both myogenic and endothelium-mediated), Systemic Blood Pressure (SBP), and exhibit properties such as anticancer, anti-viral, gastroprotective, vasorelaxant, antinociceptive, antispasmodic, and antidiabetic effects (Paduch R, et al., 2007; Edris AE, 2007; Vasconcelos CM, et al., 2018; Maia-Joca RP, et al., 2014; Cardoso-Teixeira AC, et al., 2018).
Many antioxidants, both synthetic and natural, are available. Antioxidants are classified as molecules that can terminate free radicals either by stalling or blocking the oxidation of oxidizable materials by essentially donating an electron to reactive species while eventually stabilizing themselves (Dai J and Mumper RJ, 2010). Oxidative stress generally occurs when there is a mismatch between free radicals and antioxidants in the body (Shigenaga MK, et al., 1994). Whenever there is an imbalance, free radicals can begin harming the adipose tissue, DNA, and proteins in the body when there are more of them than can be controlled by antioxidants. A growing body of knowledge has indicated that free radicals are heavily involved in many health complications such as diabetes, hypertension, coronary heart disease, cancer, aging, neurodegenerative diseases such as Parkinson and many more (Shigenaga MK, et al., 1994).
Paropsia brazzeana is a member of the family Passifloraceae, locally known as ‘Nshimonamatako’ because of its aphrodisiac potential. The plant is one of the most commonly used medicinal plants in many parts of Zambia (Maroyi A, 2022). This multistemmed shrub is also known to occur in other African countries such as Congo, Namibia, Cameroon, Central African Republic, Angola, Botswana, and Zimbabwe (Maroyi A, 2022). Ethnopharmacological studies have indicated that various parts of the plant, namely; leaves, leaf sap, root, fruit, root bark, and stem bark have been decocted and used to treat various ailments such as Venereal Diseases (VD), gastrointestinal complications, malaria, hernia, and toothaches while also possessing antibacterial, antispasmodic, and anti-amoebic activity (Maroyi A, 2022).
Molecular docking has gained widespread recognition as an important tool in drug discovery, primarily due to its relatively low cost and the perception of its ease of use. This has fueled its growing popularity among researchers. Molecular docking simulation is an in-silico method that belongs to the Structure-Based Drug Design (SBDD), typically utilized to get information on the optimal docked ligand poses to create stable complexes with the target. The widespread medicinal use of the plant under consideration motivated for molecular docking analysis of some molecules identified in the plant sample.
GC-MS and Liquid Chromatography Tandem Mass Spectrometry (LC- MS-MS) phytochemical screening is accelerating natural products research. Recent advances in natural product research include the advent of MZmine 3 and subsequent structural annotations using GNPS and METLIN tools. Some recent studies using these techniques yielded significant results (Allah AA, et al., 2023; Chibuye B, et al., 2023; Bitwell C, et al., 2023). This study aimed at evaluating the scientific credence of Paropsia brazzeana Baill root bark extracts (Passifloraceae) by conducting preliminary qualitative phytochemical screening, GC-MS phytochemical screening, and estimation of the total phenolic content, total flavonoids content and in vitro antioxidant activity of P. brazzeana. The study also used MZmine 3-assisted structural annotations using GNPS molecular networking and METLIN platforms. The study afforded significant results.
Molecular docking has gained widespread recognition as an important tool in drug discovery, primarily due to its relatively low cost and the perception of its ease of use. These factors have fueled its growing popularity among researchers. The molecular docking method belongs to the Structure-Based Drug Design (SBDD), typically used to get information on the optimal docked ligand poses to create stable complexes with the target. The widespread medicinal use of the plant under consideration motivated the molecular docking analysis of some molecules identified in the plant sample. In the human body, inflammation is a defense mechanism against infections (Simmons DL, 2006). However, uncontrolled inflammation resulting from chronic diseases like asthma and osteoarthritis can contribute to developing conditions such as multiple sclerosis and diabetic nephropathy (Navarro-González JF, et al., 2011). Through the docking studies of these plant-derived molecules, their potential as anti-inflammatory agents have been unveiled, indicated by their binding characteristics with the specific protein COX-2 (code: 5jw1). COX-2 is an enzyme primarily responsible for initiating inflammation. The results of the molecular docking signify an assuring avenue for further investigation.
Material and Methods
Collection and authentication of plant samples
The root bark of Paropsia brazzeana was collected in a clean plastic bag from Lufwanyama district (13°25ˈ60’’S and 27°45’°0’’ E° in degrees minutes seconds) located in the Copperbelt province of Zambia. The Zambia Forestry Department, Kitwe branch verified and authenticated the freshly collected plant sample. Figure 1shows Paropsia brazzeana Baill in its natural habitat prior to collection.
Figure 1: Plant sample of Paropsia brazzeana Baill
Reagents and instruments
This study used analytical grade chemicals, including sodium nitrate (97%), gallic acid (99.5%), sodium carbonate anhydrous (99.5%), aluminum chloride hexahydrate (99% AR), Folin-Ciocalteu reagent, quercetin dihydrate (98%), 1,1-Diphenyl-2-Picrylhydrazyl (DPPH), 99% methanol and sodium hydroxide pellets (97%). The chemicals were purchased from Pallav Chemicals and Solvents Pvt, Ltd, India. The laboratory equipment (Cary Agilent 60 UV-VIS Spectrophotometer) and other laboratory utensils were availed by the Chemistry Department at the Copperbelt University.
Preparation of extract for preliminary phytochemical screening and GC-MS analysis
The root bark extract was prepared using the Soxhlet extraction method, as reported by Bitwell C, et al., 2023. In brief, the freshly collected sample was dried in the shade and ground into a fine powder; 20 g of the ground root bark material was loaded in a thimble and extracted with 250 ml of 98% ethanol. The extracting process was sustained for 10 hours. Once cooled, the extract was filtered using Whatman No. 1 filter paper and finally concentrated under reduced pressure using a rotary evaporator (Büchi Rotavapour 200). The concentrated extract was then put in a sterile amber glass bottle and preserved in a refrigerator at 4°C in readiness for further analysis.
Preparation of crude extract for the estimation of total phenol content, total flavonoid content and antioxidant activity
Following the procedure described by Bitwell C, et al., 2023 the crude extract for estimating total phenols, total flavonoids, and in vitro antioxidant activities was prepared using the maceration method. For this purpose, 2.0 g of dried root bark sample was macerated with 40 ml of 99% methanol by constantly shaking the mixture for 24 hours at 100 rpm. The extract was finally obtained by filtering the mixture using Whatman No.1 filter paper and stored in sterile amber bottles in readiness for use for the various tests.
Preliminary phytochemical screening
Qualitative phytochemical screening was done following an already established procedure of Rauha JP, et al., 2000 to determine the presence of alkaloids, flavonoids, saponins, terpenoids, tannins, phenols, steroids, carbohydrates and anthraquinones in methanolic extract of Paropsia brazzeana Baill.
Test for alkaloids (Wagner’s test): To 3 ml of the crude extract in a test tube, 1 ml dilute HCl and Wagner’s reagent was added and shaken vigorously. The appearance of a reddish-brown precipitate indicated the presence of an alkaloid.
Test for saponins (Form test): About 3 ml of ethanolic extract was placed in a test tube to which 5 ml of distilled water was added, shaken vigorously, and allowed to stand for 10 minutes. The appearance of a reasonably stable emulsion indicated the presence of saponins.
Test for flavonoids (Alkaline reagent test): About 3 ml of the ethanolic extract was initially put in a test tube. A further 3 drops of dilute 20% NaOH solution were then added. An intense yellow colour changing to colorless upon adding a few drops of dilute HCl indicated the presence of flavonoids.
Test for phenols and tannins (Braymer’s test): About 3 ml of the ethanolic extract was initially placed in a test tube, and 3 drops of 10% ferric chloride solution were added. The appearance of blue-green precipitation indicated a positive test.
Test for terpenoid (Salkowski test): About 3 ml of chloroform was placed initially in a test tube, and 5 ml of plant extract was added slowly. About four drops of concentrated sulphuric acid were then added to this test tube, and the solution was allowed to stand for a few minutes. The appearance of a reddish color indicated a positive test for terpenoids.Test for anthraquinone (Borntrager’s test): About 3 ml of chloroform was placed initially in a test tube, and 5 ml of plant extract was added slowly and subsequently filtered. Further, 3 ml of 10% ammonia was then immediately added to the filtrate. A reddish colour indicated the presence of anthraquinones.
Test for steroid: (Salkowski reaction): About 3 ml of chloroform was placed initially in a test tube, and 5 ml of plant extract was added slowly. Some four drops of concentrated sulphuric acid were added to this test tube, and the solution was allowed to stand for a few minutes. The formation of red color indicated a positive test.
Test for carbohydrates (Fehling’s test): A 2 ml of the extract was initially put in a test tube, followed by adding 2 ml of Fehling’s solution. The test tube was then kept in a boiling water bath for 12 minutes. The formation of a red precipitate confirmed the presence of carbohydrates.
GC-MS phytochemical screening
The hyphenated system GC-MS was used to screen for the various bioactive compounds present in the root bark ethanolic extract of Paropsia brazzeana Baill. GC-MS analysis was conducted using Thermo GC-Trace Ultra Ver 5.0, Thermo MS DSQ II (Netherlands). The GC-MS operation used the Electron Ionization system with 70 eV ionization energy. A High-performance GC capillary column (Scion -5MS) consisting of the conditions such as; 30 m column length, 0.25 mm diameter (narrow bore), a film of 0.25 µm, and temperature limits of -60°C to 350 °C was used. Helium (99.99%) was used as carrier gas at a constant flow rate of 1 mL/ min and an injection volume of 1 µl. The injector type, S/SL, was set at a temperature of 250°C with a hold time of 20 min with a split ratio of 10:1. Autosampler (8400) and a micro syringe of size 10 µl was used. The oven temperature was initially programmed to run isothermally from 80°C for 3 min, followed by a systematic increase of 10.0°C/min to 300°C for a total GC running time of 34.00 minutes. The phytochemicals in the targeted sample were identified based on comparing their Retention Time (RT) (min) and mass spectral patterns with the spectral database in the National Institute of Standards and Technology (NIST) library (NIST, 2023).
Estimation of Total Flavonoid Content (TFC): The total flavonoid content in the root bark extracts of Paropsia brazzeana was determined by the aluminum chloride colorimetric assay as described by Zhishen J, et al., 1999. The quercetin solution to generate the calibration curve was prepared following the protocol reported by Zhishen J, et al., 1999. In brief, a stock solution of quercetin was prepared by dissolving 100 mg of it in 100 ml methanol and serially diluting it to make solutions of various concentrations (20, 40, 60, 80, 100 µg/ml). From each type of solution, 100 µl was put in 10 ml volumetric flasks, followed by adding 4 ml of distilled water. At the same time, 0.3 ml of 5% NaNO2 was added. After 5 minutes, 0.3 ml of 10% AlCl3 was added to the mixtures. Finally, after 6 minutes, 2 ml of 1 M NaOH was added to the mixtures, followed by the addition of distilled water up to the mark. The absorbance was then measured at 510 nm against the blank using a UV-Visible spectrophotometer (Agilent, Cary 60). A stock solution of the extract was initially prepared by diluting 1 ml of concentrated extract in 49 ml methanol. 0.2 ml was pipetted from the stock solution into three 10 ml volumetric flasks. These extracts were then subjected to similar conditions as the standard quercetin solution prepared earlier. The total flavonoid content was obtained via the calibration curve and expressed as mg of Quercetin Equivalent (QE) per g.
Estimation of Total Phenol Content (TPC): The total phenolic content in the root bark extracts of Paropsia brazzeana was determined by the Folin-Ciocalteu colorimetric method as outlined by Singleton VL, et al., 1999. The preparation of gallic acid solution for the calibration curve was done, as reported by Singleton VL, et al., 1999. In brief, a stock solution of gallic acid was initially prepared by dissolving 100 mg of Gallic acid into 100 ml methanol. This solution was then serially diluted to various concentrations (20, 40, 60, 80, 100 µg/ml). To 1 ml of each of these solutions, 5 ml of 10% Folin-Ciocalteu Reagent (FCR) and 4 ml of 7.5% Na2CO3 were added, making a final volume of 10 ml. The mixture was shaken well and incubated for 90 minutes at room temperature for the reaction to complete. The absorbance was then measured at 765 nm against the blank using a UV-Visible spectrophotometer (Agilent, Cary 60). A stock solution of the extract was initially prepared by diluting 1 ml of concentrated extract in 49 ml methanol. 0.2 ml was pipetted from the stock solution into three 10 ml volumetric flasks. These extracts were then subjected to similar conditions as the standard gallic acid solution earlier prepared. The total phenol content obtained was expressed as mg of Gallic Acid Equivalent (GAE) per g.
DPPH radical scavenging assay
The free radical scavenging is commonly determined by applying 2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate (DPPHH) method. DPPH radical scavenging activity was determined using a method described by Brand-williams V, et al., 1995. The DPPH test is a quick and easy way to test an antioxidant potential (Baliyan S, et al., 2022).
Preparation of DPPH solution: The DPPH solution was prepared by dissolving its 4 mg in 100 ml of 99% methanol. It was then stored in a cool and dark place after being wrapped in aluminum foil. The Control was prepared by mixing 1 ml DPPH solution and 1 ml methanol. Appropriate volumes were then transferred to a cuvette and absorbance measured at 517 nm against the blank (methanol).
Preparation of ascorbic acid standard solution: The preparation of ascorbic acid needed to determine a calibration curve was done in a manner similar to Brand-williams V, et al., 1995. Briefly, a stock solution of ascorbic acid was prepared by dissolving 100 mg of ascorbic acid in 100 ml distilled water. This solution was then serially diluted to have different concentrations of 20, 40, 60, 80, and 100 µg/ml using 99% methanol. In 10 ml volumetric flasks (volumes of 1,2,3,4,5 ml of 20 to 100 µg/ml concentrations respectively were pipetted out. 3 ml of DPPH was then added to each flask, and the solution was made to 10 ml with methanol. The mixtures were then incubated at room temperature for 30 minutes in the dark for the reaction to complete. The absorbance of each solution was measured using a Carey 60 Agilent spectrophotometer at 517 nm against the blank (methanol). The sample extracts were also treated under similar conditions as ascorbic acid. The Percentage of Inhibition (I%) was determined using the following formula as per Phuyal N, et al., 2020.
I%=AC-AS/AC × 100
Where, AC=Absorbance of the Control, AS=Absorbance of the Sample solution, and I%=Percentage of Inhibition (Phuyal N, et al., 2020).
Molecular docking
Preparation of ligands: The Structured Data File (SDF) file of 3D structures of selected molecules such as (E)-3,3’-dimethoxy-4-4’-dihydroxystilbene (L1), 2,5-diphenyl oxazole (L2), Beta-Sitosterol (L3) identified in the study plant sample and reference drug molecule Ibuprofen (R) were downloaded from PubChem. They were prepared using Avogadro 1.2.0 software by adding hydrogens and optimizing the geometry to lead to a mol2 file, which was subsequently converted to a pdbqt file by choosing torsions and selecting active bonds using autodock 4.2 tools.
Receptor preparation: COX-2 is a critical enzyme in the inflammatory process, primarily responsible for synthesizing prostaglandins, crucial lipid signaling molecules involved in various physiological processes, including inflammation. In our study, we selected COX-2 as the receptor of interest. The crystal structure of the protein complex used in this study was acquired from the Protein Data Bank at www.rcsb.org/pdb.pdb. Downloaded protein was processed using ChimeraX software by deleting nonstandard atoms and bonds from the residue and selected chain and, subsequently, using autodock 4.2 tools, deleting water, adding hydrogens, merging nonpolar hydrogens, adding Collman charges, and assigning AD4 type atoms to generate PDBQT file.Docking procedure
The grid parameters were set by modifying the dimensions of X, Y, and Z to 126. Gpf files were generated from ligand and protein pdbqt files using autodock 4.2 tools to carry out autogrid run. Dpf files were created from ligand and protein pdbqt files using autodock 4.2 by setting genetic algorithms to 70 runs to achieve desired conformations, accepting default docking parameters, and selecting Lamarckian genetic algorithm with 2500000 energy evaluations. In order to get the desired conformations, 70 runs were carried out. Further, autogrid and autodock runs were executed. The docking results were retrieved from glg files.
Results and Discussion
Qualitative preliminary phytochemical screening
Plants are known for synthesizing a broad spectrum of secondary metabolites. Some of them can be very complex and difficult to synthesize in lab. Secondary metabolites are useful to humans as flavourants, dyes, pesticides, antioxidants and pharmaceuticals (Ochatt S, et al., 2022). There is considerable focus globally on finding medicinal compounds from plant sources. Researchers worldwide are engaged in exploring bioactive compounds from medicinal plants (Fierascu RC, et al., 2018). There are several classes of phytocompounds. The preliminary phytochemical screening of Paropsia brazzeana Baill root bark revealed the presence of alkaloids, flavonoids, saponins, phenols, tannins, terpenoids, and steroids, as depicted in Table 1.
| Phytochemical class | Outcomesa |
|---|---|
| Alkaloids (1) | + |
| Saponins (2) | - |
| Flavonoids (3) | + |
| Phenols (4) | + |
| Tannins (5) | + |
| Terpenoids (6) | + |
| Steroids (7) | + |
| Carbohydrates (8) | + |
| Anthroquinones (9) | - |
Note: a: Results indicating presence (+) or absence (-) of the phytochemical of interest; Numbers 1-9 represent phytochemical of interest
Table 1: Results of qualitative phytochemical screening of root extract of Paropsia brazzaeana Baill
GC-MS phytochemical proflling
GC-MS phytochemical profiling using NIST library revealed the presence of several significant compounds such as D-allose, vanillic acid, 2,5-diphenyl-oxazole, linoelaidic acid, beta-sitosterol, and many more. Table 2 shows some compounds along with their spectral characteristics identified from NIST library. The GC-MS generated chromatogram is depicted in Figure 2.
| S.no | RT | Chemical Abstracts Service (CAS) number | Compound name | Molecular weight | Molecular formula | Molecular structure |
|---|---|---|---|---|---|---|
| 1 | 5.639 | 16326-98-0 | 1,3-Cyclopentaediol,-trans | 102 | C5H10O2 | 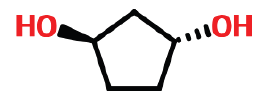 |
| 2 | 8.077 | 28564-83-2 | 4H-Pyran-one,2,3-dihydro-3,5-dihydro-6-methyl- | 144 | C6H8O4 | 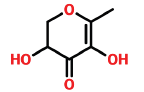 |
| 3 | 8.554 | 6973-60-0 | N-methylpyrrole-2-carboxylic acid | 125 | C6H7NO2 | 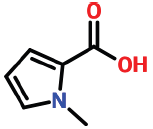 |
| 4 | 9.512 | 67-47-0 | 5-Hydromethylfurfural | 126 | C6H6O3 | 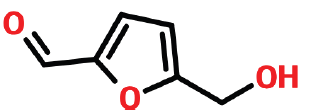 |
| 5 | 11.061 | 91-10-1 | Phenol, 2,6-dimethoxy- | 154 | C8H10O3 | 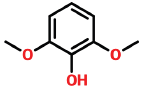 |
| 6 | 11.127 | 97-53-0 | Eugenol | 164 | C10H12O2 | 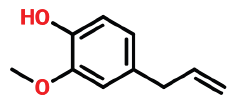 |
| 7 | 11.491 | 3856-25-5 | Copaene | 204 | C15H24 | 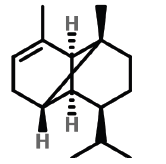 |
| 8 | 11.63 | 629-62-9 | Pentadecane | 212 | C15H32 |  |
| 9 | 12.129 | 87-44-5 | Caryophyllene | 204 | C15H2 | 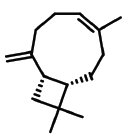 |
| 10 | 13.667 | 2595-97-3 | D-Allose | 180 | C6H12O6 | 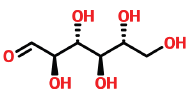 |
| 11 | 14 | 121-34-6 | Vanillic acid | 168 | C8H8O4 | 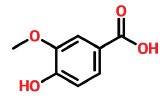 |
| 12 | 14.121 | 630-04-6 | Hentriacontane | 436 | C31H64 | 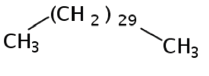 |
| 13 | 14.204 | 6627-88-9 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- | 194 | C11H14O3 | 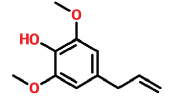 |
| 14 | 15.194 | 1367364-78-0 | 4-Hydroxy-2-methylpyrrolidine-2-carboxylic acid | 145 | C6H11NO3 | 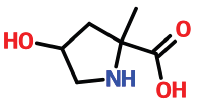 |
| 15 | 15.487 | 13096-31-6 | 5-Hydroxypipecolic acid | 145 | C6H11NO3 | 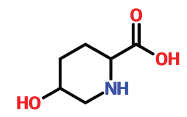 |
| 16 | 15.751 | 4899-74-5 | 2-propanone, 1-hydroxy-3-(4-hydroxy-3-methoxyphenyl | 196 | C10H12O4 | 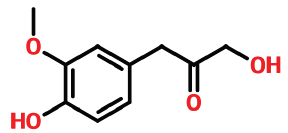 |
| 17 | 16.099 | 19037-58-2 | Syringylacetone | 210 | C11H14O4 | 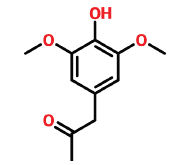 |
| 18 | 16.346 | 629-92-5 | Nonadecane | 268 | C19H4O |  |
| 19 | 16.743 | 530-57-4 | Benzoic acid, 4-hydroxy-3,5-dimethoxy- | 198 | C9H10O5 | 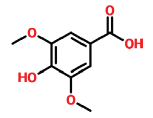 |
| 20 | 17.069 | 84-69-5 | 1,2-Benzenediccarboxylic acid, bis(2-methylpropyl) ester | 278 | C16H22O4 | 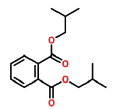 |
| 21 | 18.037 | 57-10-3 | n-Hexadecanoic acid | 256 | C16H32O2 | 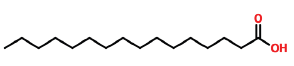 |
| 22 | 18.3 | 18281-05-5 | Eicosanoic acid, ethyl ester | 340 | C22H44O2 |  |
| 23 | 18.365 | 629-92-5 | Nonadecane | 268 | C19H4O | 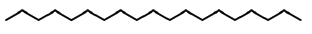 |
| 24 | 18.973 | 92-71-7 | Oxazole, 2,5-diphenyl- | 221 | C15H11NO | 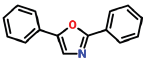 |
| 25 | 19.202 | 36653-82-4 | 1-Hexadecanol | 242 | C16H34O | 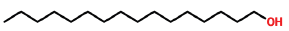 |
| 26 | 19.739 | 2416-19-5 | Cis-7-Hexadecenoic acid | 254 | C16H30O2 |  |
| 27 | 19.911 | 112-80-1 | Oleic acid | 282 | C18H34O2 | 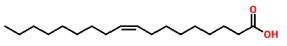 |
| 28 | 20.2 | 630-04-6 | Hentriacontane | 436 | C31H64 | 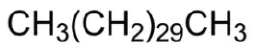 |
| 29 | 20.455 | 506-21-8 | Linoelaidic acid | 280 | C18H32O2 |  |
| 30 | 21.688 | 301-02-0 | 9-Octadecananide, (Z)- | 281 | C18H35NO | 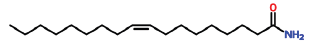 |
| 31 | 21.878 | 124-26-5 | Octadecananide | 283 | C18H37NO |  |
| 32 | 22.864 | 23470-00-0 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 330 | C19H38O4 | 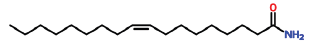 |
| 33 | 23.016 | 117-81-7 | Bis(2-ethylhexyl)Phthalate | 390 | C24H38O4 | 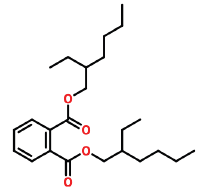 |
| 34 | 23.327 | 10417-94-4 | Cis-5,8,11,14,17-Eicosapentaenoic acid | 302 | C20H30O2 | 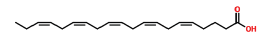 |
| 35 | 24.156 | 7329-69-3 | (E)-3,3’-dimethoxy-4-4’-dihydroxystilbene | 272 | C16H16O4 | 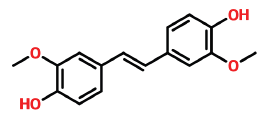 |
| 36 | 24.243 | 7459-33-8 | 9,12-Octadecadienoyl Chloride, (Z,Z)- | 298 | C18H31ClO | 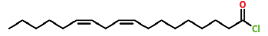 |
| 37 | 25.037 | 111-02-4 | Squalene | 410 | C30H50 | 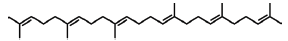 |
| 38 | 30.712 | 83-46-5 | Beta-Sitosterol | 414 | C29H50O | 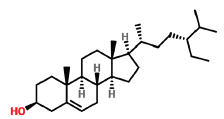 |
Table 2: Tentative phytocompounds identified in Paropsia brazzaeana root bark ethanolic extracts
Figure 2: GC-MS generated chromatogram of Paropsia brazzeana root
Bioactive compounds have been known to exert pharmacological properties. For instance, D-Allose (D-All), a D-glucose stereoisomer (Shintani T, et al., 2019). has been reported to partially reduce the production of Reactive Oxygen Species (ROS) emanating from the mitochondria and ATP (Murata A, et al., 2003). Free radicals have been implicated in development of various health conditions as a result of oxidative stress (Angelova PR, et al., 2021). Studies have also indicated that the suppression of ROS production in mitochondria is a vital step in the protection of hypertension and inhibition of ischemic diseases at the individual level (Nazarewicz RR and Dikalov SI, 2013). Similarly, in a preclinical mouse model D-All administered orally reduced the development of Bladder Cancer (BC) by potentially upregulating Thyoredoxin-Interacting Protein (TXNIP) expression while having an effect on intracellular generation of oxygen reactive species (Tohi Y, et al., 2022).
According to Kaur J, et al., 2021, Vinillic acid exerts far more pharmacologic effects other than the traditional flavouring role it has been appreciated for. For example, it has shown to have anticancer, antidiabetic, anti-obesity, antifungal, antibacterial, anti-inflammatory, anti-oxidative properties while also easing cramps and thus it can be used as a potential nutraceutical (Bin Sayeed MS, et al., 2016).
On the other hand, beta-sitosterol a plant phytosterol has shown ability to suppress or reduce the Low Density Lipoprotein (LDL) cholesterol amounts which the body takes in (Baskar AA, et al., 2010). In a certain in vivo preclinical trial involving male rats, beta-sitosterol obtained from Asclepias curassavica managed to slow down proliferation of human colon cancer cells. Ayaz M, et al., 2017 showed in vitro studies of beta-sitosterol to be a strong antioxidant and as well as having anticholinesterase properties, though the potency was less compared to standard drugs (Hwang HJ, et al., 2006).
GNPS annotated phytocompounds
The initial step involved converting raw GC-MS data into an MZML file and then processing it on the MZmine 3 platform to produce Mascot Generic Format (MGF) and Comma-Separated Values (CSV) files from the MZML data (Schmid R, et al., 2023). Subsequently, these files were uploaded to the GNPS molecular networking platform for the identification of multiple molecules, as described in Table 3. The details of the multifarious information regarding identified molecules are accessible through the link (https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=a0aa90abbe694c989555a7a48540ef69).
| S.no | Compound name | Cosine similarity | Class | Shared peaks |
|---|---|---|---|---|
| 1 | Spermine | 0.95 | Gold | 6 |
| 2 | L-Norvaline | 0.8 | Gold | 7 |
| 3 | Thiosulphate | 0.79 | Gold | 6 |
| 4 | Naphthalene, 1-phenyl- | 0.79 | Gold | 6 |
| 5 | Isoeugenol | 0.78 | Gold | 6 |
| 5 | Trans-Isoeugenol | 0.75 | Gold | 6 |
| 6 | (+-)-4-Hydroxy-alpha-(1-(methylamino)ethyl)benzyl alcohol | 0.75 | Gold | 6 |
| 7 | Linoleic acid methyl ester | 0.74 | Gold | 6 |
| 8 | Oxine-copper | 0.74 | Gold | 7 |
| 9 | 4-Butoxybenzoic acid trimethylsilyl ester | 0.72 | Gold | 7 |
| 10 | Naphthalene | 0.72 | Gold | 8 |
| 11 | N,N-Diphenyl-p-toluenesulfonamide | 0.71 | Gold | 6 |
| 12 | 4,8,14,15,15-Pentamethylbicyclo[9.3.1]pentadeca-4(E),8(E),14-triene | 0.71 | Gold | 7 |
| 13 | Pentadecafluorooctanoic acid, undecyl ester | 0.68 | Gold | 6 |
| 14 | Alpha-bisabolene | 0.66 | Gold | 6 |
| 15 | Procaine | 0.66 | Gold | 6 |
| 16 | Hexanamide, 6-chloro-N-ethyl-N-isobutyl- | 0.64 | Gold | 6 |
| 17 | 2-Cynoethyl-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran | 0.63 | Gold | 8 |
| 18 | Ethyl N-(4-amino-6-methyl-2-pyrimidinyl)anthranilate | 0.63 | Gold | 6 |
| 19 | Fiehn VocBinbase Bin #502 | 0.62 | Gold | 6 |
| 20 | Propionamide, 3-cyclopentyl-N-methyl-N-(hept-2-yl)- | 0.62 | Gold | 6 |
| 21 | Benzo[ghi]perylene | 0.62 | Gold | 6 |
| 22 | Diethyl 2-formylsuccinate | 0.61 | Gold | 6 |
| 23 | Fiehn VocBinbase Bin #481 | 0.6 | Gold | 6 |
| 24 | Cycloleucine | 0.6 | Gold | 6 |
| 25 | Cyclohexanecarboxylic acid, 4-methoxy-, tridecyl ester | 0.6 | Gold | 6 |
| 26 | 4-Acetamido-N,N-dicyclohexyl-3-nitrobenzamide | 0.6 | Gold | 8 |
| 27 | 1H-Imidazole, 2-heptadecyl-4,5-dihydro- | 0.59 | Gold | 6 |
| 28 | Methyl dihydrojasmonate (cis) | 0.59 | Gold | 6 |
| 29 | Tetrasiloxane, decamethyl- | 0.58 | Gold | 6 |
| 30 | Dibutoxyethyl Sebacate | 0.58 | Gold | 6 |
| 31 | Methyl 3-methyl-6-oxo-2-thioxo-3,6-dihydro-2H-1,3-oxazine-4-carboxylate | 0.58 | Gold | 6 |
| 32 | 1-Ethyl-2-undecylimidazole | 0.57 | Gold | 6 |
| 33 | 3-Bromo-7-methylbenzo(b)thiophene | 0.57 | Gold | 6 |
| 34 | Aspartyllysine | 0.57 | Gold | 6 |
| 35 | 9-Methyl-4-oxo-4H-pyrido(1,2-A)pyrimidine-3-acetic acid ethyl ester | 0.56 | Gold | 6 |
| 36 | 1-Methyl-3-phenylindole | 0.56 | Gold | 6 |
| 37 | 2-(p-Acetylanilino)tropone | 0.56 | Gold | 6 |
| 38 | 2,4,6-Trimethyl-4'-phenyldiphenylsulfone | 0.56 | Gold | 6 |
| 39 | Isopropyl tetradecanoate | 0.55 | Gold | 6 |
| 40 | 1,1,2-Ethanetricarboxylic acid, triethyl ester | 0.55 | Gold | 6 |
| 41 | 1-(1-Isobutoxyethyl)-4,4-dimethyl-2,5-dioxoimidazolidine | 0.55 | Gold | 6 |
| 42 | Benzene, 1,3,5-trichloro- | 0.54 | Gold | 6 |
| 43 | 5-Chloro-2-nitrobenzoic acid | 0.53 | Gold | 6 |
| 44 | 9-(Dicyanomethylene)fluorene | 0.53 | Gold | 6 |
| 45 | Copper 8-hydroxyquinolate | 0.53 | Gold | 7 |
| 46 | Fiehn VocBinbase Bin #757 | 0.52 | Gold | 6 |
| 47 | 4-Chloro-2-(2-hydroxyphenyl)-5-phenylpyrimidine | 0.52 | Gold | 6 |
| 48 | 3,4-Methylenedioxyphenyl acetone | 0.52 | Gold | 6 |
| 49 | Caffeine | 0.51 | Gold | 6 |
| 50 | Glutaric acid, 3-methylbut-2-en-1-yl but-3-en-1-yl ester | 0.51 | Gold | 6 |
| 51 | Cyclohexanecarboxylic acid, 4-butyl-, 4-methoxyphenyl ester | 0.51 | Gold | 6 |
| 52 | (3-Pyridyl)methyloctadecanoate | 0.51 | Gold | 6 |
Table 3: List of annotated molecules from Feature Based Molecular Networking (FBMN) in GNPS
Molecules mined from METLIN Gen 2
Raw GC-MC data were converted into mzML file which was subsequently processed using MZmine 3 to have MGF and Quantification table. The MGF file was subjected to METLIN Gen2 to mine metabolites. The metabolites found are depicted in Table 4. The structures of the metabolites sourced from METLIN Gen 2 are depicted in Figure 3.
| S. no | Compound name | Molecular weight | Molecular formula |
|---|---|---|---|
| 1 | 2-Amino-2-(hydromethyl)-propane-1,3-diol | 121.0739 | C4H11NO3 |
| 2 | 2-Pyrrolidine-5-carboxylic acid, methyl ester | 143.0582 | C6H9NO3 |
| 3 | Ethylenediamine | 60.0687 | C2H8N3 |
| 4 | 2,6-Diamino-1H-1,3,5-triazin-4-one | 127.0494 | C3H5N5O |
| 5 | Methyl(2S)-4-hydroxypyrrolidine-2-carboxylate | 145.0739 | C6H11NO3 |
| 6 | Methyl aminoevulinate | 145.0766 | C6H11NO3 |
| 7 | 1H-indole-4-methane | 145.0766 | C9H9N2 |
| 8 | 6-methylpyrimidine-4,5-diol | 126.043 | C5H6N2O2 |
| 9 | 4-hydroxy-2-methyl-1H-pyrimidin-6-one | 126.043 | C5H6N2O2 |
| 10 | 6-methyluracil | 126.0429 | C5H6N2O2 |
| 11 | Amidazoleacetic acid | 126.0429 | C5H6N2O2 |
| 12 | Thymine | 126.0429 | C5H6N2O2 |
| 13 | 5-Methyl-2-furancaboxylic acid | 126.0317 | C6H6O3 |
| 14 | Dimethylmaleic acic anhydride | 126.0317 | C6H6O3 |
| 15 | Allomaltol | 126.0317 | C6H6O3 |
| 16 | 5-(hydroxymethyl)furan-2-carbaldehyde | 126.0317 | C6H6O3 |
| 17 | Benzene-1,2,5-triol | 126.0317 | C6H3O3 |
| 18 | Benzene-1,2,3-triol | 126.0317 | C6H6O3 |
| 19 | 4-hydroxyl-6-methylpyran-2-one | 126.0317 | C6H6O3 |
| 20 | 3-hydroxy-2-methylpyran-4-one | 126.0317 | C6H6O3 |
| 21 | Benzene-1,2,4-triol | 126.0317 | C6H6O3 |
| 22 | 1-Ethyl-3-(prop-2-en-1-yl)thiourea | 144.072 | C6H12N2S |
| 23 | 3-Aminoquinolin | 144.0687 | C9H8N2 |
Table 4: Hit list of compounds in Paropsia brazzeana root bark identified in METLIN Gen2 (Mass Consortium Corporation) tools
Figure 3: Antioxidant activity of Paropsia brazzeana root bark methanolic extract
Total phenol content
Phenolics have previously been recognized for their wide-ranging health benefits, including antiulcer, antimicrobial, antiatherogenic, anticancer, antithrombotic, antiallergic, antidiabetic, and others (Ogasawara M, et al., 2007; Tyśkiewicz K, et al., 2018; Liu L, et al., 2004; Panche AN, et al., 2016). Total phenolic content in Paropsia brazzeana root bark extract was determined using a calibration curve (y=0.01368x+0.15211, R2=0.99188) with gallic acid as a reference (Figure 2). The results were expressed as the mass equivalent of Gallic Acid (GAE) per kilogram of the sample, yielding a value of 527.0565 ± 0.8704 mg GAE/kg of the sample. The presence of phenols in P. brazzeana root bark may explain its effectiveness in treating various ailments, consistent with the traditional use by local communities. These phytochemicals have been previously documented in the literature for their efficacy in managing various health conditions.
Total flavonoid content
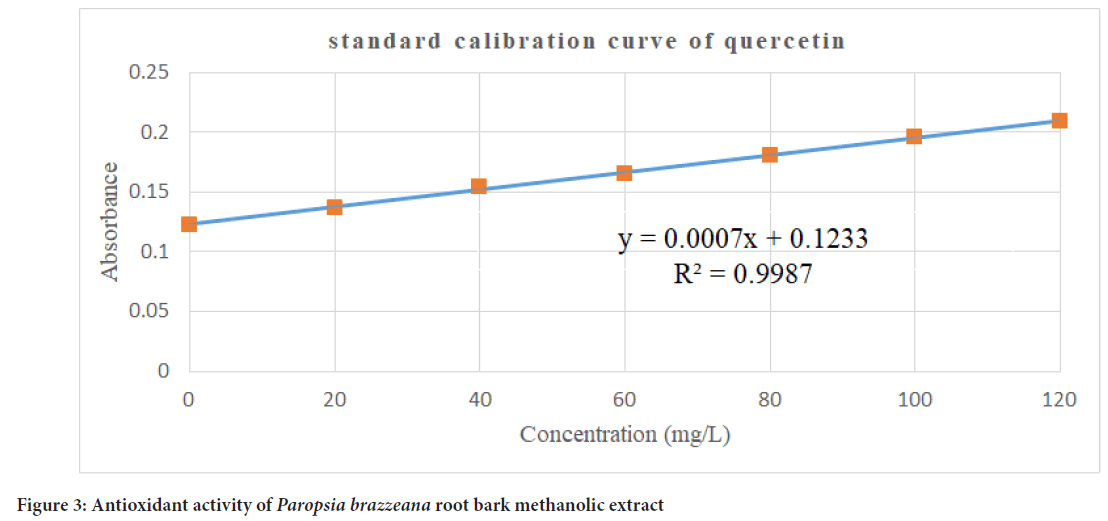
Flavonoids are regarded as crucial phytochemicals due to their wide range of applications in cosmetic, pharmacological and nutraceutical industry (Özkök A, et al., 2010). The total flavonoid content was determined with the aid of a calibration curve (y=0.0007x+0.1233, R2=0.9987) with quercetin as reference, as depicted in Figure 3. The results indicated a total flavonoid content of 587.15 ± 2.2768 QE/kg of sample expressed as mass of quercetin equivalent.
Antioxidant activity of Paropsia brazzeana root bark methanolic extract
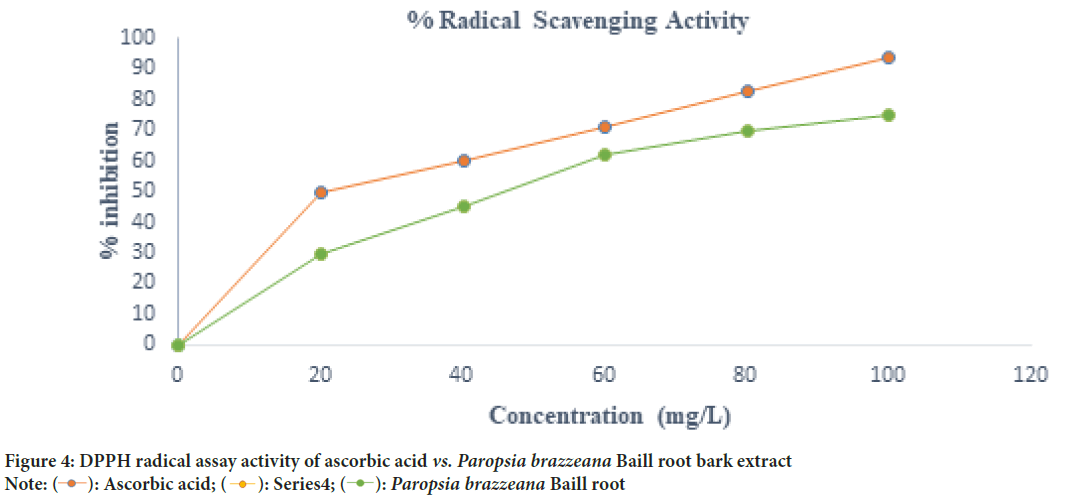
The antioxidant scavenging potential of Paropsia brazzeana Baill root bark extract was determined using DPPH assay (Brand-Williams W, et al., 1995). The results showed that the percetage radical scavenging activity increased with an increase in concentrations (Figure 4), which is in accordance with previous reported literature. The % radical scavenging activity was found to be 29.67, 45.03, 61.78, 69.77, 75.07% at 20-100 mg/l. The IC50 value which shows the concentration needed to scavenge 50% DPPH free radicals was found to be 42.37 mg/l while for standard ascorbic acid it was 23.19 mg/l. According to Molyneux (2004) a potential antioxidant having an IC50 value less than 50 mg/l is classified to be stronger (Molyneux P, 2004). A lower IC50 value indicates that a smaller concentration of the substance is required to achieve a 50% reduction in the measured oxidative activity or damage. In the case of antioxidants, a lower IC50 value suggests a more potent antioxidant effect. The DPPH solution is reduced to DPPH-H when antioxidants containing polyphenols or phenolics are present or due to an increase in number of electrons present (Aberoumand A and Deokule SS, 2008). The radical scavenging capability or potential of Paropsia brazzeana Baill root bark extract could thus be as a result of the presence of polyphenolic compounds, which have previously been reported to have free radical terminating potential.
Figure 4: DPPH radical assay activity of ascorbic acid vs. Paropsia brazzeana Baill root bark extract Note: 
Molecular docking results
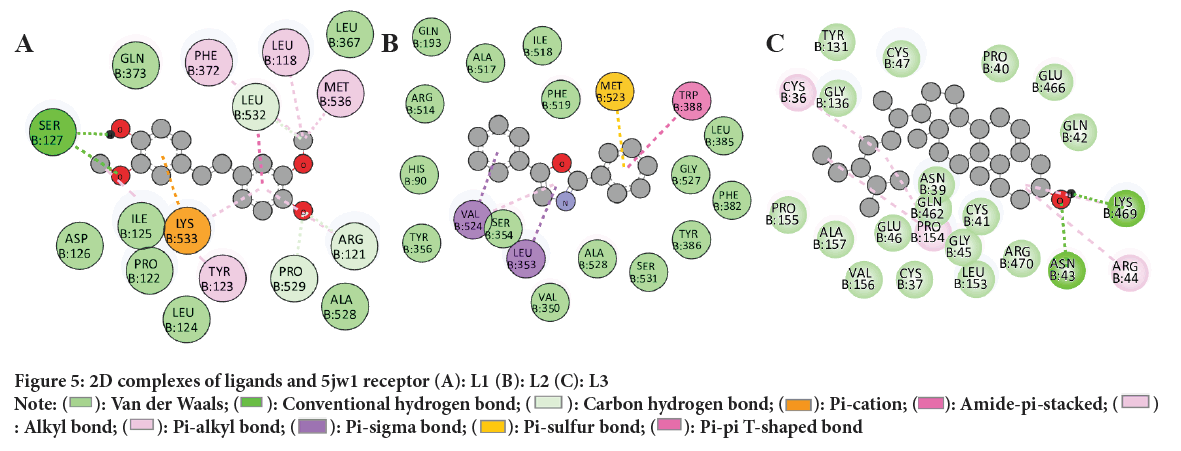
Table 5provides a visual representation of binding sites between the target protein and the selected compounds, highlighting the specific residues that are capable of forming molecular interactions. The interaction analysis using discovery studio between ligands and receptor is stipulated. The complex formation between L1 with COX-2 (code: 5jw1) (Figure 5 A-C) include conventional hydrogen bonds (SER 127), carbon hydrogen bonds (LEU 532, ARG 121, PRO 529), pi-cations (LYS 353), alkyl and pi alkyls (TYR 123, MET 536 LEU 118, PHE 372) and many van der waals interaction. Similarly complex between L2 and COX-2 (code: 5jw1) comprises pi-sigma (VAL 524, LEU 353), pi-sulfur (MET 523), pi-pi T-shaped (TRP 588) and several Van der Waals interactions. Further complex between L3 and COX-2 (code: 5jw1) include conventional hydrogen bonds (LYS 469, ASN 43), alkyl (ARG 44, CYS 36 PRO 154) and some van der waals interaction. These interactions attribute to stable ligand receptor complexes. Furthermore, complex formation between R (Ibuprofen) and COX-2 (code: 5jw1) involve conventional hydrogen bonds (GLN 373, LYS 533, GLN 371), alkyl and pi-alkyl (ARG 121, LEU 532, ILE 125) interactions. These bond interaction collectively enables a cohesive complex formation.
| Ligand | Residue | Interactions |
|---|---|---|
| (E)-3,3’-Dimethoxy-4-4’-dihydroxystilbene (L1) | LYS 353 | Pi-cation |
| SER 127 | Conventional hydrogen bonds | |
| LEU 532, ARG 121, PRO 529 | Carbon hydrogen bonds | |
| TYR 123, MET 536, LEU 118, PHE 372 | Alkyl and pi-alkyls | |
| 2,5-Diphenyl oxazole (L2) | MET 523 | Pi-sulfur |
| TRP 588 | Pi-pi T-shaped | |
| VAL 524, LEU 353 | Pi-Sigma | |
| Beta-sitosterol (L3) | LYS 469, ASN 43 | Conventional hydrogen bonds |
| ARG 44, CYS 36, PRO 154 | Alkyl | |
| Ibuprofen (R) | GLN 373, LYS 533, GLN 371 | Conventional hydrogen bonds |
| ARG 121, LEU 532, ILE 125 | Alkyls and pi-alkyls |
Table 5: Binding complexes between plant compounds (ligands) and 5jw1 receptor depicted in 2D and 3D
Figure 5: 2D complexes of ligands and 5jw1 receptor (A): L1 (B): L2 (C): L3 Note: 
The current study demonstrates strong binding affinities of selected compounds derived from the root of Paropsia brazzeana Baill root. Specifically, ligands L1, L2, and L3 exhibited notable binding energies of -6.28 kcal/ mole, -7.71 kcal/mole, and -11.43 kcal/mole, respectively (Table 6). These values are comparable to the binding energy observed for Ibuprofen, which is -6.88 kcal/mole as shown in Table 6. Moreover, the inhibition constants for L1, L2, and L3 are 25.12 µM, 2.25 µM, and 4.18 nM, respectively, indicating a similarity to the inhibition constant of Ibuprofen, which is 9.10 µM (Table 6). Using computational techniques can speed up the process of finding and creating new medicines (Khandelwal A, et al., 2007). Based on docking analysis, it was found that beta-sitosterol (L3) can firmly attach to the COX-2 receptor with a powerful binding energy of -11.43 kcal/mole and a low inhibition constant of 4.18 nM. Therefore, beta-sitosterol could be a promising candidate for making a new anti-inflammatory drug.
| Ligand | Binding energy (Kcal/mole) | Inhibition constant (µM) | Total internal energy (kcal/mol) | Torsional free energy (kcal/mol) | Unbound energy (kcal/mol) | Cluster RMSD |
|---|---|---|---|---|---|---|
| L1 | -6.28 | 25.12 | -1.38 | 1.79 | -1.38 | 0 |
| L2 | -7.71 | 2.25 | -0.45 | 0.6 | -0.45 | 0 |
| L3 | -11.43 | 4.18 | -1.04 | 2.09 | -1.04 | 0 |
| R (Ibuprofen) | -6.88 | 9.10 | -0.45 | 1.49 | -0.45 | 0 |
Table 6: Docking analysis of selected ligands from Paropsia brazeana Baill root with COX-2 enzyme
Conclusion
The preliminary screening tests on the root bark extract of Paropsia brazzeana Baill showed that it contained various beneficial compounds like alkaloids, flavonoids, phenols, tannins, terpenoids, carbohydrates, and steroids. These compounds are known for their positive effects in treating different health issues. Moreover, the methanolic root bark extract was found to have high levels of polyphenols and strong antioxidant abilities, as shown by the IC50 value. Polyphenols are known to act as powerful tools against harmful molecules in the body. This suggests that Paropsia brazzeana Baill’s root bark could be a valuable source of natural compounds beneficial to health.
Furthermore, the docking analysis study indicated that some specific compounds from the plant extract have promising properties. Among them, Beta-Sitosterol showed the most favorable binding energy and inhibition constant. This suggests that Beta-Sitosterol could be a potential candidate for further research to develop a new and promising anti-inflammatory medicine.
Acknowledgement
The authors express their profound gratitude to The Copperbelt University (CBU).
References
- Belhaj MR, Lawler NG, Hoffman NJ. Metabolomics and lipidomics: Expanding the molecular landscape of exercise biology. Metabolites. 2021; 11(3): 151.
[Crossref] [Google Scholar] [Pubmed]
- Kamboj VP. Herbal medicine. Curr Sci. 2000; 78: 35-39.
- Sharma A, Shanker C, Tyagi LK, Singh M, Rao CV. Herbal medicine for market potential in India: An overview. Acad J Plant Sci. 2008; 1(2): 26-36.
- Paduch R, Kandefer-Szerszeń M, Trytek M, Fiedurek J. Terpenes: Substances useful in human healthcare. Arch Immunol Ther Exp. 2007; 55(5): 315-327.
[Crossref] [Google Scholar] [Pubmed]
- Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res. 2007; 21(4): 308-323.
[Crossref] [Google Scholar] [Pubmed]
- Vasconcelos CM, Oliveira IS, Santos JN, Souza AA, Menezes-Filho JE, Silva Neto JA, et al. Negative inotropism of terpenes on guinea pig left atrium: Structure-activity relationships. Nat Prod Res. 2018; 32(12): 1428-1431.
[Crossref] [Google Scholar] [Pubmed]
- Maia-Joca RP, Joca HC, Ribeiro FJ, do Nascimento RV, Silva-Alves KS, Cruz JS, et al. Investigation of terpinen-4-ol effects on vascular smooth muscle relaxation. Life Sci. 2014; 115(1-2): 52-58.
[Crossref] [Google Scholar] [Pubmed]
- Cardoso-Teixeira AC, Ferreira-da-Silva FW, Peixoto-Neves D, Oliveira-Abreu K, Pereira-Gonçalves Á, Coelho-de-Souza AN, et al. Hydroxyl group and vasorelaxant effects of perillyl alcohol, carveol, limonene on aorta smooth muscle of rats. Molecules. 2018; 23(6): 1430.
[Crossref] [Google Scholar] [Pubmed]
- Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010; 15(10): 7313-7352.
[Crossref] [Google Scholar] [Pubmed]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994; 91(23): 10771-10778.
[Crossref] [Google Scholar] [Pubmed]
- Maroyi A. A synthesis and review of ethnomedicinal uses, phytochemistry and biological activities of Paropsia brazzeana baill.(passifloraceae). Res J Pharm Technol. 2022; 15(11): 5331-5336.
- Allah AA, Yousif HA, Hasaballa NO, Elkhawad EA, Abdallah RB, Ahmed HM, et al. Identification of phytochemicals from Tundub Capparis decidua (Forssk) Edgew seed oil as potential anticancer agents using gas chromatography-mass spectroscopy analysis, molecular docking, and molecular dynamics studies. Sci Afr. 2023; 19: e01517.
- Chibuye B, Singh IS, Chimuka L, Maseka K. Phytochemical and LCMS/MS screening, total phenolic and flavonoid content and antioxidant activity of the leaves of Diospyros batokana (Ebenaceae). 2023.
- Bitwell C, Sen SI, Luke C, Kakoma MK. UHPLC-MS/MS phytochemical screening, polyphenolic content and antioxidant potential of Diplorhynchus condylocarpon (Müll. Arg.) Pichon (Apocynaceae), a medicinal plant. Sci Afr. 2023; 20: e01712.
- Simmons DL. What makes a good anti-inflammatory drug target? Drug Discov Today. 2006; 11(5-6): 210-219.
[Crossref] [Google Scholar] [Pubmed]
- Navarro-González JF, Mora-Fernández C, De Fuentes MM, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011; 7(6): 327-340.
[Crossref] [Google Scholar] [Pubmed]
- Bitwell C, Sen IS, Luke C, Kakoma MK. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci Afr. 2023; 19: e01585.
- Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000; 56(1): 3-12.
[Crossref] [Google Scholar] [Pubmed]
- Metabolomics and lipidomics: Expanding the molecular landscape of exercise biology
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64(4): 555-559.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol. 1999; 299: 152-178.
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995; 28(1): 25-30.
- Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. 2022; 27(4): 1326.
[Crossref] [Google Scholar] [Pubmed]
- Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. ScientificWorldJournal. 2020; 8780704.
[Crossref] [Google Scholar] [Pubmed]
- Ochatt S, Alan AR, Bhattacharya A, Hano C, Kiselev KV, Marconi PL, et al. Secondary metabolites: A boon from plants, the best chemist in nature: Preface from the editors. Plant Cell Tissue Organ Cult. 2022; 149(1-2): 1-6.
[Crossref] [Google Scholar] [Pubmed]
- Fierascu RC, Ortan A, Fierascu IC, Fierascu I. In vitro and in vivo evaluation of antioxidant properties of wild-growing plants. A short review. Curr Opin Food Sci. 2018; 24: 1-8.
- Shintani T, Sakoguchi H, Yoshihara A, Izumori K, Sato M. D-Allose, a stereoisomer of d-glucose, extends the lifespan of Caenorhabditis elegans via sirtuin and insulin signaling. J Appl Glycosci. 2019; 66(4): 139-142.
[Crossref] [Google Scholar] [Pubmed]
- Murata A, Sekiya K, Watanabe Y, Yamaguchi F, Hatano N, Izumori K, et al. A novel inhibitory effect of D-allose on production of reactive oxygen species from neutrophils. J Biosci Bioeng. 2003; 96(1): 89-91.
[Crossref] [Google Scholar] [Pubmed]
- Angelova PR, Dinkova-Kostova AT, Abramov AY. Assessment of ROS production in the mitochondria of live cells. Methods Mol Biol. 2021; 2202: 33-42.
[Crossref] [Google Scholar] [Pubmed]
- Nazarewicz RR, Dikalov SI. Mitochondrial ROS in the prohypertensive immune response. Am J Physiol Regul Integr Comp Physiol. 2013; 305(2): R98-100.
[Crossref] [Google Scholar] [Pubmed]
- Tohi Y, Taoka R, Zhang X, Matsuoka Y, Yoshihara A, Ibuki E, et al. Antitumor effects of orally administered rare sugar D-allose in bladder cancer. Int J Mol Sci. 2022; 23(12): 6771.
[Crossref] [Google Scholar] [Pubmed]
- Kaur J, Gulati M, Gowthamarajan K, Vishwas S, Chellappan DK, Gupta G, et al. Combination therapy of vanillic acid and oxaliplatin co-loaded in polysaccharide based functionalized polymeric micelles could offer effective treatment for colon cancer: A hypothesis. Med Hypotheses. 2021; 156: 110679.
[Crossref] [Google Scholar] [Pubmed]
- Bin Sayeed MS, Karim SM, Sharmin T, Morshed MM. Critical analysis on characterization, systemic effect, and therapeutic potential of beta-sitosterol: A plant-derived orphan phytosterol. Medicines. 2016; 3(4): 29.
[Crossref] [Google Scholar] [Pubmed]
- Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Chemopreventive potential of β-sitosterol in experimental colon cancer model-an in vitro and in vivo study. BMC Complement Altern Med. 2010; 10(1): 1-10.
[Crossref] [Google Scholar] [Pubmed]
- Ayaz M, Junaid M, Ullah F, Subhan F, Sadiq A, Ali G, et al. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front Pharmacol. 2017; 8: 697.
[Crossref] [Google Scholar] [Pubmed]
- Hwang HJ, Park HJ, Chung HJ, Min HY, Park EJ, Hong JY, et al. Inhibitory effects of caffeic acid phenethyl ester on cancer cell metastasis mediated by the down-regulation of matrix metalloproteinase expression in human HT1080 fibrosarcoma cells. J Nutr Biochem. 2006; 17(5): 356-362.
[Crossref] [Google Scholar] [Pubmed]
- Schmid R, Heuckeroth S, Korf A, Smirnov A, Myers O, Dyrlund TS, et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat Biotechnol. 2023; 41(4): 447-449.
[Crossref] [Google Scholar] [Pubmed]
- Ogasawara M, Matsunaga T, Suzuki H. Differential effects of antioxidants on the in vitro invasion, growth and lung metastasis of murine colon cancer cells. Biol Pharm Bull. 2007; 30(1): 200-204.
[Crossref] [Google Scholar] [Pubmed]
- Tyśkiewicz K, Konkol M, Rój E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules. 2018; 23(10): 2625.
[Crossref] [Google Scholar] [Pubmed]
- Liu L, Zubik L, Collins FW, Marko M, Meydani M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis. 2004; 175(1): 39-49.
[Crossref] [Google Scholar] [Pubmed]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016; 5: e47.
[Crossref] [Google Scholar] [Pubmed]
- Özkök A, Darcy B, Sorkun K. Total phenolic acid and total flavonoid content of turkish pine honeydew honey. J ApiProduct ApiMedical Sci. 2010; 2(2): 65-71.
- Molyneux P. The use of the stable free radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004; 26(2): 211-219.
- Aberoumand A, Deokule SS. Comparison of phenolic compounds of some edible plants of Iran and India. Pak J Nutr. 2008; 7(4): 582-585.
- Khandelwal A, Bahadduri PM, Chang C, Polli JE, Swaan PW, Ekins S. Computational models to assign biopharmaceutics drug disposition classification from molecular structure. Pharm Res. 2007; 24(12): 2249-2262.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Indra Sen Singh* and Sidney MuzyambaCitation: Singh IS: GC-MS, GNPS and METLIN Assisted Phytochemical Profiling, Bioactivity Study and Molecular Docking Analysis of Paropsia brazzeana Root Bark, a Medicinal Plant in Zambia
Received: 18-Dec-2023 Accepted: 02-Jan-2024 Published: 10-Jan-2024, DOI: 10.31858/0975-8453.15.1.1-14
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3