Mini Review - (2024) Volume 15, Issue 1
Abstract
Although the experimental attempts to measure a single molecule/particle, i.e., an individual molecule/ particle, in dilute liquids or live cells without immobilization on a surface or hydrodynamic flow at room temperature or under physiological conditions have failed so far, this failure spurred the theory on Brownian molecular motion based on the stochastic nature of diffusion. This new physical theory for the quantifying the thermodynamic jitter of molecules/particles is inspiring for many and forms the theoretical basis of single-molecule biophysics and biochemistry, which underlies the stochastic nature of diffusion. Theoretical considerations for analyzing mobility data are summarized. Measuring the individual molecule or the individual particle is considered among the most challenging trends of research in spectroscopy, microscopy and nanoscopy.
Keywords
Diffusion, Thermodynamic jitter, Nanoscopy, Ergodicity
Introduction
The transport and binding of molecules to specific targets are necessary for the assembly and function of ordered supramolecular structures in cells. Analysis of these processes in vitro and in vivo is central in life sciences including pharmaceutical sciences. In cellular compartments transport is frequently driven by diffusion, which tends to balance concentration gradients. Mobility has been studied during the last decades by different experimental technologies, mainly based on fluorescence techniques with confocal or wide-field optics including laser scanning microscopy. Thus, time-resolved protein mobility data are related to images of cellular structures from fluorescence confocal laser scanning microscopy to identify localization-specific dynamics and interactions of a fluorescently labeled species. The spatial resolution of the mobility and interaction analysis in super-resolution microscopy is below the diffraction limit and it is not anymore limited by the diffraction-limited size of the excitation volume (observation/detection volume) in a confocal fluorescence microscopy/ spectroscopy setup. For many of these approaches, the Green Fluorescent Protein (GFP) and its spectral relatives are particularly well suited in the intracellular environment due to their high-fluorescence yield, their photobleaching properties (Harms GS, et al., 2001), and because they can be fused to proteins in vivo (White J and Stelzer E, 1999). As a result of these technological and biotechnological developments, single-molecule studies in liquids and live cells have entered the scene a couple years ago. Such studies are related to measurements of individual molecules (individual particles), but they are not the same. Fortunately, in cases of diffusion-controlled reactions and systems, respectively, there are physical criteria (see below the criteria 1 to 4) to make the decision. The figures summarizes the most important theoretical results for measuring just one molecule at a time (individual molecule/particle) in dilute liquids at room temperature or live cells under physiological conditions without immobilization on a surface or hydrodynamic flow, i.e., the self-same molecule/particle. Here, we contribute in the field for the benefit of researchers, for example mainly in pharmacy, who have no deep experience with the single-molecule detection in liquids and live cells without immobilization on a surface or without significant hydrodynamic flow.
Literature Review
The main idea of single-molecule detection without immobilization on a surface or without hydrodynamic flow in liquids at room temperature or live cells under physiological conditions is simple. A single-molecule/single-particle experiment is an experiment that investigates the properties of individual molecules/particles. Two means are of great importance. First, only one probe molecule/particle should diffuse in the excited volume, and second, the light emitted from this single molecule/particle, that is the individual molecule/particle or the selfsame molecule/particle, should be distinguishable from the experimental noise. So far so simple, but it becomes much more difficult when such a scenario is quantified in terms of the temporal motion (time resolution) of individual molecules/individual particles. The thermodynamic concept of diffusion (fluctuations, thermodynamic jitter) due to the same molecule, that is the individual molecule, was developed by Foldes-Papp Z, 2007; Foldes-Papp Z, 2007; Földes-Papp Z, 2006; Földes-Papp Z, et al., 2005; Földes-Papp Z, 2013) and from the mathematical core to the physical theory of single-molecule biophysics and biochemistry based on the stochastic nature of diffusion (Földes-Papp Z, 2015; Földes-Papp Z, et al., 2021) and it was considered pioneering work (Digman MA and Gratton E, 2011). The conceptual and theoretical basis of thermodynamic jitter has been established in series of publications.
Physical criteria are required to decide whether it is a single molecule/a single particle (an individual molecule/an individual particle) or not, as we illustrate in Figure 1. These criteria (criteria 1 to 3) result in the time-resolution of an individual molecule/individual particle in optical microscopy/nanoscopy (that is the criterion 4), which covers the most important families of dynamical systems with randomness, that means here molecule number fluctuations are currently of interest. Main theoretical results for measuring just one molecule/particle at a time (individual molecule/particle) in liquids or live cells without immobilization or hydrodynamic flow, i.e. the self-same molecule/particle (Foldes-Papp Z, 2013; Földes-Papp Z, 2021; Baumann G and Foldes-Papp Z, 2022).
Discussion
Stochastic thermodynamics rules the physical formulation of the single-molecule time resolution (Tm) which can be explained by the below mentioned equation-
Tm=τdif(t)/cm.△V.K
Where, K stands for the proportionality factor of the equation with K=e(cm.NA. △V)/NA
Here e=Natural exponential function
Tm=Single molecule time resolution, i.e., the meaningful time of measuring just the same molecule in dilute solutions and live cells without immobilization or hydrodynamic flow.
τdif(t)=Diffusion time of the molecule
cm=Molar concentration of molecules of the same kind in the bulk (bulk phase).
△V=Observation/detection volume
NA=Avagadro constant number and it is defined as NA=6.02214076 × 1023 mol-1
Obviously the dimension of Tm is the dimension of τdif
The number of molecules averaged over the measurement time T is gives as mentioned below-
Nlmax=T/Tm
The basic results are due to Markov processes which are the door to dynamical systems that fluctuate, for example in their number of molecules under observation. The probability of separating two individual molecules or two individual particles as independent molecular units during the measurement time is the criterion 3 as demonstrated by Földes-Papp Z, 2021 in the Table 1 there under different experimental measurement conditions.
| Experimental criteria | Formulas |
|---|---|
| Criterion 1 | N ≤ 1 |
| N is the absolute number of the specific molecules (labeled, studied) in the observation/detection volume | It is the Poisson probability of detecting single molecules of the same kind in the observation/detection volume |
| Criterion 2 | ln(C)-ln(2)-c |
| C denotes the (true) mean value of the population (subpopulation) of specific (e.g. fluorescent) molecules of the same kind (the average molecule number) in the observation/detection volume | It describes the analytical sensitivity that the observation/detection volume contains a single molecule of the same kind |
| Criterion 3 | |
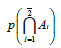 Denotes the selfsame molecule likelihood estimators which are the probabilities that a second molecule of the same kind (e.g. a second fluorescent molecule) is outside a boundary at time, meaning outside the lower limit of distance of the observation/detection volume |
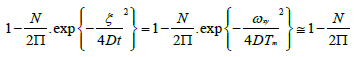 It shows the likelihood to really see the selfsame molecule that is the individual molecule in the observation/detection volume |
Table 1: Experimental conditions that must be set up in the “single-molecule experiments” to analyze the meaningful times as shown in the figures 1 and 2 in liquids, living cells and artificial as well as biological membranes without immobilization and without hydrodynamic flow (Foldes-Papp Z, 2007; Földes-Papp Z, et al., 2005; Földes-Papp Z, 2006; Földes-Papp Z, 2021)
Looking deeper into this physical theory of single-molecule detection of one and the same molecule/particle (the individual molecule/particle) that is the theory of single molecule biophysics and biochemistry based on the stochastic nature of diffusion in dilute liquids and single live cells without immobilization on artificial or biological surfaces/membranes or without significant hydrodynamic flow is merely the way to understand what occurs during diffusion of an individual molecule/particle in the observation/detection volume embedded into a bulk phase such as liquids as well as live cell compartment or membrane (Földes-Papp Z and Baumann G, 2011; Baumann G, et al., 2010). Let us now focus on this in a more broadly comprehensible way.
So, we have diffused to about 1 nanometer from another protein. Our encounter has begun, and in roughly a nanosecond we will either touch the surface of the other protein, or be repelled by it. What happens in this nanosecond occupied several publications. We got into the ergodic hypothesis where it was clear that ‘single molecule’ behavior should not be extrapolated to all molecule behavior (Földes-Papp Z and Baumann G, 2011). By simulation experiments we found the minimal variation if the number of randomly selected single-molecule tracks is Nℓmax=32. All other values of Nℓ deliver only a local minimum instead of a global minimum. The graphs in Figure 1 also showed that the variation approaches a stable value if Nℓ approaches large values; i.e. only a small subpopulation of single molecules delivers the minimal variation (Földes-Papp Z and Baumann G, 2011). In other words, the most striking feature of performing ensemble averaging in sparse subpopulations of single molecules, however, is the same mean value obtained in an ergodic system that is a many molecule system, if the number of randomly selected single-molecule tracks is Nℓmax=32. Hence, broken ergodicity and unbroken ergodicity are not anymore distinguishable. In addition, when averaging procedures are carried out without knowing whether the underlying molecular system behaves in ergodic or non-ergodic ways, each measurement can be related to an ergodic or a non-ergodic behavior unless one is able to show the single-molecule fingerprint of non-ergodicity (Földes-Papp Z and Baumann G, 2011).
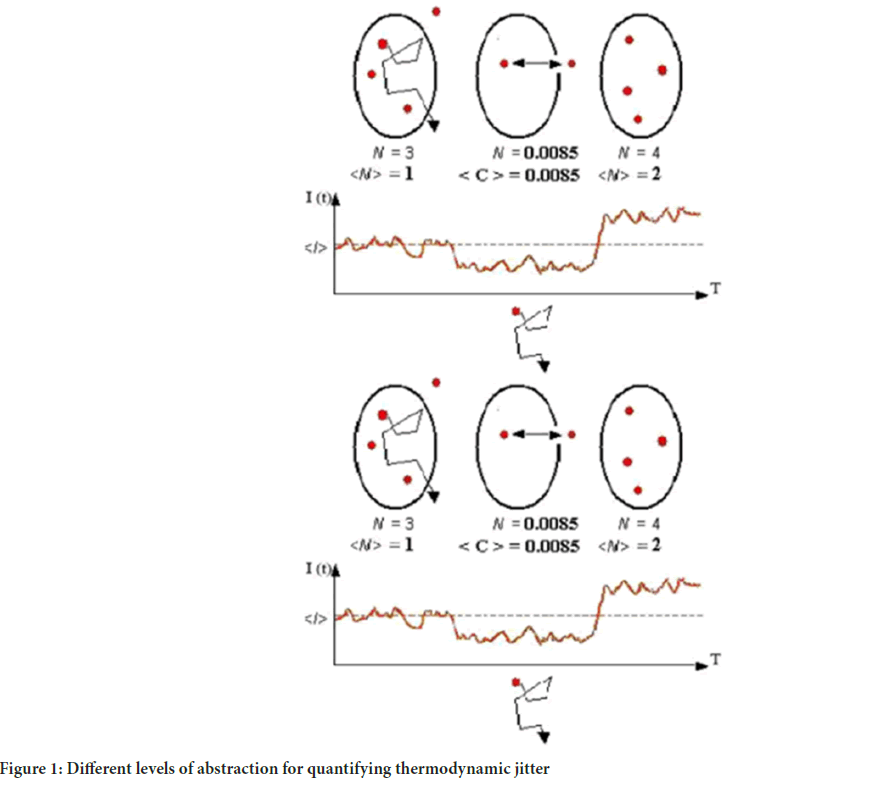
Figure 1: Different levels of abstraction for quantifying thermodynamic jitter
The single-molecule/particle fingerprint of non-ergodicity is the thermodynamic fingerprint (signature) of an individual molecule/particle in liquids or live cells without immobilization or without significant hydrodynamic flow (Földes-Papp Z, 2023). The novel physical theory presented offers a new way to understand the molecular behavior when single biomacromolecules are trapped in interactions with their neighboring ligands and reaction partner(s), respectively, in a crowded environment at room temperature or at physiological conditions in live cells and their cellular compartments (Baumann G and Földes-Papp Z, 2022). Therefore, single-molecule studies may be contrasted with measurements on an ensemble or bulk collection of molecules, where the individual behavior of molecules cannot be distinguished and only average characteristics can be measured. It clearly follows from these results that the biggest breakthrough in microscopy/nanoscopy and spectroscopy would be a breakthrough in sensitivity for measuring an individual single molecule (one and the same molecule) over several milliseconds to seconds and even minutes without immobilization on artificial surfaces or biological membranes as well as without significant hydrodynamics.
The measurement time for measuring just one single molecule (individual molecule, selfsame molecule) or one single particle (individual particle, selfsame particle) is a meaningful time in contrast to the measurement times for the many-molecule measurements. From the inspection of the mathematically derived and well experimentally founded physical relationships on the basis of stochastic translational diffusion (Baumann G and Földes-Papp Z, 2022), we can finally distinguish three types of meaningful times-
• The meaningful time as single molecule/single particle time- resolution discussed here in detail (Földes-Papp Z, 2013),
• The meaningful time as limits in measurement time that should not be exceeded in order to follow the same single molecule/same single particle with high probability in one, two or three dimensions also discussed above (Table 1) (Földes-Papp Z, 2021)
• The meaningful time as quantitative measure for the meaningful reentries of the same single molecule/same single particle in the observation/detection volume (Foldes-Papp Z, 2007).
The dimensions of the meaningful times are the dimensions of the diffusion times of the molecule/particle in liquids and live cells without immobilization or without significant hydrodynamic flow. The measurement of the individual molecule or the individual particle is considered one of the most demanding research trends in spectroscopy, microscopy and nanoscopy because individual molecules (oligonucleotides and single-stranded DNA sequences) were theoretically described in chemical oligonucleotide syntheses on solid supports after release from the solid phase as early as 1994 for the first time (Földes-Papp Z, et al., 1994).
Conclusion
Not least because individual molecules (oligonucleotides and single-stranded DNA sequences) were theoretically described in chemical oligonucleotide syntheses on solid supports after release from the solid phase as early as 1994, the biggest breakthrough would be increasing the sensitivity of measurements in liquids (solution) and live cells including membranes without immobilization or hydrodynamic flow. To avoid the single molecule expert in own measurements; use the formulas and relationships provided in this paper. These can be easily and safely applied to determine how many molecules in the measurements are averaged during the measurement of the time.
References
- Harms GS, Cognet L, Lommerse PH, Blab GA, Schmidt T. Autofluorescent proteins in single-molecule research: Applications to live cell imaging microscopy. Biophys J. 2001; 80(5): 2396-2408.
[Crossref] [Google scholar] [Pubmed]
- White J, Stelzer E. Photobleaching GFP reveals protein dynamics inside live cells. Trends Cell Biol. 1999; 9(2): 61-65.
[Crossref] [Google scholar] [Pubmed]
- Foldes-Papp Z. Fluorescence fluctuation spectroscopic approaches to the study of a single molecule diffusing in solution and a live cell without systemic drift or convection: A theoretical study. Curr Pharm Biotechnol. 2007; 8(5): 261-273.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z. ‘True’single-molecule molecule observations by fluorescence correlation spectroscopy and two-color fluorescence cross-correlation spectroscopy. Exp Mol Pathol. 2007; 82(2): 147-155.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z. What it means to measure a single molecule in a solution by fluorescence fluctuation spectroscopy. Exp Mol Pathol. 2006; 80(3): 209-218.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z, Baumann G, Kinjo M, Tamura M. Single-Phase Single-Molecule Fluorescence Correlation Spectroscopy (SPSM-FCS). Encyclopedia of medical genomics and proteomics. 2005.
- Foldes-Papp Z. Measurements of single molecules in solution and live cells over longer observation times than those currently possible: The meaningful time. Curr Pharm Biotechnol. 2013; 14(4): 441-444.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z. Individual macromolecule motion in a crowded living cell. Curr Pharm Biotechnol. 2015; 16(1): 1-2.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z, Baumann G, Li LC. Visualization of subdiffusive sites in a live single cell. J Biol Methods. 2021;8(1): 1-8.
[Crossref] [Google scholar] [Pubmed]
- Digman MA, Gratton E. Lessons in fluctuation correlation spectroscopy. Annu Rev Phys Chem. 2011; 62: 645-668.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z. Single-molecule time resolution in dilute liquids and live cells at the molecular scale: Constraints on the measurement time. Am J Transl Res. 2021; 5(3): 154-165.
- Baumann G, Foldes-Papp Z. Study on single-molecule biophysics and biochemistry in dilute liquids and live cells without immobilization or significant hydrodynamic flow: The thermodynamic single-molecule demon. Curr Pharm Biotechnol. 2022; 23(14): 1750-1757.
[Crossref] [Google scholar] [Pubmed]
- Foldes-Papp Z, Baumann G. Fluorescence molecule counting for single-molecule studies in crowded environment of living cells without and with broken ergodicity. Curr Pharm Biotechnol. 2011; 12(5): 824-833.
[Crossref] [Google scholar] [Pubmed]
- Baumann G, Place RF, Foldes-Papp Z. Meaningful interpretation of subdiffusive measurements in living cells (crowded environment) by fluorescence fluctuation microscopy. Curr Pharm Biotechnol. 2010; 11(5): 527-543.
[Crossref] [Google scholar] [Pubmed]
- Földes-Papp Z. The thermodynamic signature of a single molecule or a single particle in dilute liquids and live cells: Single-molecule biophysics and biochemistry based on the stochastic nature of diffusion. Am J Transl Med. 2023; 7(2): 74-77. Am J Transl Med. 2023; 7(2): 74-77.
- Földes-Papp Z, Herold A, Seliger H, Kleinschmidt AK. Error propagation theory of chemically solid phase synthesized oligonucleotides and DNA sequences for biomedical application. Fractals in Biology and Medicine. 1994.
Author Info
Franz Proust*Citation: Földes-Papp Z: Single-Molecule Detection in Dilute Liquids and Live Cells without Immobilization or Significant Hydrodynamic Flow at Room Temperature or under Physiological Conditions: Too Much Thermodynamic Jitter
Received: 18-Dec-2023 Accepted: 02-Apr-2024 Published: 10-Jan-2024, DOI: 10.31858/0975-8453.15.1.29-32
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






